Thermoplasmonic Nano–Hybrid Core@Shell Ag@SiO2 Films Engineered via One–Step Flame Spray Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. In-Situ Film Deposition Engineering via Flame Spray Pyrolysis
2.2. Characterization of the Ag@SiO2 Nanofilms
2.3. Monitoring Plasmonic Heating (ΔT) Dynamics
2.4. Theoretical Background
3. Results and Discussion
3.1. FSP Engineering of Ag@SiO2 Films on Plasmonic Glass Substrates (PGSs) and Plasmonic Glass Fiber Filters (PGFFs)
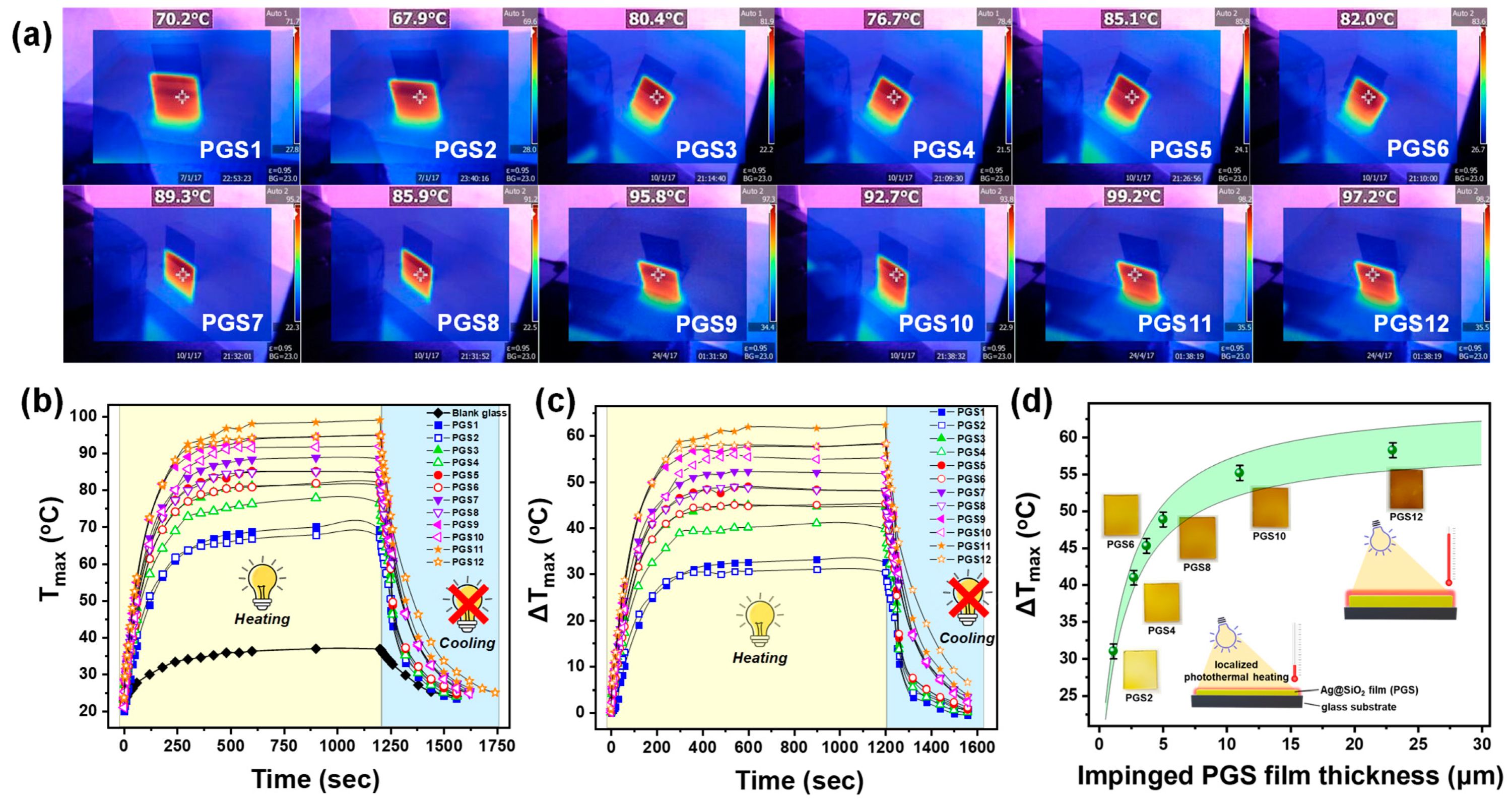
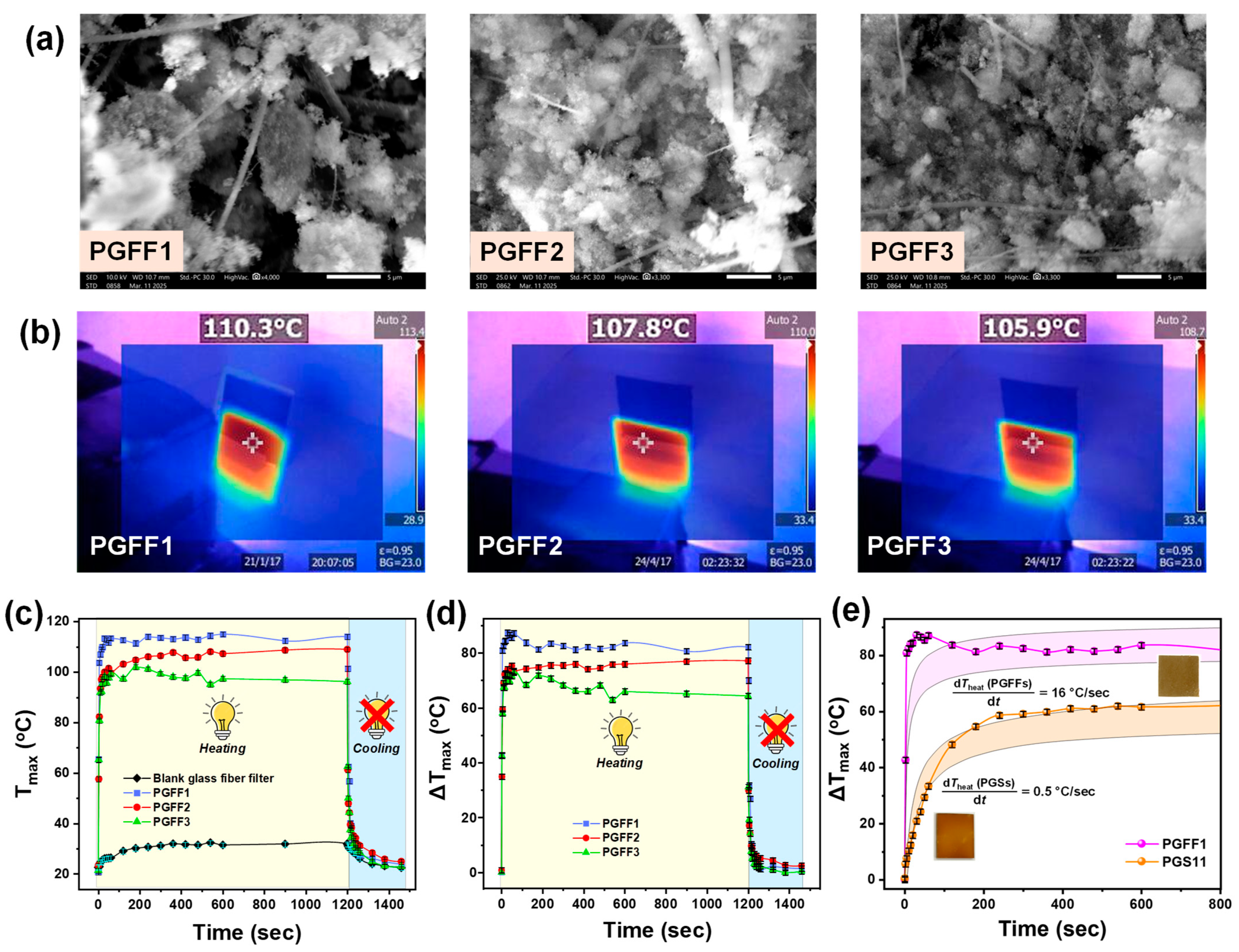
3.2. Thermoplasmonic Performance of Ag@SiO2 PGSs and PGFFs: Impact of Impinging and Substrate Type on Heat Generation Dynamics
3.2.1. Irradiation of Ag@SiO2 PGSs with 405 nm LED Light
3.2.2. Irradiation of Ag@SiO2 PGFFs with 405 nm LED Light
3.2.3. Effects of Impinging
3.2.4. Effect of Substrate Type
3.2.5. Theoretical Evaluation
3.2.6. Determination of Photothermal Conversion Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stockman, M.I. Nanoplasmonics: The Physics behind the Applications. Phys. Today 2011, 64, 39–44. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light, New edition; Wiley-VCH: New York, NY, USA, 2012. [Google Scholar]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.-S.; Link, S.; Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Besteiro, L.V.; Kong, X.-T.; Wang, Z.; Hartland, G.; Govorov, A.O. Understanding Hot-Electron Generation and Plasmon Relaxation in Metal Nanocrystals: Quantum and Classical Mechanisms. ACS Photonics 2017, 4, 2759–2781. [Google Scholar] [CrossRef]
- Cortés, E.; Xie, W.; Cambiasso, J.; Jermyn, A.S.; Sundararaman, R.; Narang, P.; Schlücker, S.; Maier, S.A. Plasmonic Hot Electron Transport Drives Nano-Localized Chemistry. Nat. Commun. 2017, 8, 14880. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-Induced Hot Carriers in Metallic Nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef]
- Moularas, C.; Dimitriou, C.; Georgiou, Y.; Evangelakis, G.; Boukos, N.; Deligiannakis, Y. Electron Paramagnetic Resonance Quantifies Hot-Electron Transfer from Plasmonic Ag@SiO2 to Cr6+/Cr5+/Cr3+. J. Phys. Chem. C 2023, 127, 2045–2057. [Google Scholar] [CrossRef]
- Lee, S.J.; Morrill, A.R.; Moskovits, M. Hot Spots in Silver Nanowire Bundles for Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2006, 128, 2200–2201. [Google Scholar] [CrossRef]
- Li, W.; Camargo, P.H.C.; Lu, X.; Xia, Y. Dimers of Silver Nanospheres: Facile Synthesis and Their Use as Hot Spots for Surface-Enhanced Raman Scattering. Nano Lett. 2009, 9, 485–490. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Thermo-Plasmonics: Using Metallic Nanostructures as Nano-Sources of Heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Toennies, J.P., Gonser, U., Osgood, R.M., Panish, M.B., Sakaki, H., Lotsch, H.K.V., Eds.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25. [Google Scholar] [CrossRef]
