Activation of Persulfates Using Alkali-Modified Activated Coke to Promote Phenol Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Alkali-Modified AC Preparation
2.3. Experimental Process
2.3.1. Batch Experiment
2.3.2. Radical Quenching Experiments
2.4. Characterizations
3. Result and Discussions
3.1. Characterizations Results
3.1.1. BET Analysis Results
3.1.2. SEM
3.1.3. XRD
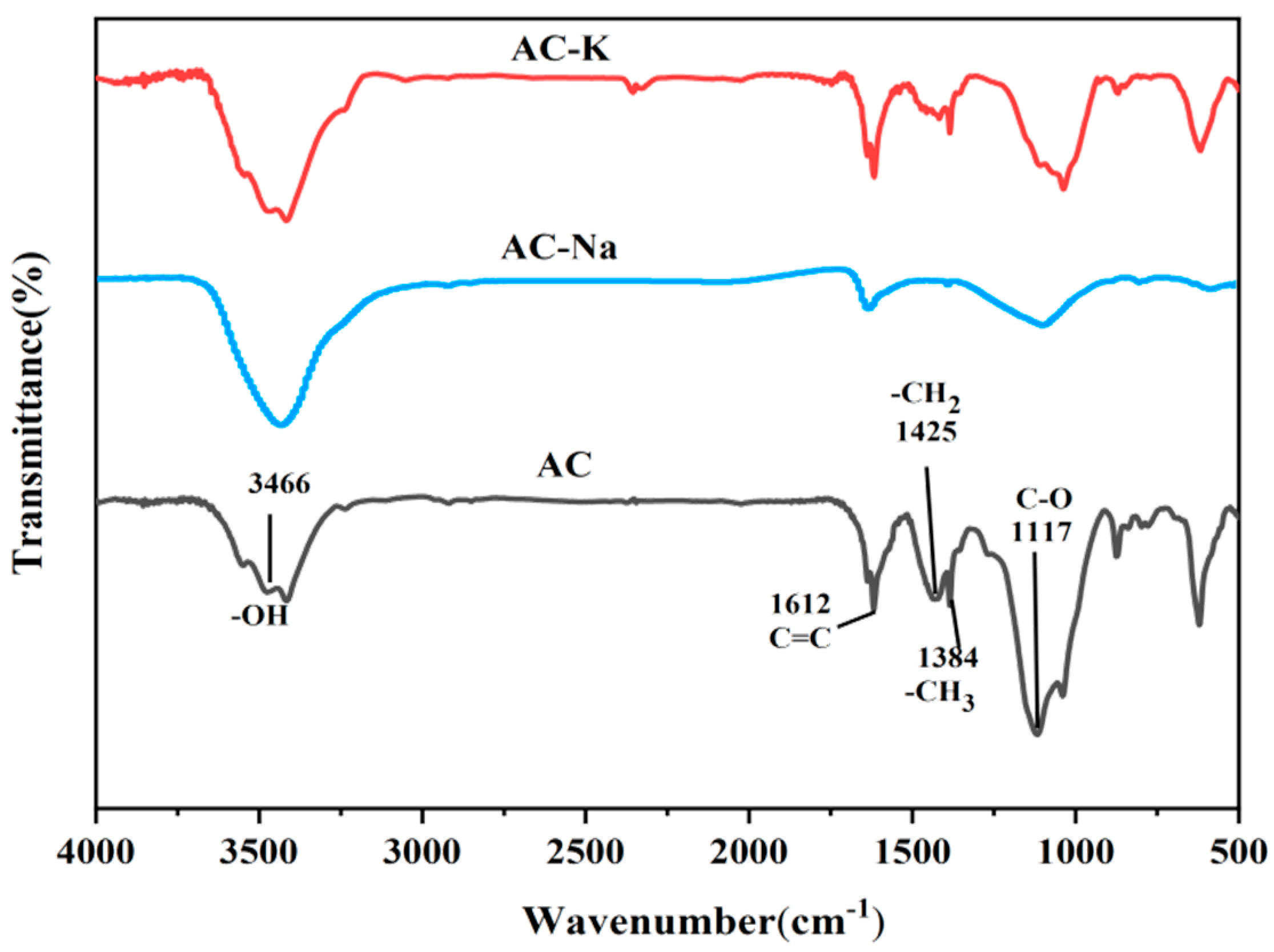
3.1.4. FTIR
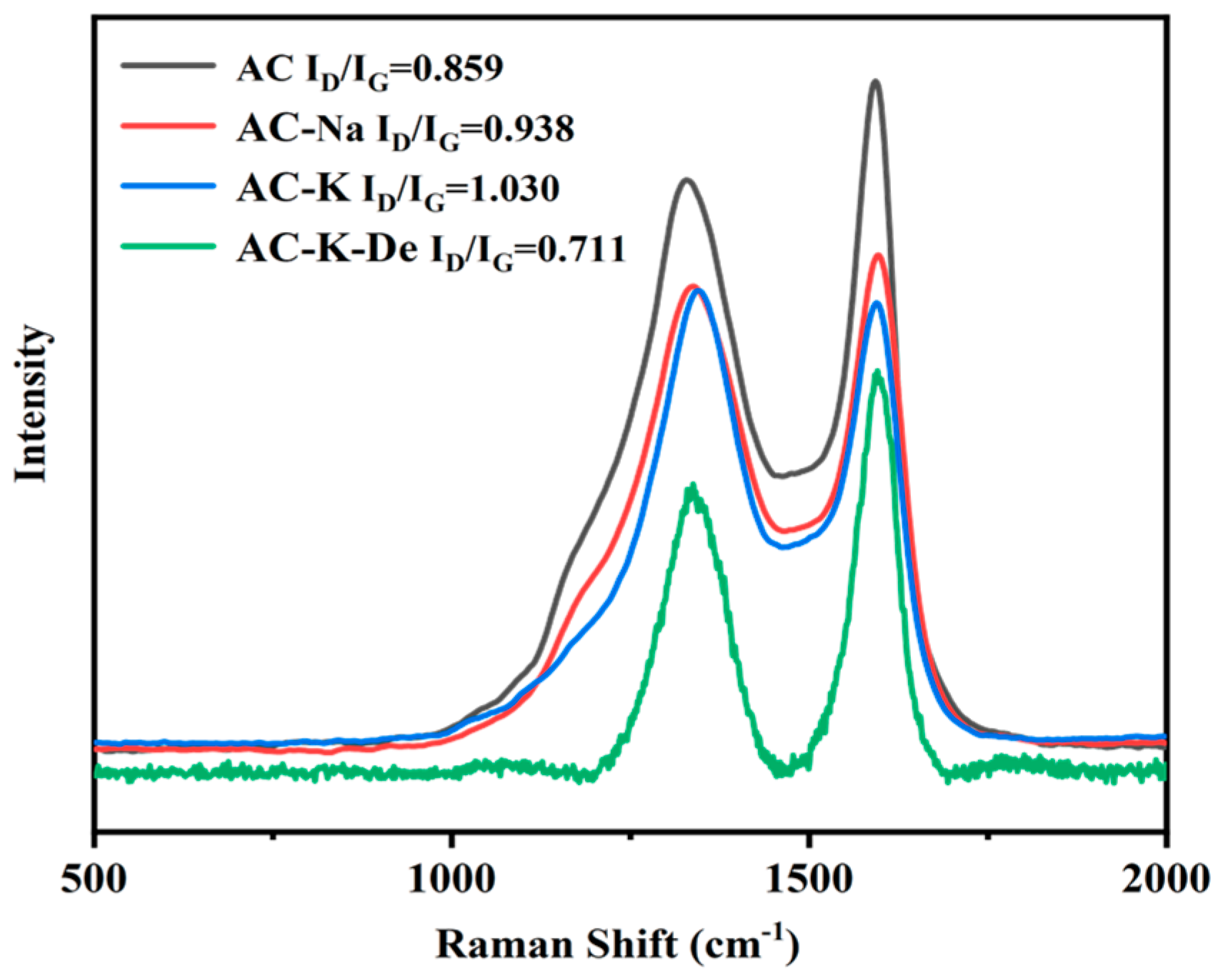
3.1.5. Raman Spectroscopy
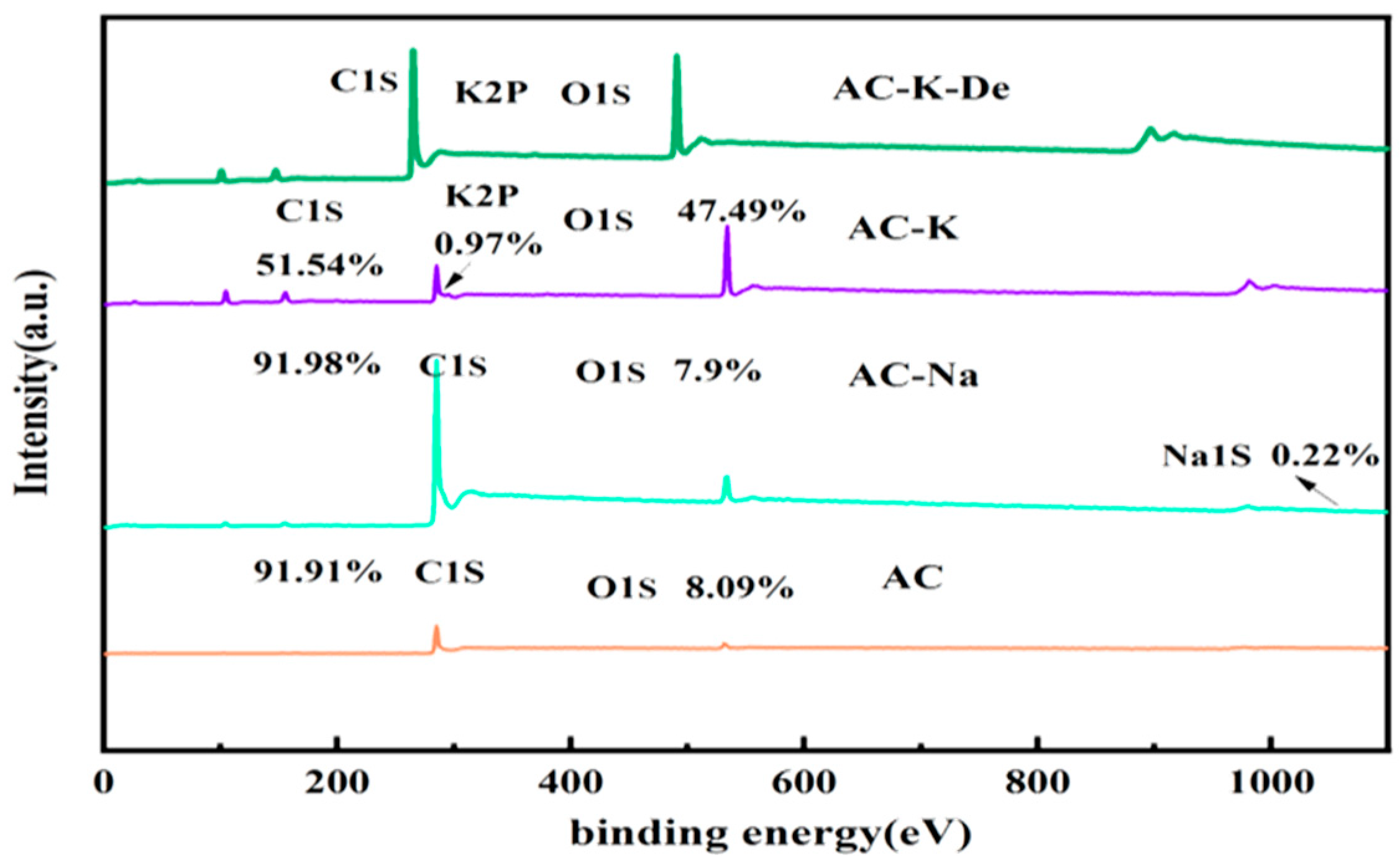
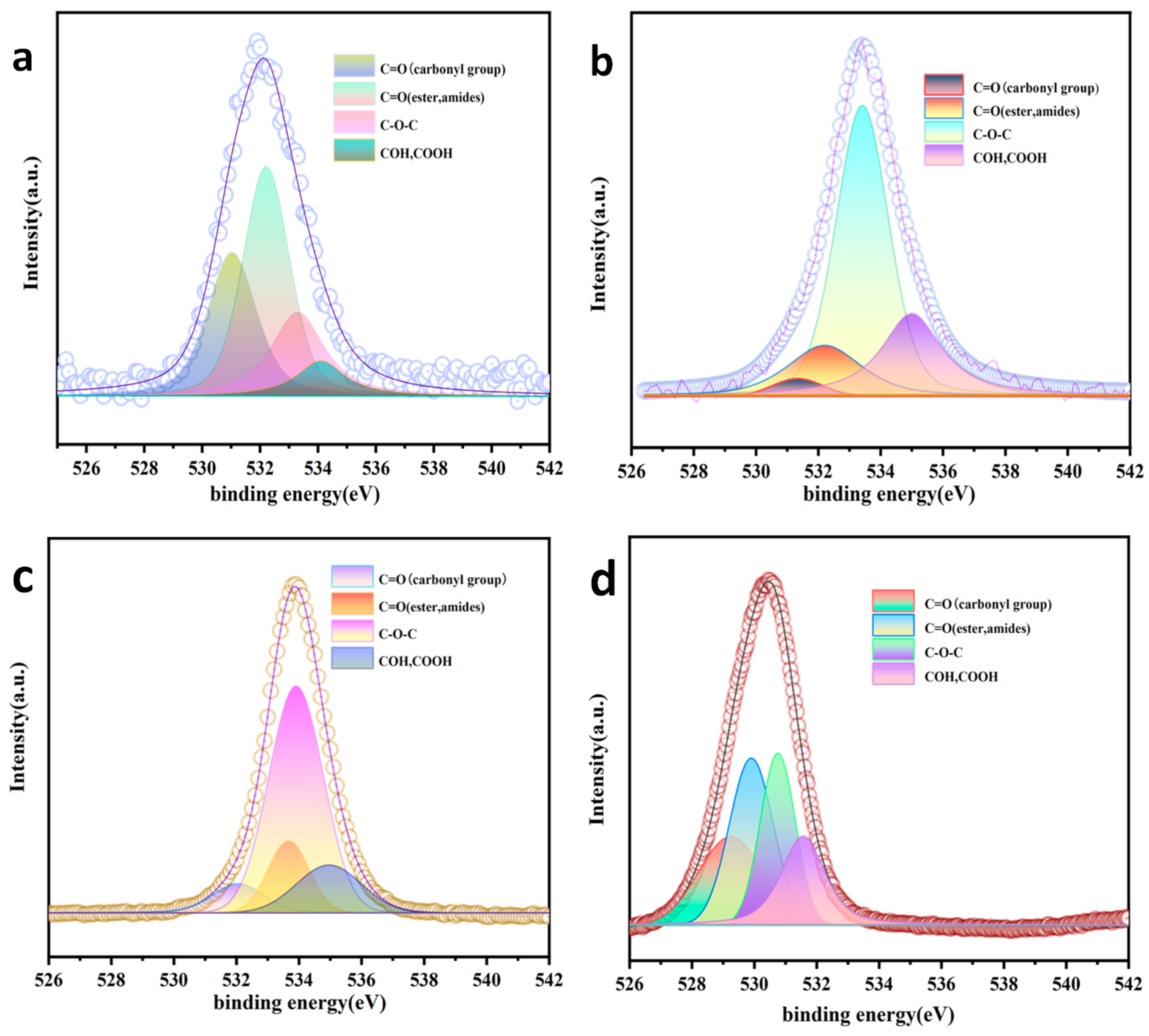
3.1.6. XPS
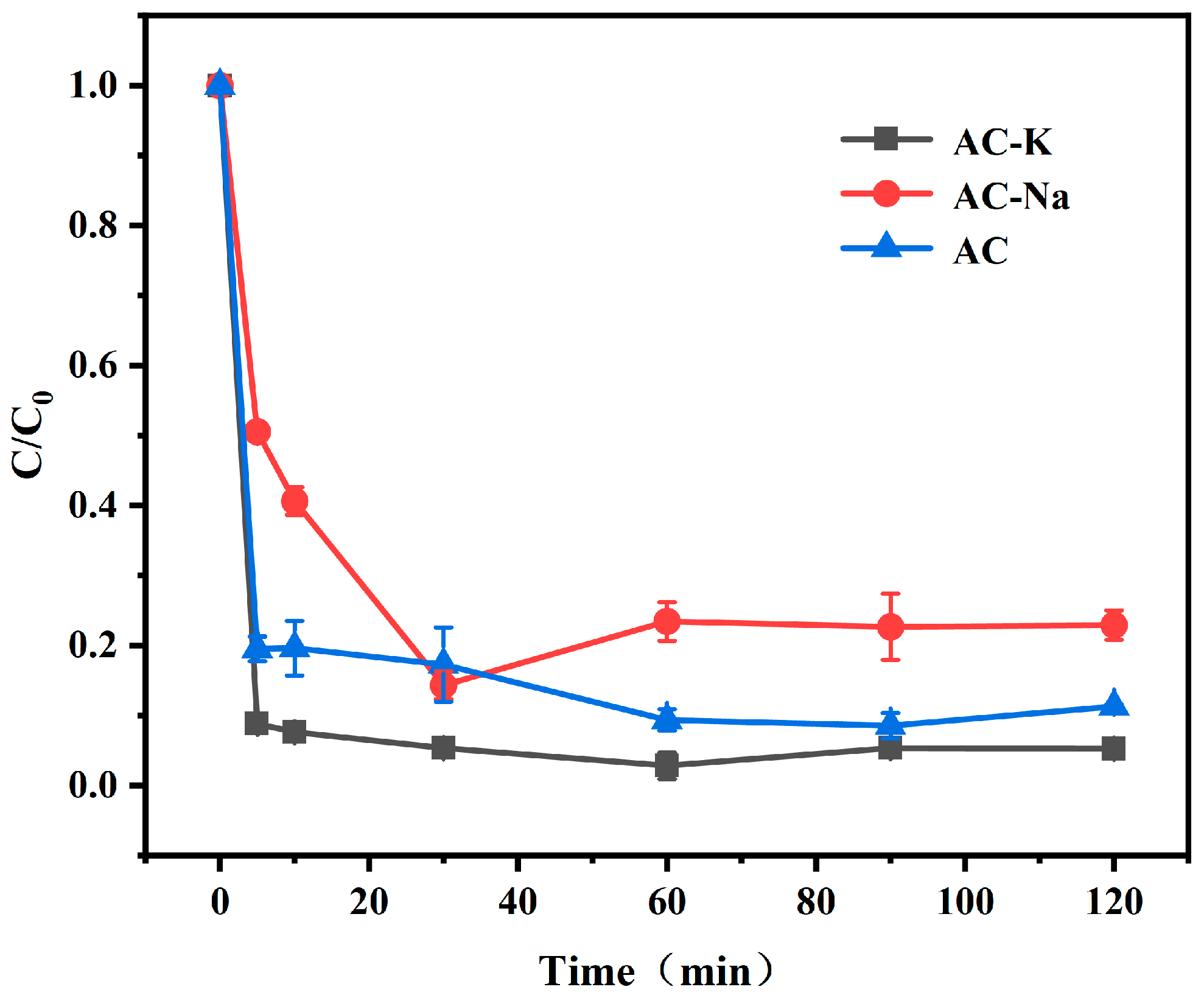
3.2. Determination of Alkali Modifier
3.3. Factors Influencing Ph Removal of MAC/PS Systems
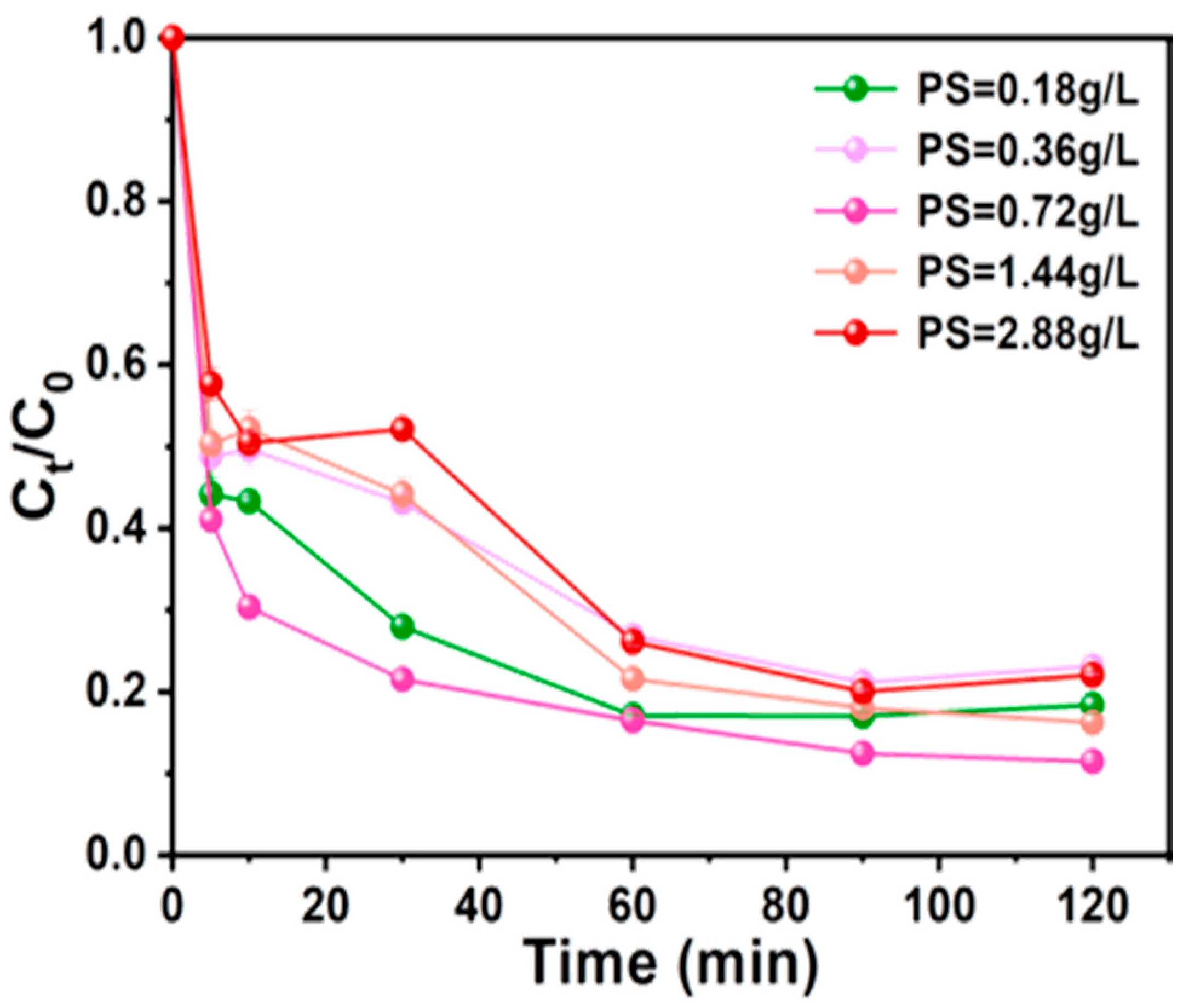
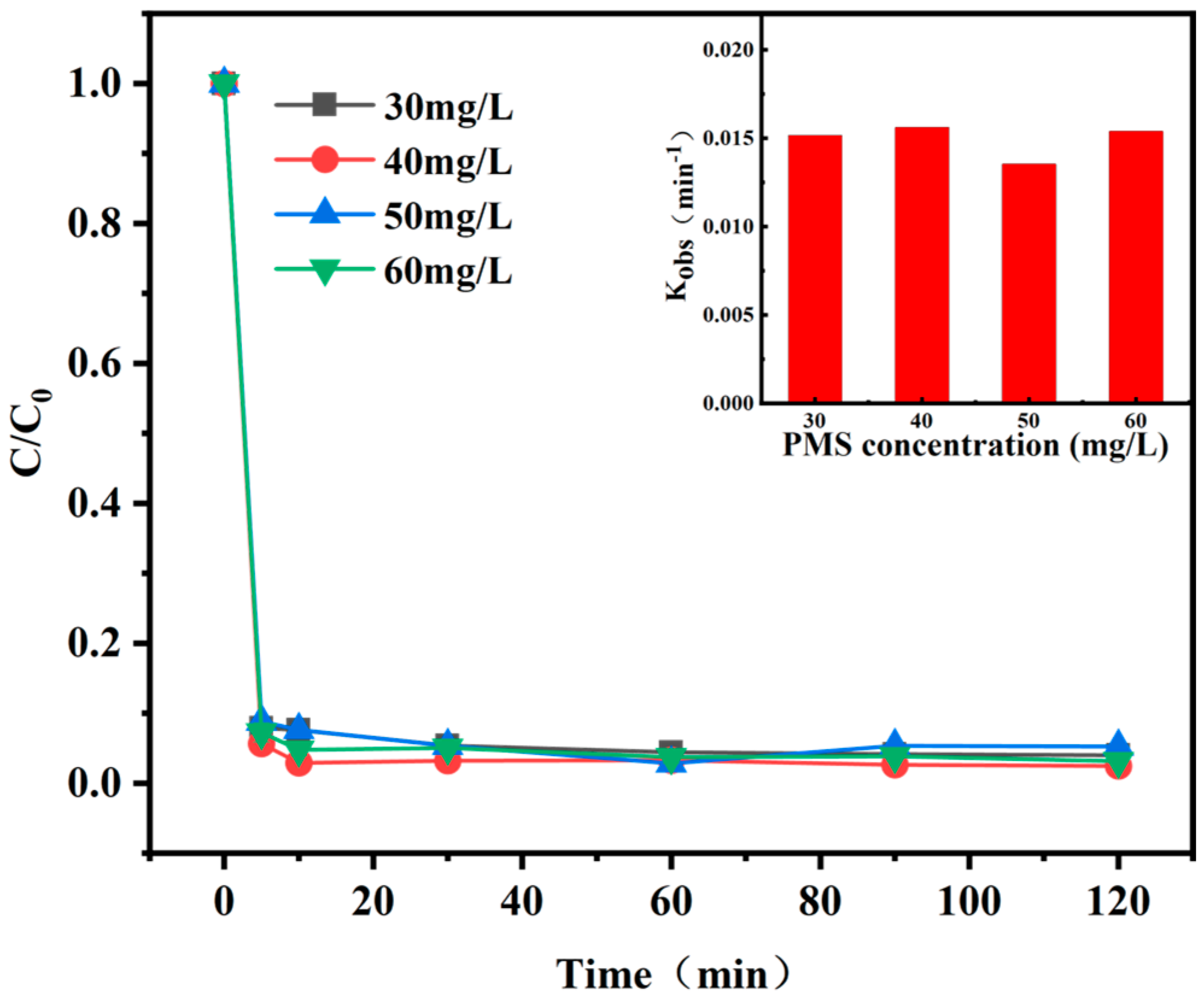
3.3.1. PS Dosage
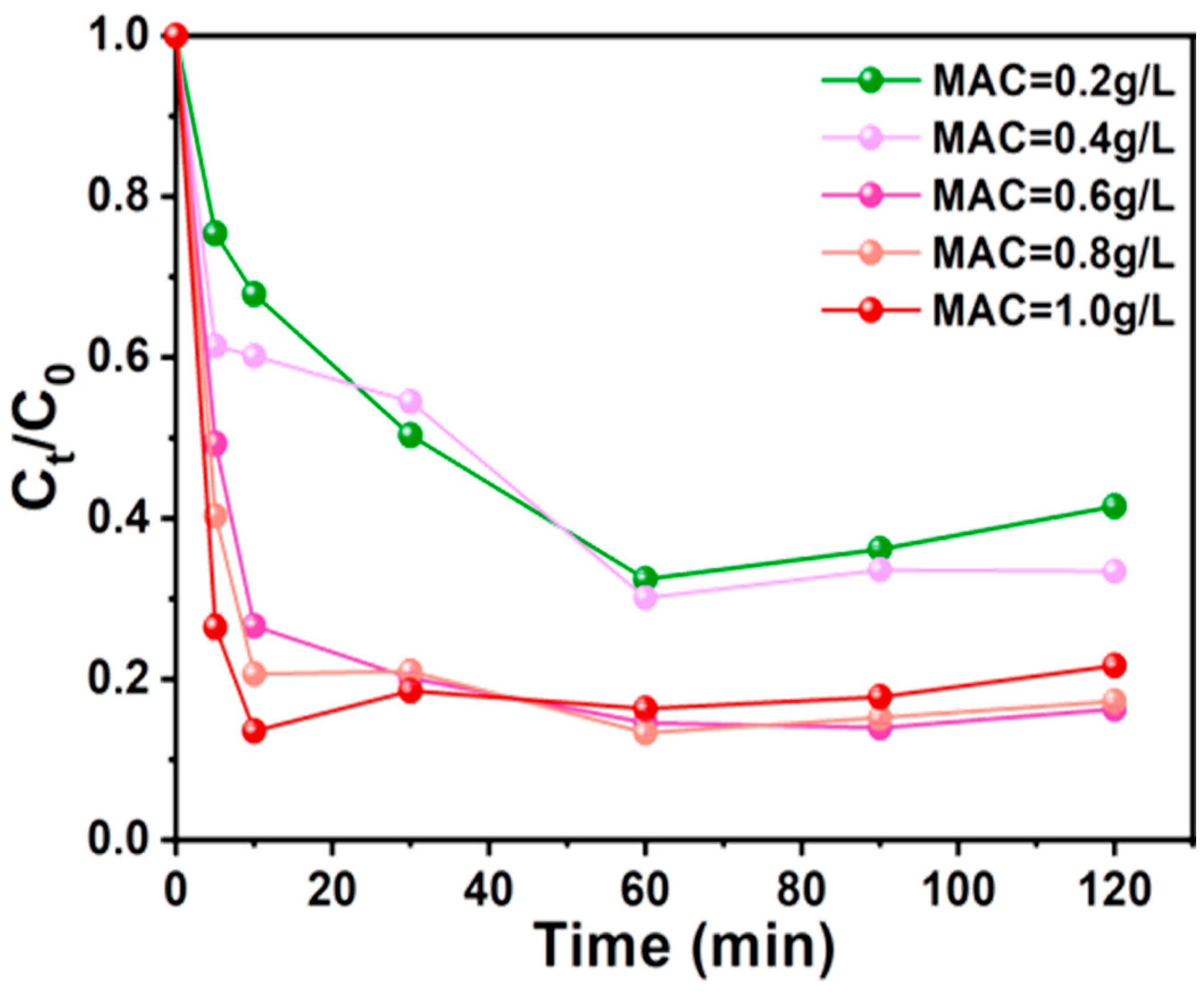
3.3.2. AC-K Dosage
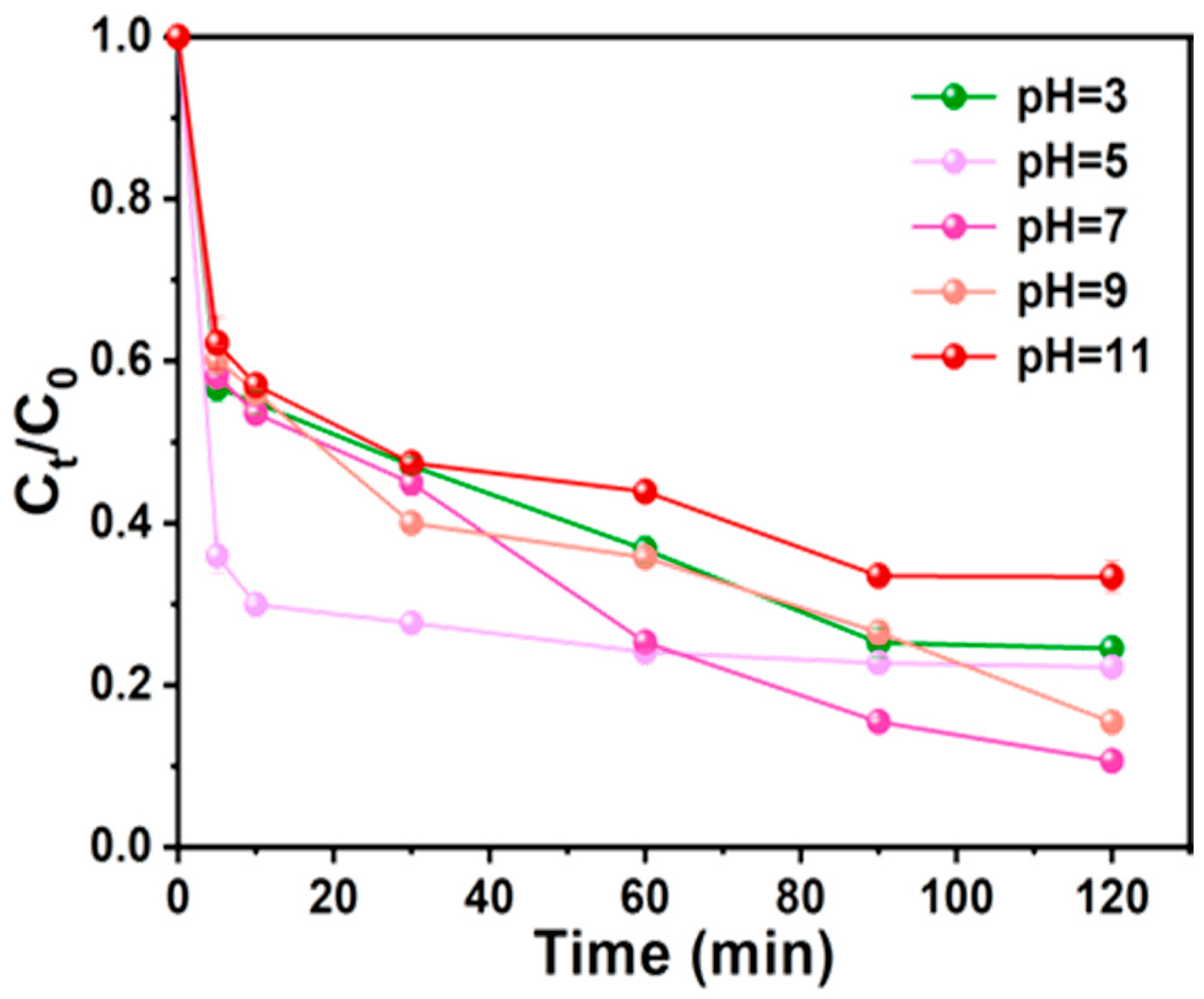
3.3.3. Effect of pH
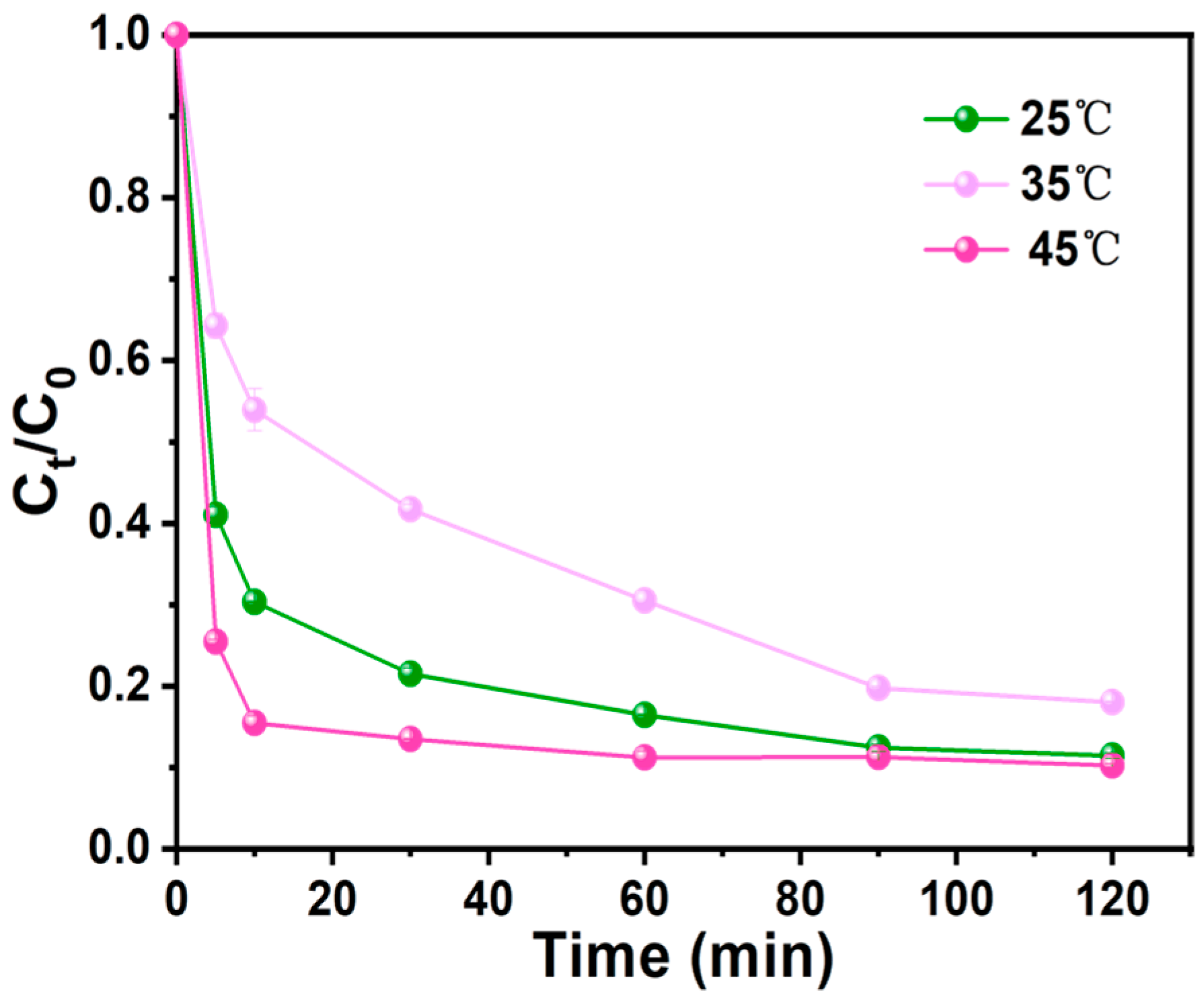
3.3.4. Effect of Temperature
3.3.5. Effect of Initial Concentration of Ph
3.4. Reactivity and Reusability of MAC
3.5. Identification of Primary ROS
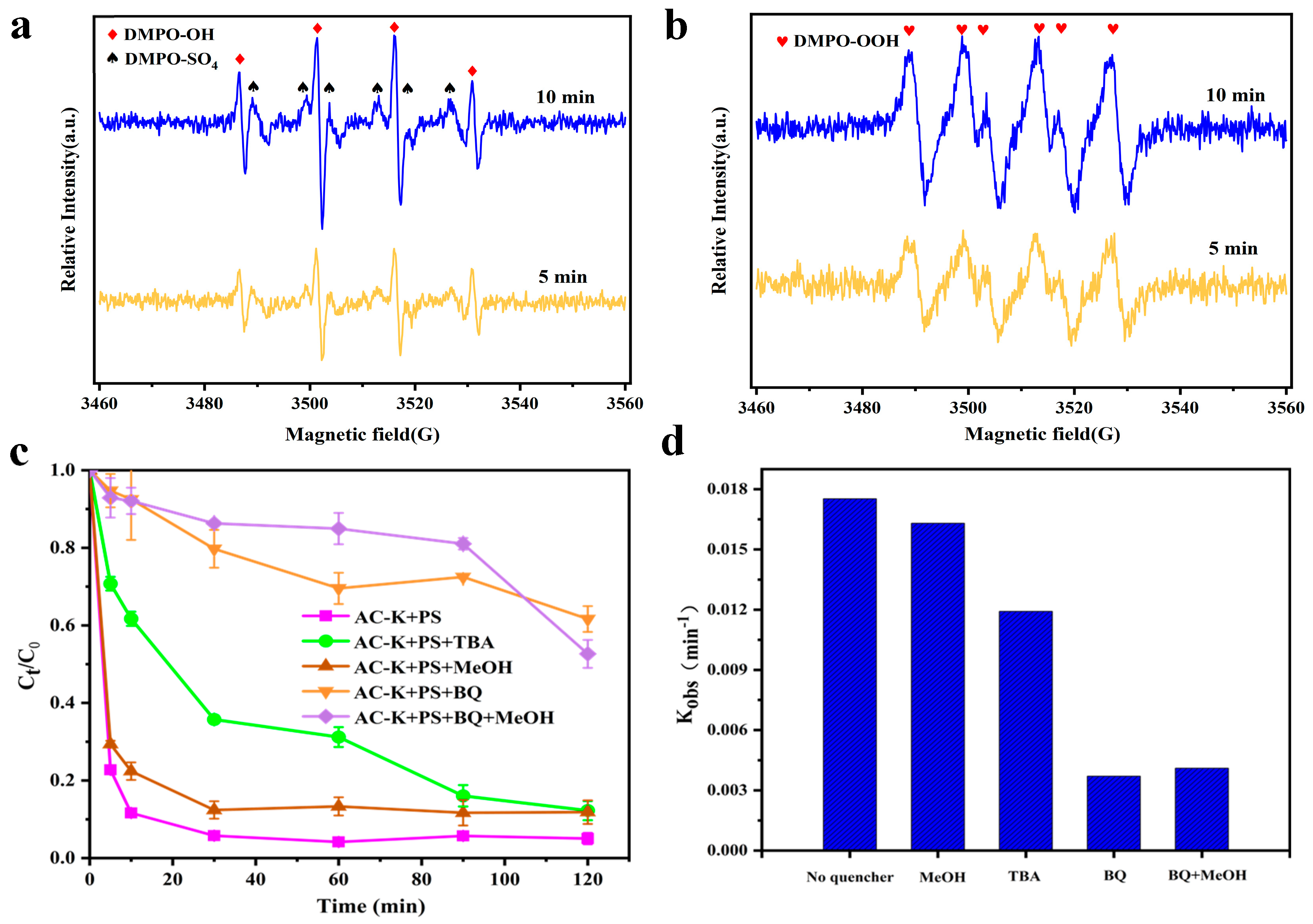
3.5.1. Radical Scavenging
3.5.2. EPR Analysis
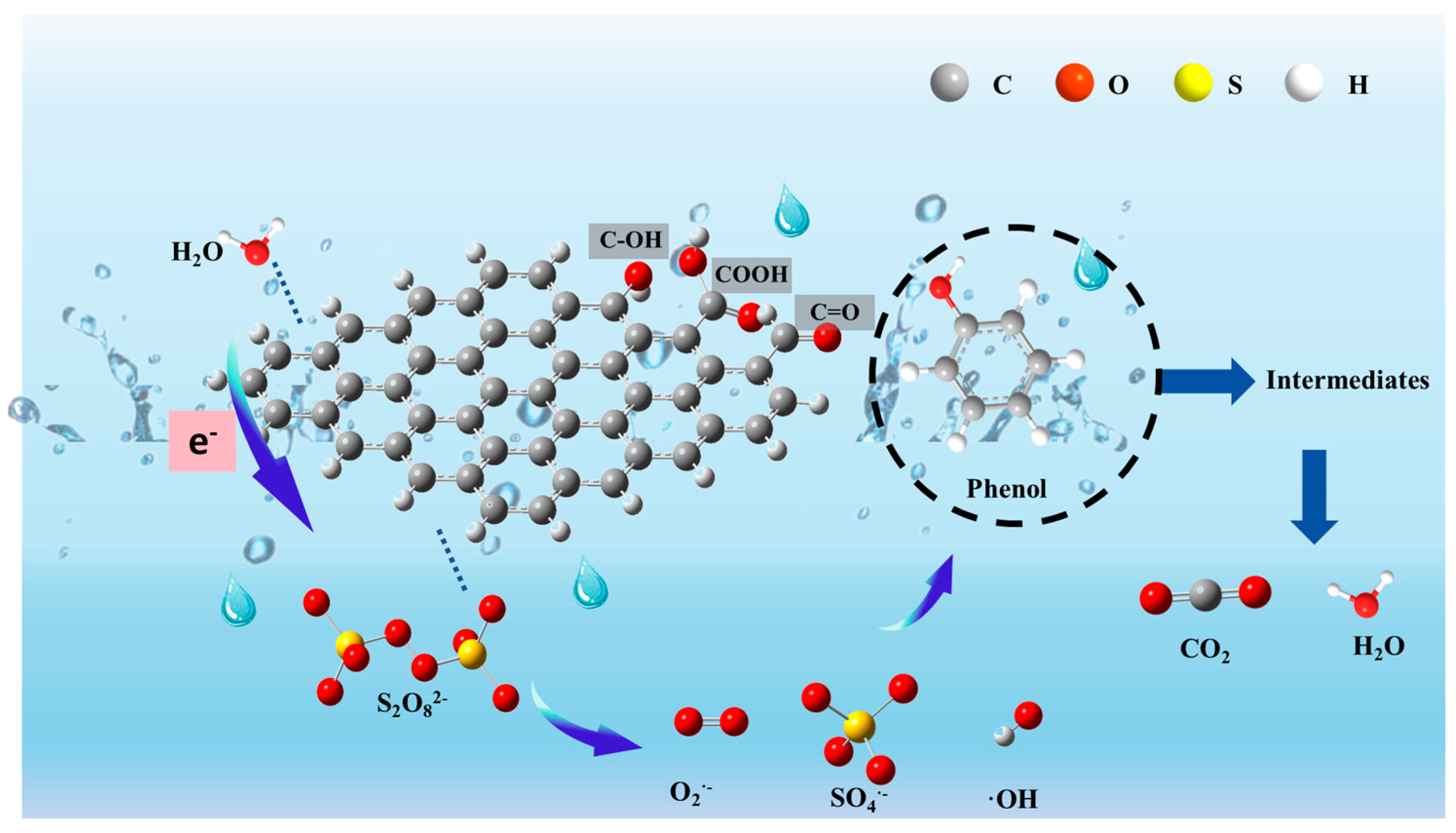
3.6. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samsnavith, S.; Varun, A.; Gowtham, N.; Samdavid, S. Removal of phenol from aqueous phenol solution using bio-emulsion. Sep. Purif. Technol. 2021, 275, 119138. [Google Scholar]
- Acosta, C.A.; Pasquali, C.E.L.; Paniagua, G.; Garcinuño, R.M.; Hernando, P.F. Evaluation of total phenol pollution in water of San Martin Canal from Santiago del Estero Argentina. Environ. Pollut. 2018, 236, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M. The functions of taste and olfaction. Ann. N. Y. Acad. Sci. 1989, 575, 353–362. [Google Scholar] [CrossRef]
