Insights on Polyidide Shuttling of Zn-I2 Batteries by I3−/I− Electrolytes Based on the Dual-Ion Battery System
Abstract
1. Introduction
2. Experimental Section
2.1. Chemical
2.2. Electrochemical Measurements
3. Results and Discussion
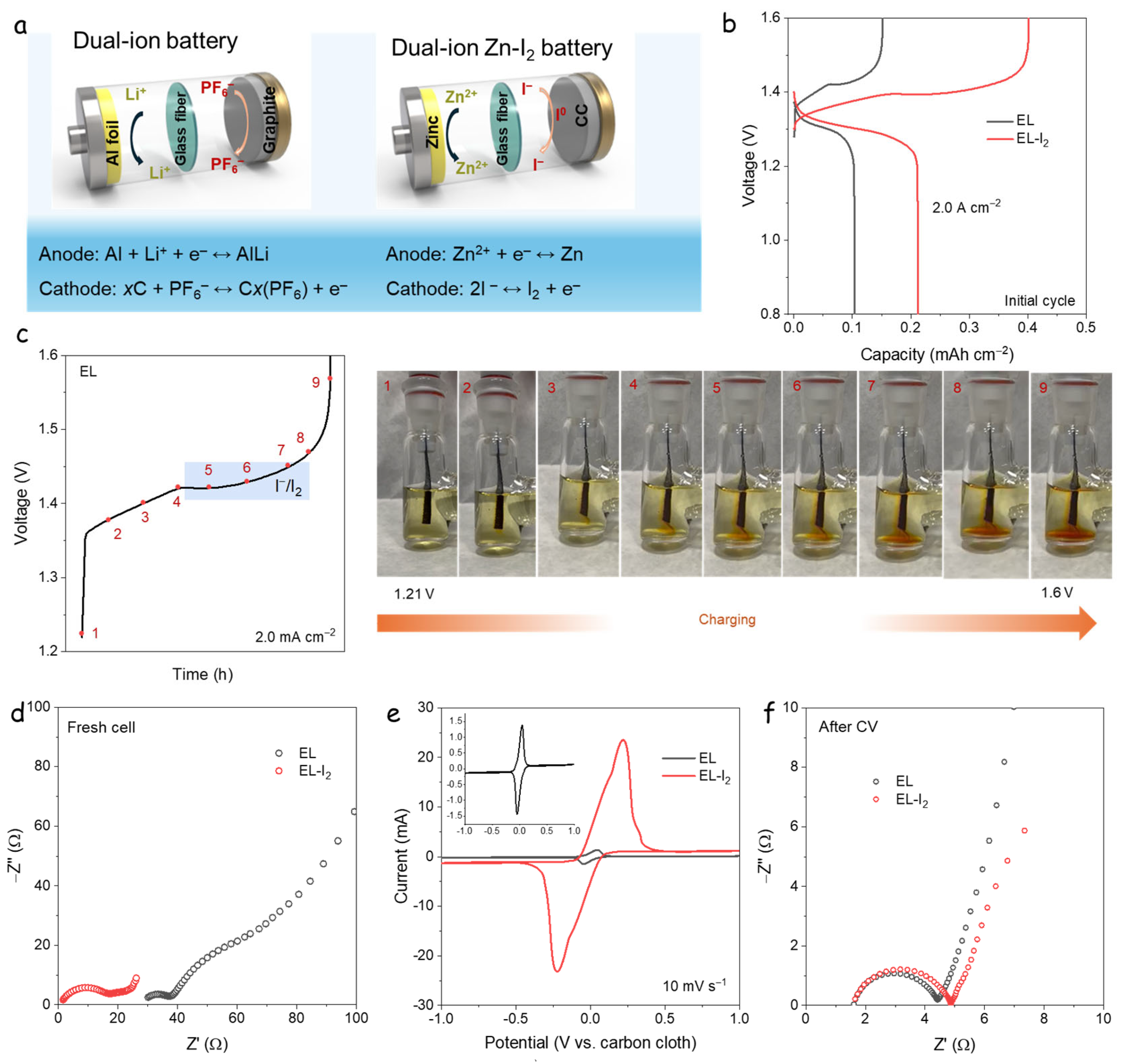
3.1. Dual-Ion Zn-I2 Battery
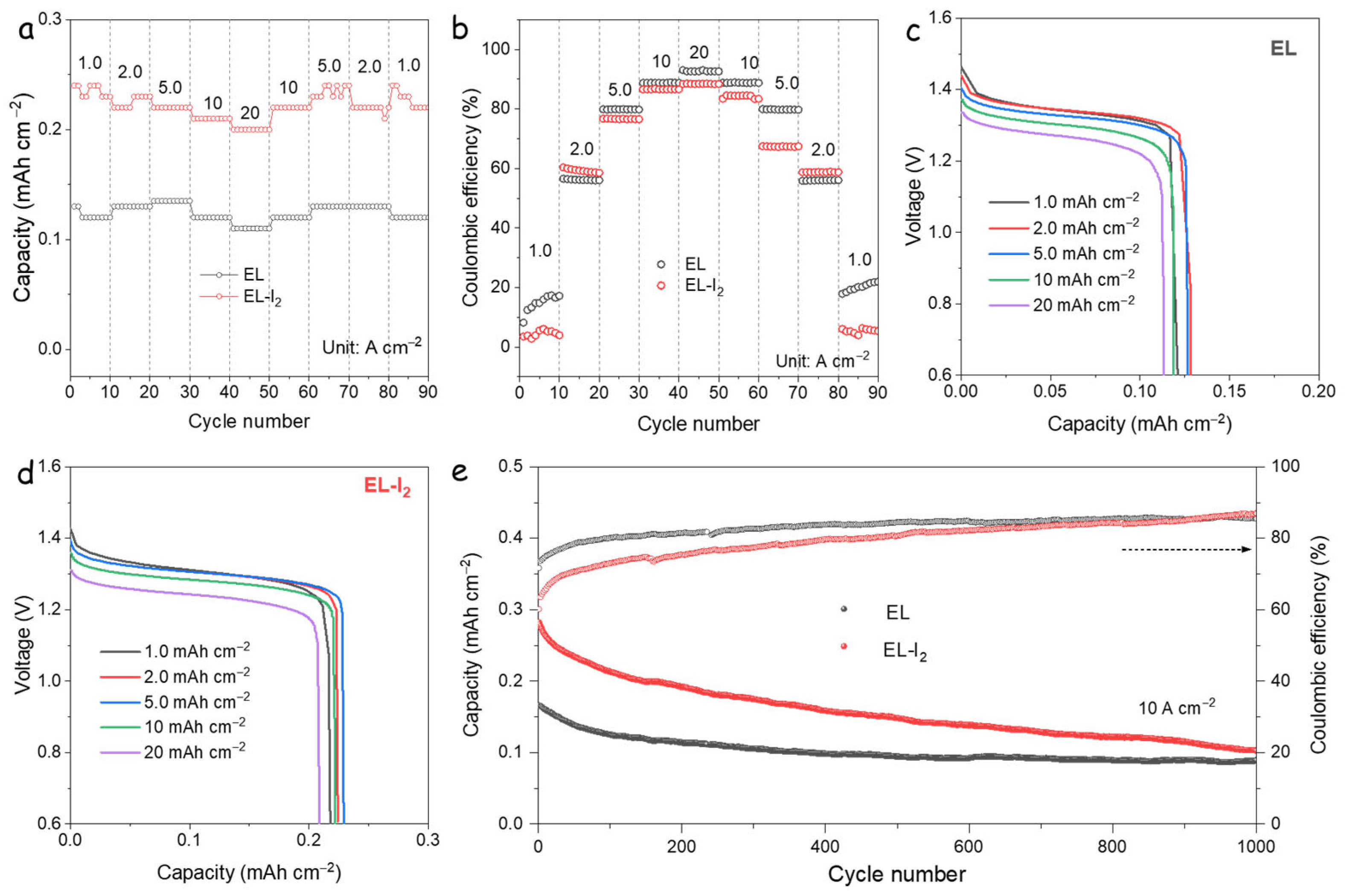
3.2. Electrochemical Performance of DIZIBs
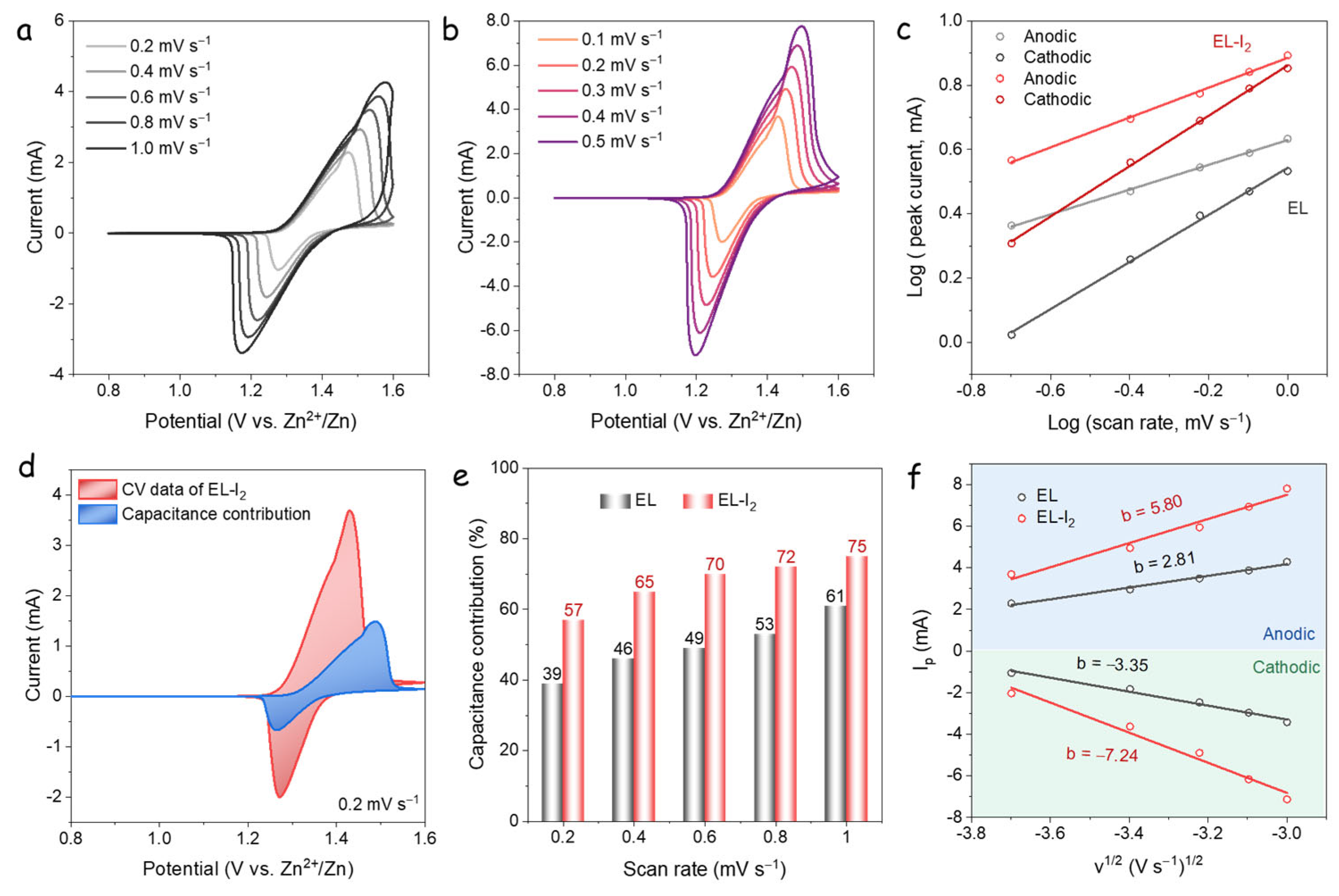
3.3. Kinetic Analysis
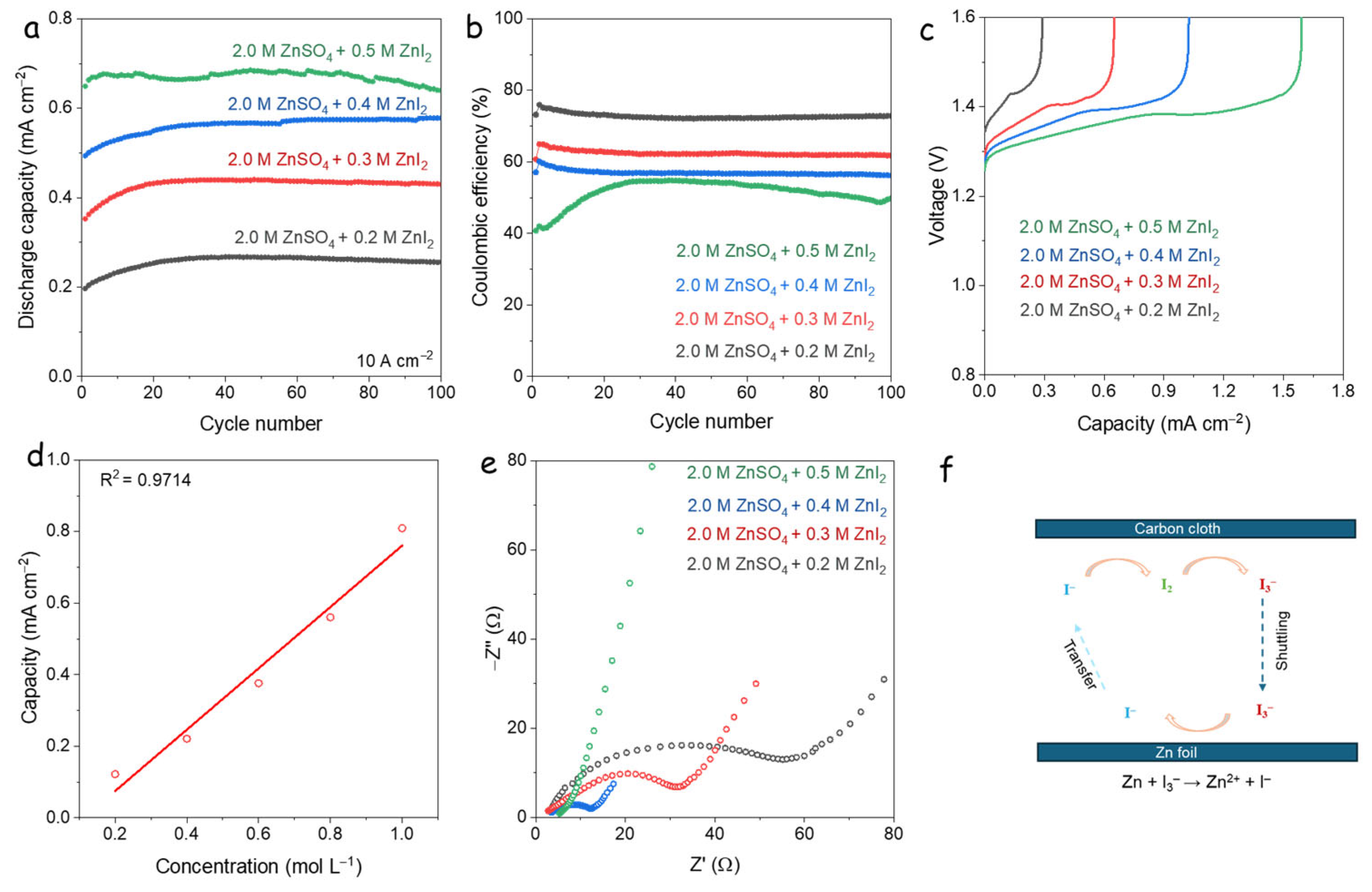
3.4. Electrochemical Behavior of High Concentration I-Additives
3.5. Self-Discharge Analysis
3.6. Prospects of Strategies for Mitigating Self-Discharge in Zn-I2 Batteries
3.7. Distinction Between Dual-Ion and Rocking Chair Zn-I2 Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and characterization of Li (Co0.5Ni0.5) PO4 cathode for Li-ion aqueous battery applications. Electrochem. Solid-State Lett. 2011, 14, A86. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Unlocking Layered Double Hydroxide as a High-Performance Cathode Material for Aqueous Zinc-Ion Batteries. Adv. Mater. 2022, 34, e2204320. [Google Scholar] [CrossRef]
- Xue, T.; Fan, H.J. From aqueous Zn-ion battery to Zn-MnO2 flow battery: A brief story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery: Part II. Comparison of the behavior of EMD and battery grade MnO2 in Zn|MnO2| aqueous LiOH electrolyte. Journal of power sources 2004, 138, 319–322. [Google Scholar] [CrossRef]