- Baffou, G. Thermoplasmonics: Heating Metal Nanoparticles Using Light; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Rodrigo, S.G.; García-Vidal, F.J.; Martín-Moreno, L. Influence of Material Properties on Extraordinary Optical Transmission through Hole Arrays. Phys. Rev. B 2008, 77, 075401. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Quantifying the Efficiency of Plasmonic Materials for Near-Field Enhancement and Photothermal Conversion. J. Phys. Chem. C 2015, 119, 25518–25528. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-Driven Plasmon Enhanced Proton-Coupled Electron Transfer. Nanoscale 2015, 8, 796–803. [Google Scholar] [CrossRef]
- Sotiriou, G.; Gass, S.; Pratsinis, S.E. Hermetically Coated Nanosilver: No Ag+ Ion Leaching. MRS Online Proc. Libr. 2012, 1386, 924. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Pratsinis, S.E. Composite Nanosilver Structures Suitable for Plasmonic Biosensors. MRS Online Proc. Libr. 2012, 1416, 25–30. [Google Scholar] [CrossRef]
- Stathi, P.; Deligiannakis, Y.; Avgouropoulos, G.; Louloudi, M. Efficient H2 Production from Formic Acid by a Supported Iron Catalyst on Silica. Appl. Catal. Gen. 2015, 498, 176–184. [Google Scholar] [CrossRef]
- Gemenetzi, A.; Deligiannakis, Y.; Louloudi, M. Controlled Photoplasmonic Enhancement of H2 Production via Formic Acid Dehydrogenation by a Molecular Fe Catalyst. ACS Catal. 2023, 13, 9905–9917. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.-Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, Silica-Coated, Janus-Like Plasmonic-Magnetic Nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of Some Interesting Surface Plasmon Resonance-Enhanced Properties of Noble Metal Nanoparticles and Their Applications to Biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Merkl, P.; Zhou, S.; Zaganiaris, A.; Shahata, M.; Eleftheraki, A.; Thersleff, T.; Sotiriou, G.A. Plasmonic Coupling in Silver Nanoparticle Aggregates and Their Polymer Composite Films for Near-Infrared Photothermal Biofilm Eradication. ACS Appl. Nano Mater. 2021, 4, 5330–5339. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Zhang, J.; Ma, R.; Gao, J.; Liu, Y.; Zhang, C.; Zhang, Z. A Tumor-Targeting near-Infrared Laser-Triggered Drug Delivery System Based on GO@Ag Nanoparticles for Chemo-Photothermal Therapy and X-Ray Imaging. Biomaterials 2014, 35, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Jauffred, L.; Samadi, A.; Klingberg, H.; Bendix, P.M.; Oddershede, L.B. Plasmonic Heating of Nanostructures. Chem. Rev. 2019, 119, 8087–8130. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Saleh, L.; Tang, J.; Gui, T.; Kullak-Ublick, G.A.; Wang, J. Thermoplasmonic-Assisted Cyclic Cleavage Amplification for Self-Validating Plasmonic Detection of SARS-CoV-2. ACS Nano 2021, 15, 7536–7546. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef]
- Mascaretti, L.; Naldoni, A. Hot Electron and Thermal Effects in Plasmonic Photocatalysis. J. Appl. Phys. 2020, 128, 041101. [Google Scholar] [CrossRef]
- Mascaretti, L.; Schirato, A.; Fornasiero, P.; Boltasseva, A.; Shalaev, V.M.; Alabastri, A.; Naldoni, A. Challenges and Prospects of Plasmonic Metasurfaces for Photothermal Catalysis. Nanophotonics 2022, 11, 3035–3056. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Wang, Z.; Dai, Q. Thermoplasmonics in Solar Energy Conversion: Materials, Nanostructured Designs, and Applications. Adv. Mater. 2022, 34, 2107351. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Linic, S. Visible-Light-Enhanced Catalytic Oxidation Reactions on Plasmonic Silver Nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Moularas, C.; Gemenetzi, A.; Deligiannakis, Y.; Louloudi, M. Nanoplasmonics in Catalysis for Energy Technologies: The Concept of Plasmon-Assisted Molecular Catalysis (PAMC). Nanoenergy Adv. 2024, 4, 25–44. [Google Scholar] [CrossRef]
- De Sio, L.; Placido, T.; Comparelli, R.; Lucia Curri, M.; Striccoli, M.; Tabiryan, N.; Bunning, T.J. Next-Generation Thermo-Plasmonic Technologies and Plasmonic Nanoparticles in Optoelectronics. Prog. Quantum Electron. 2015, 41, 23–70. [Google Scholar] [CrossRef]
- Hanauer, S.; Massiot, I.; Mlayah, A.; Carcenac, F.; Doucet, J.-B.; Beldjoudi, S.; Faniayeu, I.; Dmitriev, A. Photothermal Conversion of Solar Infrared Radiation by Plasmonic Nanoantennas for Photovoltaic-Thermoelectric Hybrid Devices. ACS Appl. Energy Mater. 2023, 6, 2128–2133. [Google Scholar] [CrossRef]
- Shin, D.; Kang, G.; Gupta, P.; Behera, S.; Lee, H.; Urbas, A.M.; Park, W.; Kim, K. Thermoplasmonic and Photothermal Metamaterials for Solar Energy Applications. Adv. Opt. Mater. 2018, 6, 1800317. [Google Scholar] [CrossRef]
- Epifani, M.; Giannini, C.; Tapfer, L.; Vasanelli, L. Sol-Gel Synthesis and Characterization of Ag and Au Nanoparticles in SiO2, TiO2, and ZrO2 Thin Films. J. Am. Ceram. Soc. 2004, 83, 2385–2393. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Cheng, B.; Ong, H.C. Microwave-Hydrothermal Preparation and Visible-Light Photoactivity of Plasmonic Photocatalyst Ag-TiO2 Nanocomposite Hollow Spheres. Chem.—Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct Evidence and Enhancement of Surface Plasmon Resonance Effect on Ag-Loaded TiO2 Nanotube Arrays for Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Beyene, H.T.; Chakravadhanula, V.S.K.; Hanisch, C.; Elbahri, M.; Strunskus, T.; Zaporojtchenko, V.; Kienle, L.; Faupel, F. Preparation and Plasmonic Properties of Polymer-Based Composites Containing Ag–Au Alloy Nanoparticles Produced by Vapor Phase Co-Deposition. J. Mater. Sci. 2010, 45, 5865–5871. [Google Scholar] [CrossRef]
- Chen, T.; Pourmand, M.; Feizpour, A.; Cushman, B.; Reinhard, B.M. Tailoring Plasmon Coupling in Self-Assembled One-Dimensional Au Nanoparticle Chains through Simultaneous Control of Size and Gap Separation. J. Phys. Chem. Lett. 2013, 4, 2147–2152. [Google Scholar] [CrossRef]
- Dimitriou, C.; Psathas, P.; Solakidou, M.; Deligiannakis, Y. Advanced Flame Spray Pyrolysis (FSP) Technologies for Engineering Multifunctional Nanostructures and Nanodevices. Nanomaterials 2023, 13, 3006. [Google Scholar] [CrossRef]
- Dimitriou, C.; Belles, L.; Boukos, N.; Deligiannakis, Y. {TiO2/TiO2(B)} Quantum Dot Hybrids: A Comprehensible Route toward High-Performance [>0.1 Mol Gr–1 h–1] Photocatalytic H2 Production from H2O. ACS Catal. 2024, 14, 17919–17934. [Google Scholar] [CrossRef]
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled Synthesis of Nanostructured Particles by Flame Spray Pyrolysis. J. Aerosol. Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Li, H.; Dumont, E.; Slipets, R.; Thersleff, T.; Boisen, A.; Sotiriou, G.A. Democratizing Robust SERS Nano-Sensors for Food Safety Diagnostics. Chem. Eng. J. 2023, 470, 144023. [Google Scholar] [CrossRef]
- Li, H.; Merkl, P.; Sommertune, J.; Thersleff, T.; Sotiriou, G.A. SERS Hotspot Engineering by Aerosol Self-Assembly of Plasmonic Ag Nanoaggregates with Tunable Interparticle Distance. Adv. Sci. 2022, 9, 2201133. [Google Scholar] [CrossRef] [PubMed]
- Bletsa, E.; Merkl, P.; Thersleff, T.; Normark, S.; Henriques-Normark, B.; Sotiriou, G.A. Highly Durable Photocatalytic Titanium Suboxide–Polymer Nanocomposite Films with Visible Light-Triggered Antibiofilm Activity. Chem. Eng. J. 2023, 454, 139971. [Google Scholar] [CrossRef]
- Blattmann, C.O.; Sotiriou, G.A.; Pratsinis, S.E. Rapid Synthesis of Flexible Conductive Polymer Nanocomposite Films. Nanotechnology 2015, 26, 125601. [Google Scholar] [CrossRef]
- Blattmann, C.O.; Pratsinis, S.E. In Situ Measurement of Conductivity during Nanocomposite Film Deposition. Appl. Surf. Sci. 2016, 371, 329–336. [Google Scholar] [CrossRef]