- Danny, V.; Yuhe, L.; Wouter, S.; Bert, F.S. Alkylphenols to phenol and olefins by zeolite catalysis: A pathway to valorize raw and fossilized lignocellulose. Green Chem. 2016, 18, 297–306. [Google Scholar]
- Otto, M. Phenol-soluble modulins. Int. J. Med. Microbiol. 2014, 304, 164–169. [Google Scholar] [CrossRef]
- Schmidt, R.J. Industrial catalytic processes—Phenol production. Appl. Catal. A-Gen. 2005, 280, 89–103. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols—Sources and Toxicity. Pol. J. Environ. Stud. Stud. 2007, 16, 347–362. [Google Scholar]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Debjani, M.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Frascari, D.; Bacca, A.E.M.; Zama, F.; Bertin, L.; Fava, F.; Pinelli, D. Olive mill wastewater valorisation through phenolic compounds adsorption in a continuous flow column. Chem. Eng. J. 2016, 283, 293–303. [Google Scholar] [CrossRef]
- Gümüş, D.; Akbal, F. Comparison of Fenton and electro-Fenton processes for oxidation of phenol. Process Saf. Environ. Prot. 2016, 103, 252–258. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Madeira, L.M. p-Nitrophenol removal by activated persulfate. Environ. Technol. Innov. 2021, 210, 101265. [Google Scholar] [CrossRef]
- Zhang, J.K.; Chen, L.; Zhang, X.Y. Removal of P-nitrophenol by nano zero valent iron-cobalt and activated persulfate supported onto activated carbon. Water 2022, 14, 1387. [Google Scholar] [CrossRef]
- Shi, C.F.; Li, Y.M.; Feng, H.Y.; Jia, S.M.; Xue, R.J.; Li, G.; Wang, G.X. Removal of p-nitrophenol using persulfate activated by biochars prepared from different biomass materials. Chem. Res. Chin. Univ. 2018, 34, 39–43. [Google Scholar] [CrossRef]