- Guo, H.; Montes-García, V.; Peng, H.; Samorì, P.; Ciesielski, A. Molecular connectors boosting the performance of MoS2 cathodes in zinc-ion batteries. Small 2024, 20, 2310338. [Google Scholar] [CrossRef]
- Lin, D.; Li, Y. Recent advances of aqueous rechargeable zinc-iodine batteries: Challenges, solutions, and prospects. Adv. Mater. 2022, 34, 2108856. [Google Scholar] [CrossRef]
- Chen, M.; Xie, S.; Zhao, X.; Zhou, W.; Li, Y.; Zhang, J.; Chen, Z.; Chao, D. Aqueous zinc-ion batteries at extreme temperature: Mechanisms, challenges, and strategies. Energy Storage Mater. 2022, 51, 683–718. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Fang, K.; Wang, H.; Ning, J.; Hu, Y. Aqueous Zinc-Iodine Batteries: From Electrochemistry to Energy Storage Mechanism. Adv. Energy Mater. 2023, 13, 2302187. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, W.; Guo, H.; Tian, Z.; Zhang, L.; Wang, J.; Liu, T.; Lai, F.; Huang, J. Tightly confined iodine in surface-oxidized carbon matrix toward dual-mechanism zinc-iodine batteries. Energy Storage Mater. 2023, 59, 102760. [Google Scholar] [CrossRef]
- Wang, F.; Tseng, J.; Liu, Z.; Zhang, P.; Wang, G.; Chen, G.; Wu, W.; Yu, M.; Wu, Y.; Feng, X. A Stimulus-Responsive Zinc–Iodine Battery with Smart Overcharge Self-Protection Function. Adv. Mater. 2020, 32, 2000287. [Google Scholar] [CrossRef]
- Pan, H.; Li, B.; Mei, D.; Nie, Z.; Shao, Y.; Li, G.; Li, X.S.; Han, K.S.; Mueller, K.T.; Sprenkle, V.; et al. Controlling Solid–Liquid Conversion Reactions for a Highly Reversible Aqueous Zinc-Iodine Battery. ACS Energy Lett. 2017, 2, 2674–2680. [Google Scholar] [CrossRef]