- Moularas, C.; Georgiou, Y.; Adamska, K.; Deligiannakis, Y. Thermoplasmonic Heat Generation Efficiency by Nonmonodisperse Core–Shell Ag0@SiO2 Nanoparticle Ensemble. J. Phys. Chem. C 2019, 123, 22499–22510. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Sannomiya, T.; Teleki, A.; Krumeich, F.; Vörös, J.; Pratsinis, S.E. Non-Toxic Dry-Coated Nanosilver for Plasmonic Biosensors. Adv. Funct. Mater. 2010, 20, 4250–4257. [Google Scholar] [CrossRef]
- Teleki, A.; Heine, M.C.; Krumeich, F.; Akhtar, M.K.; Pratsinis, S.E. In Situ Coating of Flame-Made TiO2 Particles with Nanothin SiO2 Films. Langmuir 2008, 24, 12553–12558. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering Nanosilver as an Antibacterial, Biosensor and Bioimaging Material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef]
- Yaws, C.L. The Yaws Handbook of Vapor Pressure: Antoine Coefficients, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Teleki, A.; Akhtar, M.K.; Pratsinis, S.E. The Quality of SiO2-Coatings on Flame-Made TiO2-Based Nanoparticles. J. Mater. Chem. 2008, 18, 3547–3555. [Google Scholar] [CrossRef]
- Hahnemühle FineArt, Inc. Filter Papers & Membranes; Industry & Laboratory|Product Profile & Application. Available online: https://www.hahnemuehle.com/fileadmin/user_upload/bilder/Filtration/pdf/web-10603856_Katalog_Filtration_EN.pdf (accessed on 12 March 2025).
- Tricoli, A.; Elmøe, T.D. Flame Spray Pyrolysis Synthesis and Aerosol Deposition of Nanoparticle Films. AIChE J. 2012, 58, 3578–3588. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and Application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Kühne, S.; Graf, M.; Tricoli, A.; Mayer, F.; Pratsinis, S.E.; Hierlemann, A. Wafer-Level Flame-Spray-Pyrolysis Deposition of Gas-Sensitive Layers on Microsensors. J. Micromech. Microeng. 2008, 18, 035040. [Google Scholar] [CrossRef]
- Chen, H.; Mulmudi, H.K.; Tricoli, A. Flame Spray Pyrolysis for the One-Step Fabrication of Transition Metal Oxide Films: Recent Progress in Electrochemical and Photoelectrochemical Water Splitting. Chin. Chem. Lett. 2020, 31, 601–604. [Google Scholar] [CrossRef]
- Tricoli, A.; Graf, M.; Mayer, F.; Kuühne, S.; Hierlemann, A.; Pratsinis, S.E. Micropatterning Layers by Flame Aerosol Deposition-Annealing. Adv. Mater. 2008, 20, 3005–3010. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Baffou, G.; Berto, P.; Bermúdez Ureña, E.; Quidant, R.; Monneret, S.; Polleux, J.; Rigneault, H. Photoinduced Heating of Nanoparticle Arrays. ACS Nano 2013, 7, 6478–6488. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. American Mineralogist Crystal Structure Database. Department of Geosciences, University of Arizona, Tucson, Arizona. Available online: https://rruff.geo.arizona.edu/AMS/minerals/Silver (accessed on 12 March 2025).