- Li, W.L.; Zhang, Q.Y.; Zhang, J.; Zheng, Y.H.; Zhang, H.; Liu, J.; Cui, Y.B. Fabrication of hydrophobic regenerated activated carbon with high specific surface area. J. Mater. Sci. 2021, 56, 19969–19982. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.F.; Chen, Z.L.; Cheng, F.Q. Factors controlling adsorption of recalcitrant organic contaminant from bio-treated coking wastewater using lignite activated coke and coal tar-derived activated carbon. J. Chem. Technol. Biotechnol. 2018, 93, 112–120. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Bhatia, S.K. Characterization of heat-treated porous carbons using argon adsorption. Carbon 2006, 44, 646–652. [Google Scholar] [CrossRef]
- Li, S.J.; Han, K.H.; Li, J.X.; Li, M.; Lu, C.M. Preparation and characterization of super activated carbonproduced from gulfweed by KOH activation. Microporous Mesoporous Mater. 2017, 243, 291–300. [Google Scholar] [CrossRef]
- Bai, H.F.; Wang, B.; Talifu, D.; Abulizi, A.; Maihemuti, M. Treatment on thiodicarb in pesticide wastewater with walnut shells-derived carbon and its improved modification: Adsorption behavior. Water Sci. Technol. 2022, 85, 2682–2692. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Juang, R.S. Surface modifications of carbonaceous materials forcarbon dioxide adsorption: A review. J. Taiwan Inst. Chem. E 2017, 71, 214–234. [Google Scholar] [CrossRef]
- Yang, S.Y.; Li, L.; Xiao, T.; Zheng, D.; Zhang, Y.T. Role of surface chemistry in modified ACF (activated carbon fiber)-catalyzed peroxymonosulfate oxidation. Appl. Surf. Sci. 2016, 383, 142–150. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.F.; Cheng, H.F.; Hu, M.S.; Zhang, S. Classification and carbon structural transformation from anthracite to natural coaly graphite by XRD, Raman spectroscopy, and HRTEM. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119286. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Xu, S.P.; Gan, Y.X.; Liu, S.Q.; Liu, C.H. Effect of Precarbonization of Petroleum Cokes on Chemical Activation Process with KOH. Carbon 2005, 43, 2295–2301. [Google Scholar]
- Lillo-Ródenas, M.A.; Juan-Juan, J.; Cazorla-Amorós, D.; Linares-Solano, A. About reactions occurring during chemical activation with hydroxides. Carbon 2004, 42, 1371–1375. [Google Scholar] [CrossRef]
- Qiang, X.; Chen, Q.R. Approaches to lmprove the Quality of Activated Carbon from Coal. Coal Convers. 1996, 19, 46–52. [Google Scholar]
- Li, W.; Zhu, Y.M. Structural characteristics of coal vitrinite during pyrolysis. Energy Fuel 2014, 28, 3645–3654. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.L.; Xue, L.H. Calibration of binding energy positions with C1s for XPS results. J. Wuhan Univ. Technol. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organization. Carbon 2005, 43, 43786–43795. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- He, L.; Li, M.X.; Chen, F.; Yang, S.S.; Ding, J.; Ding, L.; Ren, N.Q. Novel coagulation waste-based Fe-containing carbonaceous catalyst as peroxymonosulfate activator for pollutants degradation: Role of ROS and electron transfer pathway. J. Hazard. Mater. 2021, 417, 126113. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zeng, Z.Q.; Liu, Y.J.; Huang, Z.G.; Cui, Y.M.; Yang, J.Y. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics. J. Mater. Chem. A 2018, 6, 4055–4067. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zeng, Z.Q.; Zhu, Y.C.; Huang, Z.G.; Cui, Y.M.; Yang, J.Y. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulture—Doped hierarchically porous carbon derived from thiophene. Appl. Catal. B Environ. 2018, 220, 635–644. [Google Scholar] [CrossRef]