- Wu, H.; Hao, J.; Zhang, S.; Jiang, Y.; Zhu, Y.; Liu, J.; Davey, K.; Qiao, S.-Z. Aqueous Zinc–Iodine Pouch Cells with Long Cycling Life and Low Self-Discharge. J. Am. Chem. Soc. 2024, 146, 16601–16608. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, J.; Wang, W.; Zhai, Z.; Liu, G.; Ge, Y.; Wang, L.; Wang, C.; Lu, H. Dynamic Cathode Interlayer for Ultralow Self-Discharge and High Iodide Utilization in Zinc-Iodine Batteries. Energy Environ. Sci. 2025. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Z.; Cao, B.; Xin, Z.; Lai, H.; Gao, S.; Xu, B.; Yang, J.L.; Xiao, T.; Zhang, B. Marangoni-driven self-assembly MXene as functional membrane enables dendrite-free and flexible zinc–iodine pouch cells. Adv. Energy Mater. 2024, 14, 2400318. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Shang, W.; Ma, J.; Zhang, J. In Situ Formation of 3D ZIF-8/MXene Composite Coating for High-Performance Zinc-Iodine Batteries. Adv. Funct. Mater. 2025, 35, 2422081. [Google Scholar] [CrossRef]
- Yan, L.; Liu, T.; Zeng, X.; Sun, L.; Meng, X.; Ling, M.; Fan, M.; Ma, T. Multifunctional porous carbon strategy assisting high-performance aqueous zinc-iodine battery. Carbon 2022, 187, 145–152. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Wang, S.; Sun, W.; Yu, D.; Li, N.; Zhou, H.; Zhang, X.; Pang, H. Nano/Micro Metal-Organic Framework-Derived Porous Carbon with Rich Nitrogen Sites as Efficient Iodine Hosts for Aqueous Zinc-Iodine Batteries. Adv. Sci. 2025, e2502563. [Google Scholar] [CrossRef]
