The Role of the Surface Functionalities in the Electrocatalytic Activity of Cytochrome C on Graphene-Based Materials
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
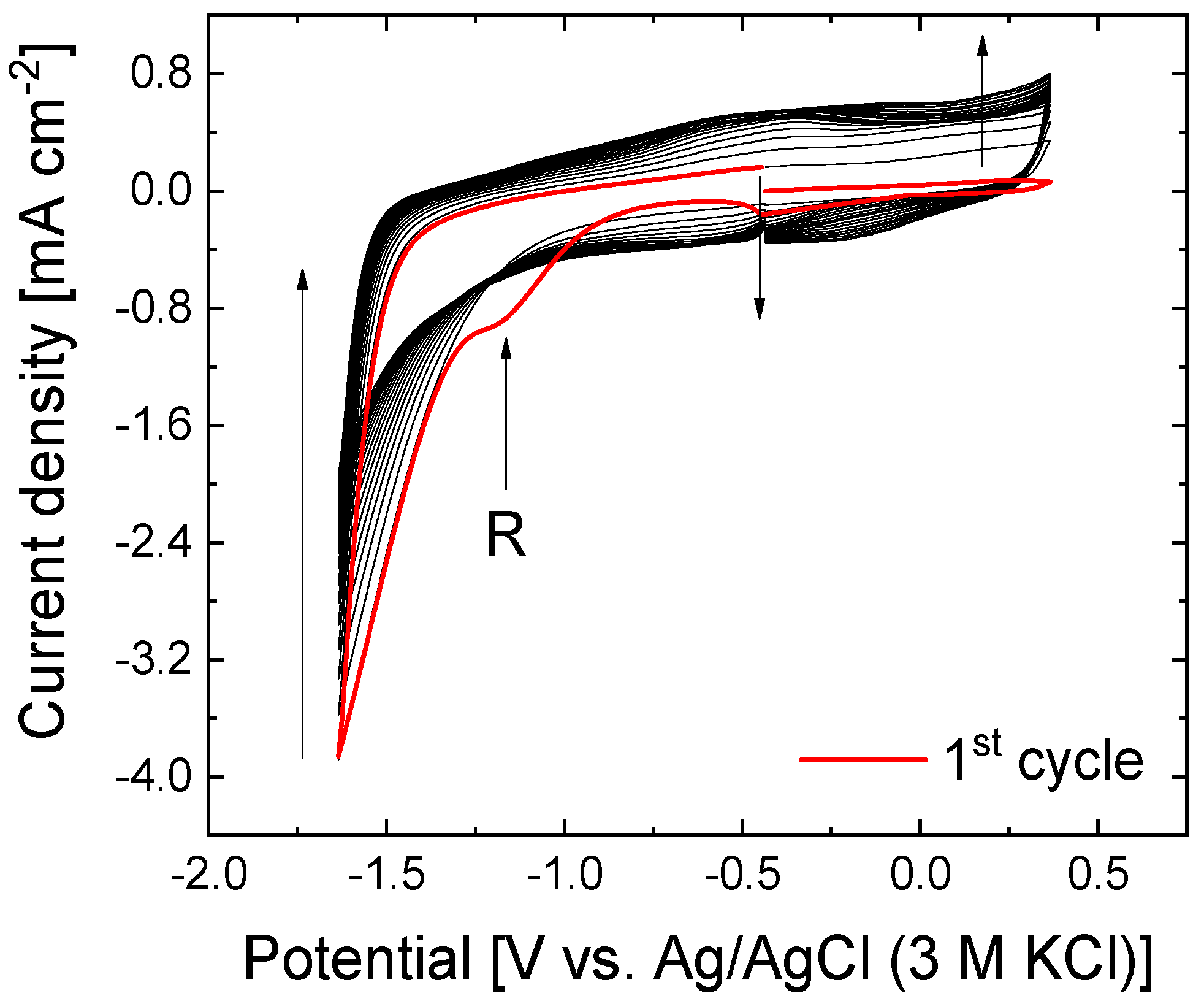