- Zhao, C.K.; Zhong, S.; Li, C.Y.; Zhou, H.Y.; Zhang, S.Y. Property and mechanism of phenol degradation by biochar activated persulfate. J. Mater. Res. Technol. 2020, 9, 601–609. [Google Scholar] [CrossRef]
- Wang, S.; Lu, H.; Wang, J. Nitrogen, sulfur and oxygen co-doped carbon-armored Co/Co9S8 rods (Co/Co9S8@N-S-O-C) as efficient activator of peroxymonosulfate for sulfamethoxazole degradation. J. Hazard. Mater. 2020, 387, 121669–121679. [Google Scholar] [CrossRef]
- Weng, X.; Owens, G.; Chen, Z. Synergetic adsorption and Fenton-like oxidation for simultaneous removal of ofloxacin and enrofloxacin using green synthesized Fe NPs. Chem. Eng. J. 2020, 382, 122871–122882. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.J.; Jeong, J.; Lee, J.; Park, N.B.; Lee, C. Activation of persulfates bycarbon nanotubes: Oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 2015, 266, 28–33. [Google Scholar] [CrossRef]
- Pang, E.; Zhao, S.; Wang, B.; Niu, G.; Song, X.Z.; Lan, M.H. Strategies to construct efficient singlet oxygen-generating photosensitizers. J. Coord. Chem. Rev. 2022, 472, 214780. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.; Watts, R. Mechanism of base activation of persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef]
- Belver, C.; Aranda, P.; Ruiz-Hitzky, E. Silica–alumina/sepiolite nanoarchitectures. J. Mater. Chem. A 2013, 1, 7477. [Google Scholar] [CrossRef]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Mechanism of persulfate activation by phenols. Environ. Sci. Technol. 2013, 47, 5864–5871. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yang, X.; Zhang, T.; Tang, H. 3D reduced graphene oxide aerogelmediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B Environ. 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Murugananthan, M.; Sun, P.; Dionysiou, D.D.; Zhang, K.; Khan, A.; Zhang, Y. Glucose and melamine derived nitrogen-doped carbonaceous catalyst for nonradical peroxymonosulfate activation process. Carbon 2020, 156, 399–409. [Google Scholar] [CrossRef]
- Wang, N.; Ma, W.; Ren, Z.; Du, Y.; Xu, P.; Han, X. Prussian blue analogues derived porous nitrogen-doped carbon microspheres as high-performance metal-free peroxymonosulfate activators for non-radical-dominated degradationof organic pollutants. J. Mater. Chem. A 2018, 6, 884–895. [Google Scholar] [CrossRef]
- Zhu, S.S.; Huang, X.C.; Ma, F.; Wang, L.; Duan, X.G.; Wang, S.B. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Waldemer, R.H.; Tratnyek, P.G.; Johnson, R.L.; James, T.N. Oxidation of chlorinated ethenes by heat-activated persulfate: Kinetics and products. Environ. Sci. Technol. 2007, 41, 1010–1015. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Wang, J.J.; Yu, J.F.; Feng, H.P.; Lu, Y.; Chen, Y.; Liu, Y.N.; Wang, J.J.; Xie, Q.Q. Enhanced surface activation process of persulfate by modified bagasse biochar for removal of phenol in water and soil: Active sites and electron transfer mechanism. Colloid Surface A 2020, 599, 124904. [Google Scholar] [CrossRef]
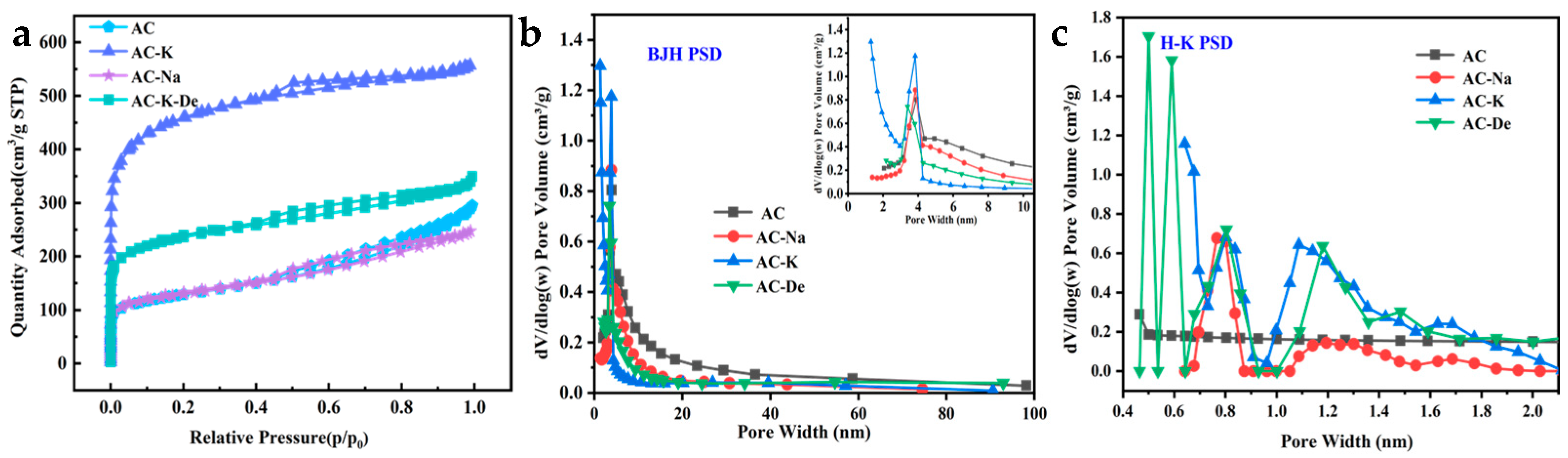
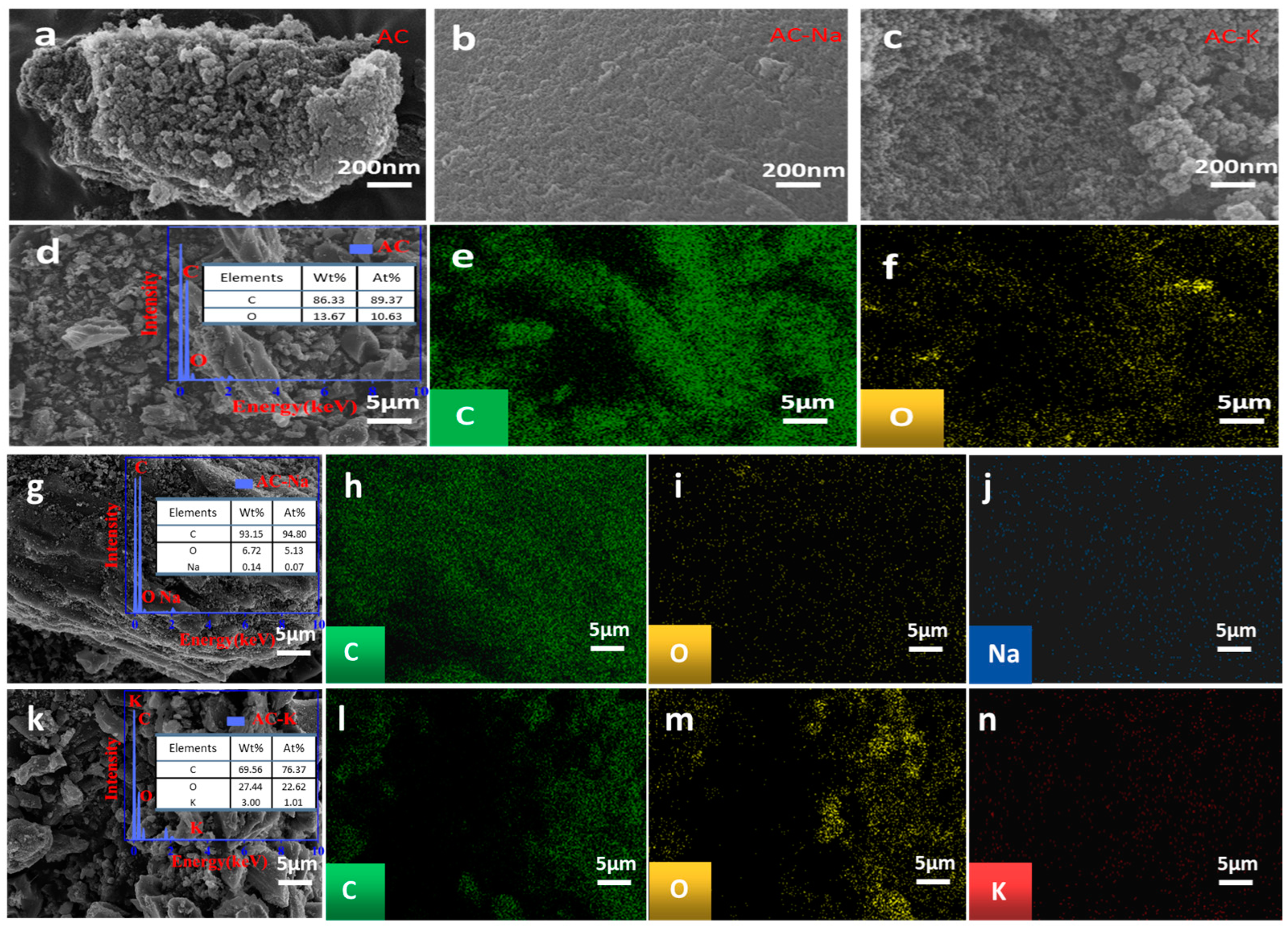
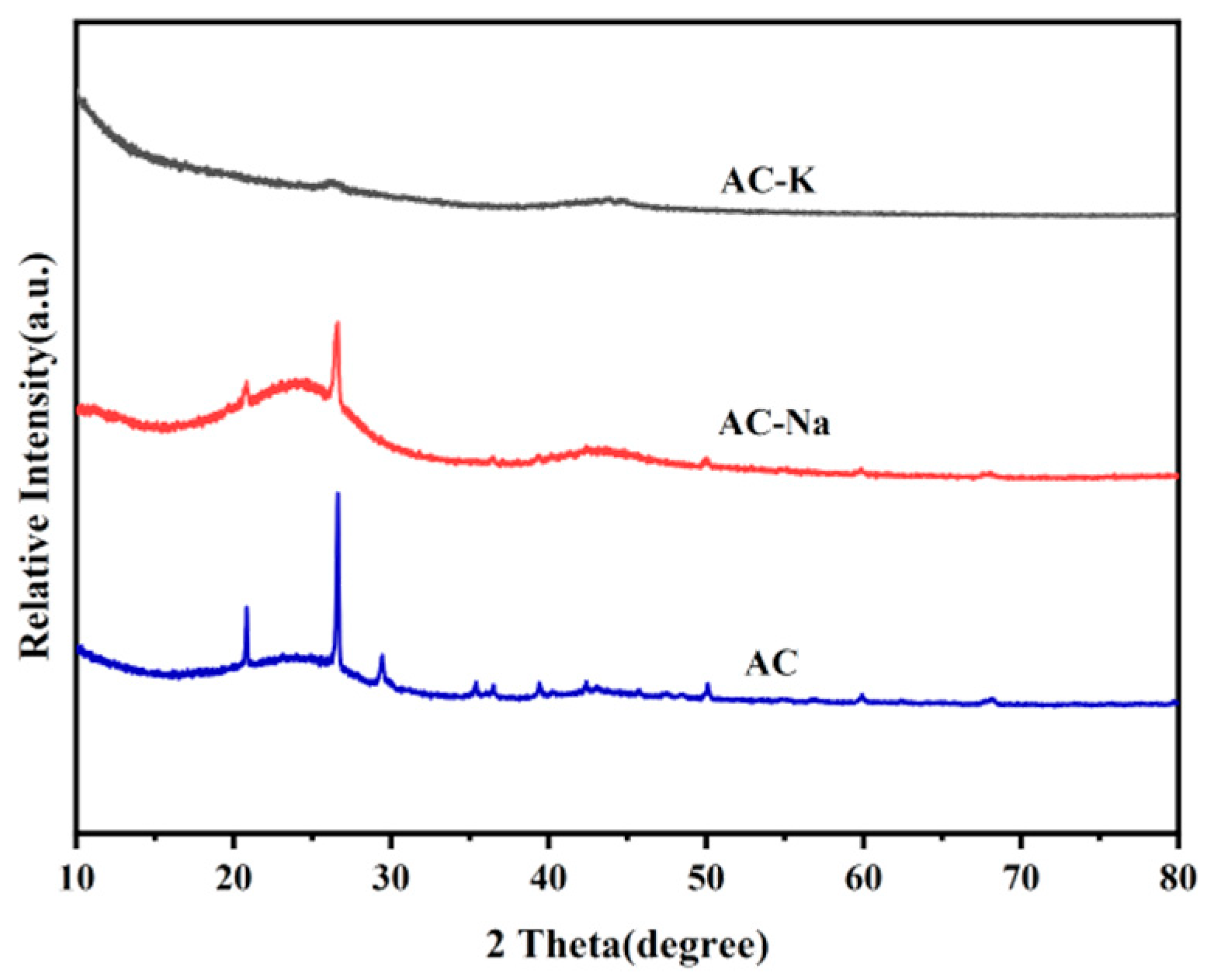
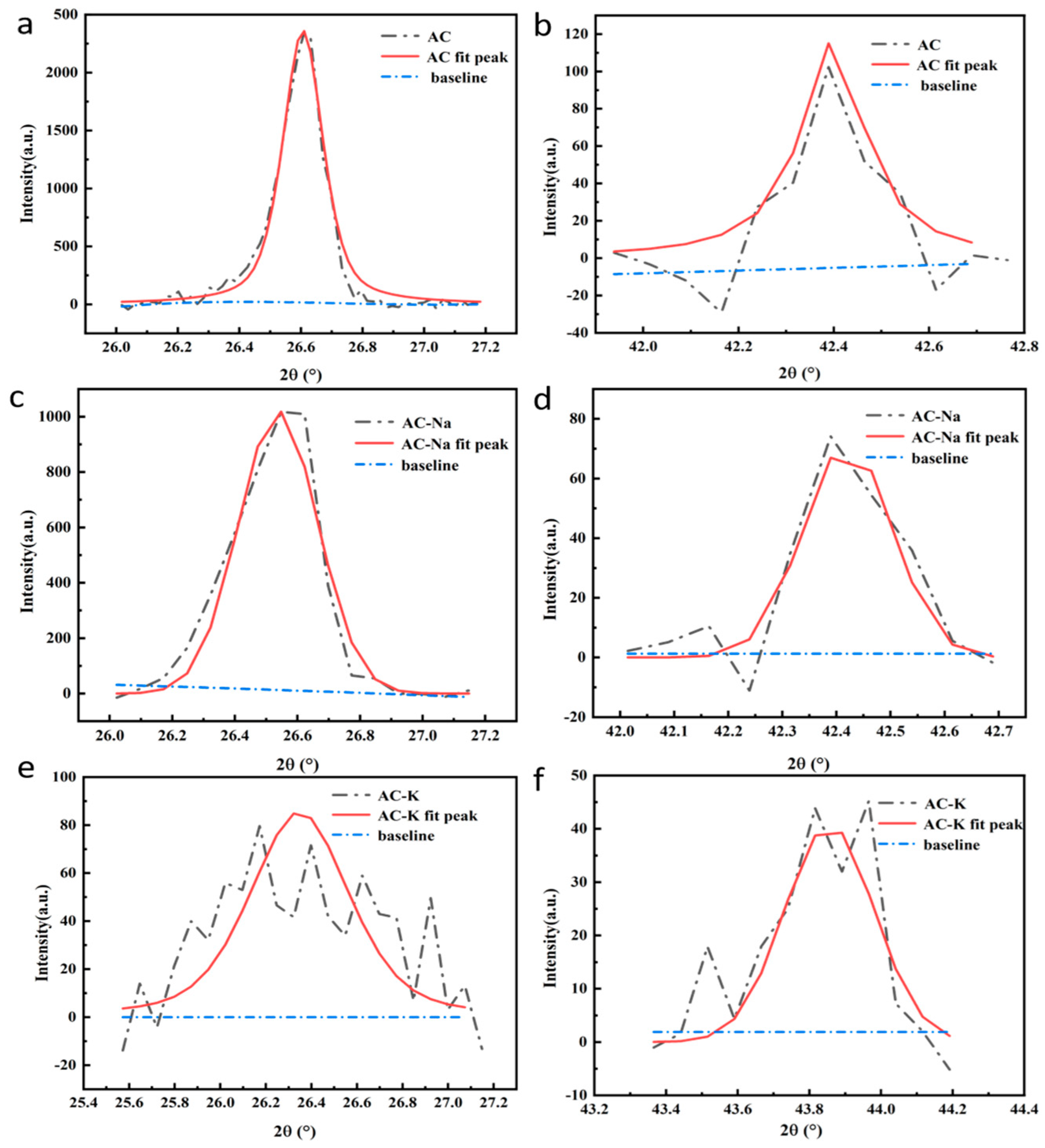


| Samples | SBET (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Dav (nm) |
|---|---|---|---|---|
| AC | 462.8 | 0.456 | 0.085 | 3.9 |