- Pei, L.H.; Xu, D.M.; Luo, Y.Z.; Guo, S.J.; Liu, D.R.; Jiang, S.J.; Zhang, W.J.; Cao, F.F. Zinc Single-Atom Catalysts Encapsulated in Hierarchical Porous Bio-Carbon Synergistically Enhances Fast Iodine Conversion and Efficient Polyiodide Confinement for Zn-I2 Batteries. Adv. Mater. 2025, 37, 2420005. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, S.; Ao, Z.; Lin, X.; Yan, L.; Liu, C.; Lin, Z. Dual-Mechanism Anchoring of Iodine Species by Pitch-Derived Porous Carbon for Enhanced Zinc–Iodine Battery Performance. Small 2025, 21, 2501695. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, G.; Liu, H.; Lou, Y.; Wang, N.; Liu, H.; Dou, S. Advancements in aqueous zinc–iodine batteries: A review. Chem. Sci. 2024, 15, 3071–3092. [Google Scholar] [CrossRef]
- Ou, X.; Gong, D.; Han, C.; Liu, Z.; Tang, Y. Advances and prospects of dual-ion batteries. Adv. Energy Mater. 2021, 11, 2102498. [Google Scholar] [CrossRef]
- Placke, T.; Heckmann, A.; Schmuch, R.; Meister, P.; Beltrop, K.; Winter, M. Perspective on Performance, Cost, and Technical Challenges for Practical Dual-Ion Batteries. Joule 2018, 2, 2528–2550. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.-S. A novel aluminum–graphite dual-ion battery. Adv. energy mater. 2016, 6, 1502588. [Google Scholar] [CrossRef]
- Lyu, Y.; Yuwono, J.A.; Wang, P.; Wang, Y.; Yang, F.; Liu, S.; Zhang, S.; Wang, B.; Davey, K.; Mao, J. Organic pH Buffer for Dendrite-Free and Shuttle-Free Zn-I2 Batteries. Angewandte Chemie 2023, 135, e202303011. [Google Scholar] [CrossRef]