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Romero, I.; Aizpurua, J.; Bryant, G.W.; de Abajo, F.J.G. Plasmons in Nearly Touching Metallic Nanoparticles: Singular Response in the Limit of Touching Dimers. Opt. Express 2006, 14, 9988–9999. [Google Scholar] [CrossRef]
- Mie, G. Beiträge Zur Optik Trüber Medien, Speziell Kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Jiang, M.-M.; Chen, H.-Y.; Li, B.-H.; Liu, K.-W.; Shan, C.-X.; Shen, D.-Z. Hybrid Quadrupolar Resonances Stimulated at Short Wavelengths Using Coupled Plasmonic Silver Nanoparticle Aggregation. J. Mater. Chem. C 2013, 2, 56–63. [Google Scholar] [CrossRef]
- Bastús, N.G.; Piella, J.; Puntes, V. Quantifying the Sensitivity of Multipolar (Dipolar, Quadrupolar, and Octapolar) Surface Plasmon Resonances in Silver Nanoparticles: The Effect of Size, Composition, and Surface Coating. Langmuir 2016, 32, 290–300. [Google Scholar] [CrossRef]
- Kreibig, U.; Schmitz, B.; Breuer, H.D. Separation of Plasmon-Polariton Modes of Small Metal Particles. Phys. Rev. B 1987, 36, 5027–5030. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Setoura, K.; Tamura, M.; Oshikiri, T.; Iida, T. Switching Nanoscale Temperature Fields with High-Order Plasmonic Modes in Transition Metal Nanorods. RSC Adv. 2023, 13, 34489–34496. [Google Scholar] [CrossRef]
- Newport Corporation. Introduction to Solar Radiation. Available online: https://www.newport.com/t/introduction-to-solar-radiation (accessed on 21 March 2025).
- Ghini, M.; Curreli, N.; Camellini, A.; Wang, M.; Asaithambi, A.; Kriegel, I. Photodoping of Metal Oxide Nanocrystals for Multi-Charge Accumulation and Light-Driven Energy Storage. Nanoscale 2021, 13, 8773–8783. [Google Scholar] [CrossRef]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and Theoretical Studies of Light-to-Heat Conversion and Collective Heating Effects in Metal Nanoparticle Solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 8th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ulazia, A.; Sáenz, J.; Ibarra-Berastegi, G.; González-Rojí, S.J.; Carreno-Madinabeitia, S. Global Estimations of Wind Energy Potential Considering Seasonal Air Density Changes. Energy 2019, 187, 115938. [Google Scholar] [CrossRef]
- Earth Science Data Systems, N. Air Mass/Density|NASA Earthdata. Available online: https://www.earthdata.nasa.gov/topics/atmosphere/air-mass-density (accessed on 3 May 2025).
- Pilkington North America. Properties of Soda-Lime-Silica Float Glass (ATS-129). Technical Bulletin. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiV17zpnomNAxX0cfEDHQCOAcYQFnoECBYQAQ&url=https%3A%2F%2Fwww.pilkington.com%2F-%2Fmedia%2Fpilkington%2Fsite-content%2Fusa%2Fwindow-manufacturers%2Ftechnical-bulletins%2Fats129propertiesofglass20130114.pdf&usg=AOvVaw1QcY7Pwa7wjo8gAsis4j3d&opi=89978449 (accessed on 4 May 2025).
- McLellan, G.W.; Shand, E.B. Glass Engineering Handbook, Subsequent edition; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Varshneya, V.K.; Mauro, J.C. Fundamentals of Inorganic Glasses, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hampton Research Corp. Soda-Lime Glass|Technical Data Sheet. Available online: https://hamptonresearch.com/uploads/support_materials/Glass_10_Soda_lime_glass_Glass_0100_Technical_Data_Sheet.pdf (accessed on 4 May 2025).
- Modarresifar, F.; Bingham, P.A.; Jubb, G.A. Thermal Conductivity of Refractory Glass Fibres. J. Therm. Anal. Calorim. 2016, 125, 35–44. [Google Scholar] [CrossRef]
- Continental Trade Sp. z o.o. Borosilicate Glass|Technical Data. Available online: https://www.continentaltrade.com.pl/en/our-offer/technical-glass/types-of-materials/borosilicate-glass (accessed on 4 May 2025).
- Tamura, S. Low-Dielectric-Constant Glass Fiber and Glass Fiber Fabric Made Thereof. US6846761B2, 25 January 2005. Available online: https://patents.google.com/patent/US6846761B2/en (accessed on 4 May 2025).