| AC-Na | 426.3 | 0.383 | 0.098 | 3.6 |
| AC-K | 1468.8 | 0.861 | 0.527 | 2.3 |
| AC-K-De | 840.7 | 0.538 | 0.205 | 2.6 |
| Sample | d002 (Å) | Lα (Å) | Lc (Å) | N |
|---|---|---|---|---|
| AC | 3.350 | 18.767 | 8.895 | 2.656 |
| AC-Na | 3.355 | 15.815 | 4.725 | 1.408 |
| AC-K | 3.400 | 10.362 | 2.586 | 0.761 |
| Sample | Relative Content (%) | Relative Content (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | O | K | Na | C=O | C=O | C-O-C | O-C=O | |
| AC | 91.91 | 8.09 | - | - | 29.3 | 41.0 | 21.0 | 8.7 |
| AC-Na | 91.98 | 7.8 | - | 0.22 | 3.0 | 15.8 | 57.1 | 24.1 |
| AC-K | 51.54 | 47.49 | 0.97 | - | 8.3 | 14.7 | 61.5 | 15.5 |
| AC-K-De | 72.38 | 21.98 | 5.16 | - | 23.6 | 30.4 | 25.3 | 20.7 |
| Sample | Functional Groups | FWHM (eV) | Position (eV) | Area (%) |
|---|---|---|---|---|
| AC | C=O (carbonyl group) | 2.00 | 531.03 | 3949 |
| C=O (ester amides) | 1.89 | 532.21 | 5528 | |
| C–O–C | 2.16 | 533.30 | 2840 | |
| COH, COOH | 2.00 | 534.10 | 1160 | |
| AC-K | C=O (carbonyl group) | 1.95 | 532.06 | 15,080 |
| C=O (ester amides) | 1.52 | 533.67 | 26,710 | |
| C–O–C | 2.05 | 533.91 | 112,100 | |
| COH, COOH | 2.47 | 534.95 | 28,220 | |
| AC-Na | C=O (carbonyl group) | 1.9 | 531.33 | 699 |
| C=O (ester amides) | 2.8 | 532.19 | 3758 | |
| C–O–C | 2.0 | 533.40 | 13,570 | |
| COH, COOH | 2.29 | 534.99 | 5726 | |
| AC-K-De | C=O (carbonyl group) | 2.41 | 529.24 | 64,360 |
| C=O (ester amides) | 1.66 | 529.90 | 83,750 | |
| C–O–C | 1.33 | 530.75 | 69,080 | |
| COH, COOH | 1.66 | 531.56 | 56,540 |
| Sample | Relative Content (%) | Relative Content (%) | |||||
|---|---|---|---|---|---|---|---|
| C | O | K | Na | sp2-C/C=C | C–OH | C=O | |
| AC | 91.91 | 8.09 | - | - | 78.5 | 8.4 | 13.1 |