- Chen, C.; Lee, C.-S.; Tang, Y. Fundamental Understanding and Optimization Strategies for Dual-Ion Batteries: A Review. Nano-Micro Lett. 2023, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.; Rana, P.J.S.; Ni, Z.; Yang, G.; Li, M.; Wang, M.; Gu, H.; Zhang, H.; Dou, B.D.; Huang, J. Iodide manipulation using zinc additives for efficient perovskite solar minimodules. Nat. Commun. 2024, 15, 1355. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, Y.; Zhang, Y.; Xu, H.; Huang, W.; He, X.; Chen, Q.; Dong, H.; Li, L.; Wu, X. Industrial-scale hard carbon designed to regulate electrochemical polarization for fast sodium storage. Angew. Chem. Int. Ed. 2024, 63, e202406889. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, X.; Ou, X.; Lee, C.; Zhou, J.; Tang, Y. Ultrahigh Nitrogen Doping of Carbon Nanosheets for High Capacity and Long Cycling Potassium Ion Storage. Adv. Energy Mater. 2019, 9, 1902672. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Liang, X.; Feng, X.; Xiang, H. Chromium-Modified Li4Ti5O12 with a Synergistic Effect of Bulk Doping, Surface Coating, and Size Reducing. ACS Appl. Mater. Interfaces 2016, 8, 21407–21416. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Sun, Y.; Xiang, H.; Liang, X.; Yu, Y. 3D Amorphous Carbon with Controlled Porous and Disordered Structures as a High-Rate Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2017, 8, 1702434. [Google Scholar] [CrossRef]