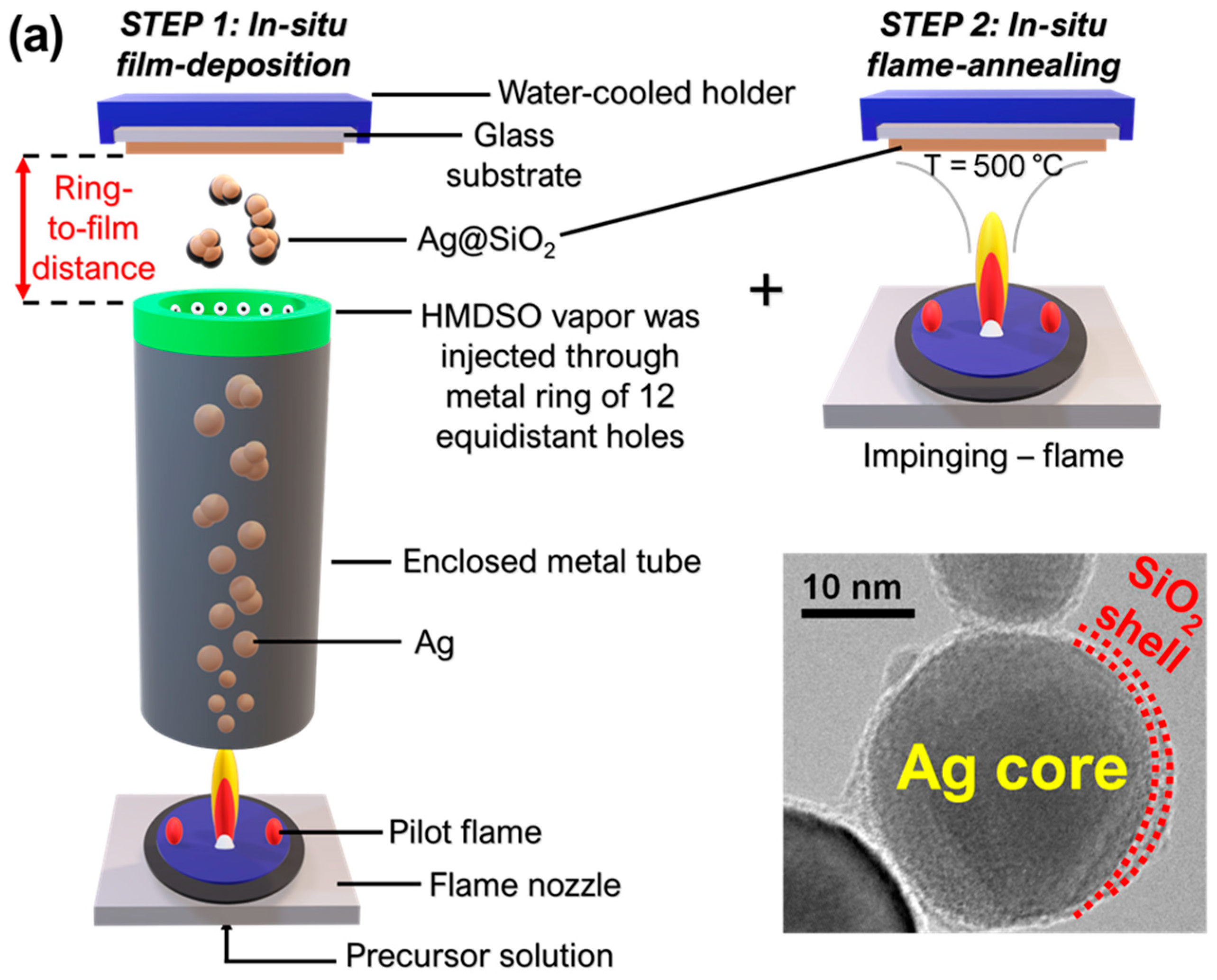
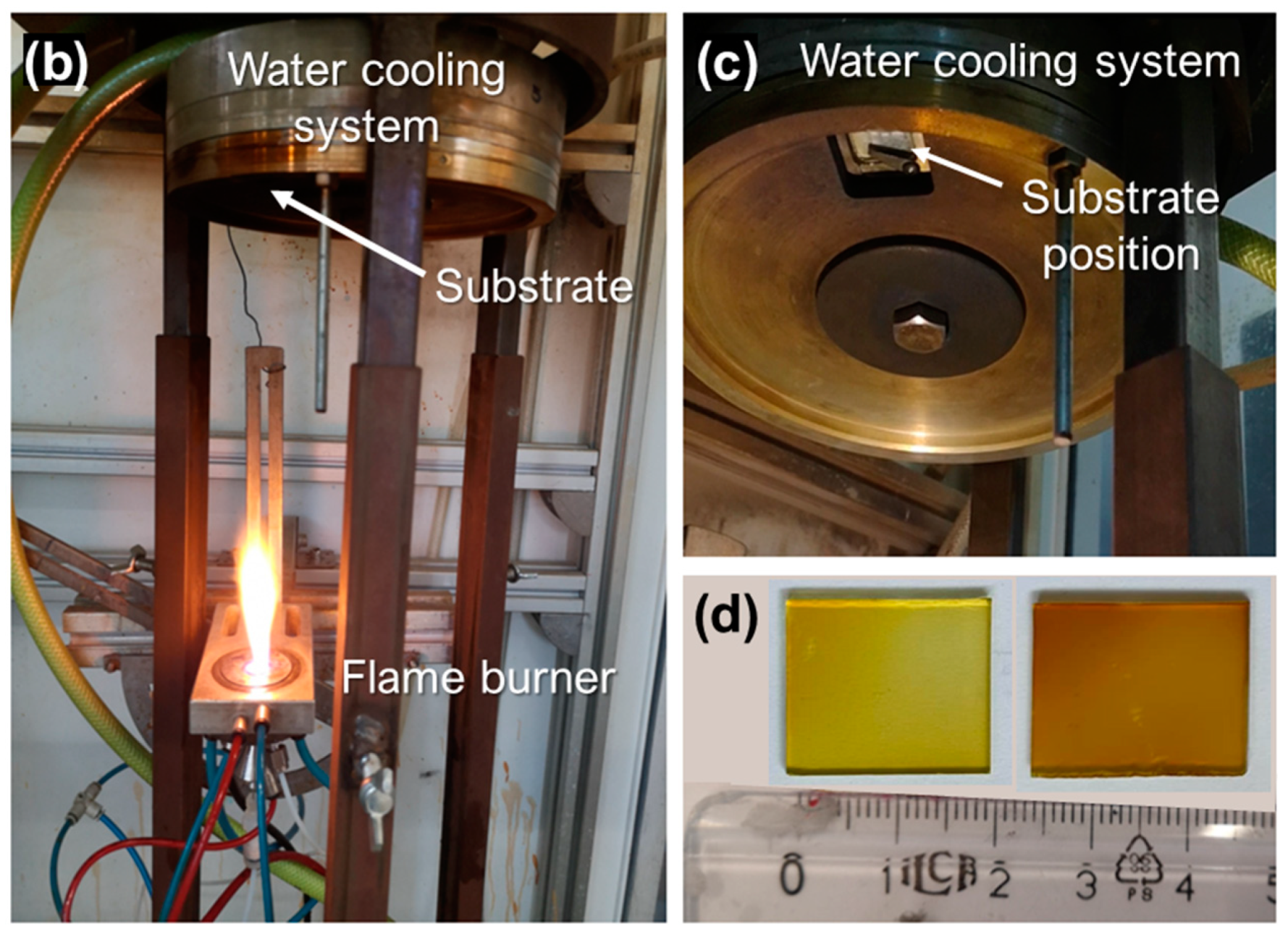


| Materials | Ring-to-Substrate/ Filter Distance (cm) | Deposition Time tdeposition (s) | Flame-Annealing/ Impinging Time timpinging (s) | Ag Particle Diameter dXRD (nm) (±1) | Ag@SiO2 Film Thickness Lz (μm) |
|---|---|---|---|---|---|
| Plasmonic Glass Substrates (PGSs) | |||||
| PGS1 | 5 | 15 | – | 20 | 0.8–3 (irregular) |
| PGS2 | 5 | 15 | 30 | 24 | 1.1 ± 0.3 |
| PGS3 | 5 | 45 | – | 24 | 1.5–3.5 (irregular) |
| PGS4 | 5 | 45 | 30 | 25 | 2.7 ± 0.5 |
| PGS5 | 5 | 60 | – | 22 | 2–5 (irregular) |
| PGS6 | 5 | 60 | 30 | 26 | 3.7 ± 0.7 |
| PGS7 | 5 | 90 | – | 20 | 3–7 (irregular) |
| PGS8 | 5 | 90 | 30 | 24 | 5.0 ± 0.8 |
| PGS9 | 5 | 180 | – | 20 | 6–12 (irregular) |
| PGS10 | 5 | 180 | 30 | 22 | 11.0 ± 1.2 |
| PGS11 | 5 | 360 | – | 22 | 10–20 (irregular) |
| PGS12 | 5 | 360 | 30 | 24 | 23.0 ± 1.6 |
| Plasmonic Glass Fiber Filters (PGFFs) | |||||
| PGFF1 | 50 | – | – | 20 | – |