| AC-Na | 91.98 | 7.8 | - | 0.22 | 55.7 | 25.2 | 19.1 |
| AC-K | 51.54 | 47.49 | 0.97 | - | 65.0 | 15.8 | 19.2 |
| AC-K-De | 72.38 | 21.98 | 5.16 | - | 46.6 | 25.3 | 28.1 |
| Sample | Types of Functional Groups | FWHM (eV) | Position (eV) | Area (%) |
|---|---|---|---|---|
| AC | sp2-C/C=C | 1.12 | 284.81 | 38,550 |
| C–OH | 1.34 | 286.10 | 4137 | |
| C=O | 2.28 | 287.73 | 6448 | |
| AC-K | sp2-C/C=C | 1.15 | 284.78 | 57,300 |
| C–OH | 1.50 | 286.23 | 13,890 | |
| C=O | 2.3 | 288.25 | 16,950 | |
| AC-Na | sp2-C/C=C | 1.03 | 284.77 | 66,780 |
| C–OH | 2.59 | 285.77 | 30,080 | |
| C=O | 2.40 | 288.10 | 22,930 | |
| AC-K-De | sp2-C/C=C | 0.93 | 281.33 | 160,500 |
| C–OH | 1.01 | 281.97 | 86,900 | |
| C=O | 1.83 | 282.89 | 96,890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shi, S.; Wei, J.; Ma, Q.; Wang, X.; Zhang, X.; Hao, H.; Yang, C. Activation of Persulfates Using Alkali-Modified Activated Coke to Promote Phenol Removal. Nanomaterials 2025, 15, 744. https://doi.org/10.3390/nano15100744
Zhang Y, Shi S, Wei J, Ma Q, Wang X, Zhang X, Hao H, Yang C. Activation of Persulfates Using Alkali-Modified Activated Coke to Promote Phenol Removal. Nanomaterials. 2025; 15(10):744. https://doi.org/10.3390/nano15100744
Chicago/Turabian StyleZhang, Yan, Shuang Shi, Jianxiong Wei, Qiang Ma, Xiaoxue Wang, Xingyu Zhang, Huarui Hao, and Chen Yang. 2025. "Activation of Persulfates Using Alkali-Modified Activated Coke to Promote Phenol Removal" Nanomaterials 15, no. 10: 744. https://doi.org/10.3390/nano15100744
APA StyleZhang, Y., Shi, S., Wei, J., Ma, Q., Wang, X., Zhang, X., Hao, H., & Yang, C. (2025). Activation of Persulfates Using Alkali-Modified Activated Coke to Promote Phenol Removal. Nanomaterials, 15(10), 744. https://doi.org/10.3390/nano15100744