- Sonigara, K.K.; Vaghasiya, J.V.; Pumera, M. Corrosion-Resistant Shape-Programmable Zn-I2 Battery. Adv. Energy Mater. 2024, 2401321. [Google Scholar] [CrossRef]
- Oldham, K.B. A Gouy–Chapman–Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal Chem. 2008, 613, 131–138. [Google Scholar] [CrossRef]
- Wang, C.; Gao, G.; Su, Y.; Xie, J.; He, D.; Wang, X.; Wang, Y.; Wang, Y. High-voltage and dendrite-free zinc-iodine flow battery. Nat. Commun. 2024, 15, 6234. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S batteries: Challenges, achievements and opportunities. Electrochem. Energy Rev. 2023, 6, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Cabot, A. Insights on Polyidide Shuttling of Zn-I2 Batteries by I3−/I− Electrolytes Based on the Dual-Ion Battery System. Nanomaterials 2025, 15, 738. https://doi.org/10.3390/nano15100738
Chang X, Cabot A. Insights on Polyidide Shuttling of Zn-I2 Batteries by I3−/I− Electrolytes Based on the Dual-Ion Battery System. Nanomaterials. 2025; 15(10):738. https://doi.org/10.3390/nano15100738
Chicago/Turabian StyleChang, Xingqi, and Andreu Cabot. 2025. "Insights on Polyidide Shuttling of Zn-I2 Batteries by I3−/I− Electrolytes Based on the Dual-Ion Battery System" Nanomaterials 15, no. 10: 738. https://doi.org/10.3390/nano15100738
APA StyleChang, X., & Cabot, A. (2025). Insights on Polyidide Shuttling of Zn-I2 Batteries by I3−/I− Electrolytes Based on the Dual-Ion Battery System. Nanomaterials, 15(10), 738. https://doi.org/10.3390/nano15100738