| PGFF2 | 50 | – | 60 | 20 | – |
| PGFF3 | 50 | – | 120 | 21 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, C.; Deligiannakis, Y. Thermoplasmonic Nano–Hybrid Core@Shell Ag@SiO2 Films Engineered via One–Step Flame Spray Pyrolysis. Nanomaterials 2025, 15, 743. https://doi.org/10.3390/nano15100743
Dimitriou C, Deligiannakis Y. Thermoplasmonic Nano–Hybrid Core@Shell Ag@SiO2 Films Engineered via One–Step Flame Spray Pyrolysis. Nanomaterials. 2025; 15(10):743. https://doi.org/10.3390/nano15100743
Chicago/Turabian StyleDimitriou, Christos, and Yiannis Deligiannakis. 2025. "Thermoplasmonic Nano–Hybrid Core@Shell Ag@SiO2 Films Engineered via One–Step Flame Spray Pyrolysis" Nanomaterials 15, no. 10: 743. https://doi.org/10.3390/nano15100743
APA StyleDimitriou, C., & Deligiannakis, Y. (2025). Thermoplasmonic Nano–Hybrid Core@Shell Ag@SiO2 Films Engineered via One–Step Flame Spray Pyrolysis. Nanomaterials, 15(10), 743. https://doi.org/10.3390/nano15100743