3.1. Electrochemical Reduction of GO Under Neutral Conditions
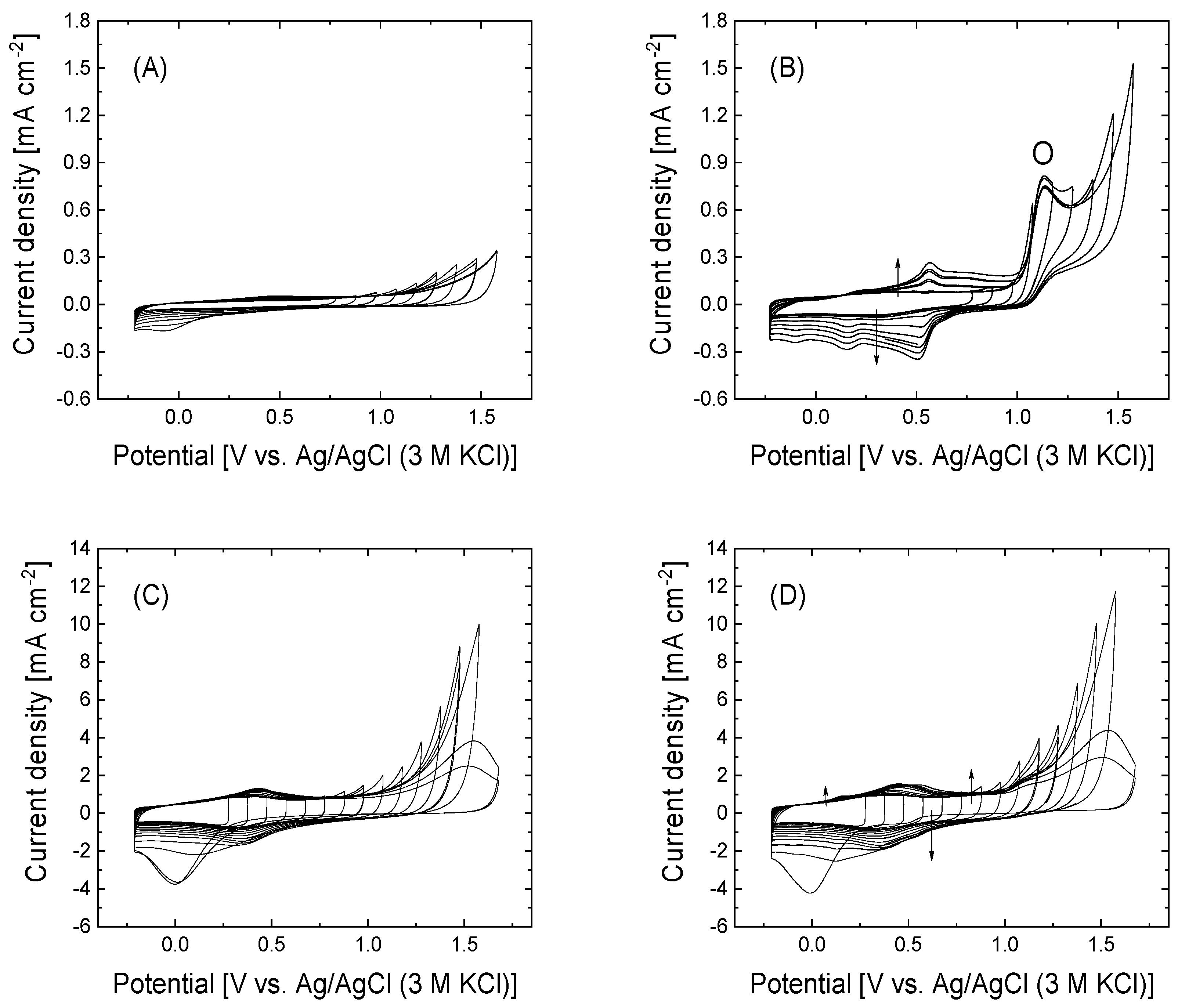
3.2. Electrochemical Surface Functionalization with 4-APPA of GO and rGO
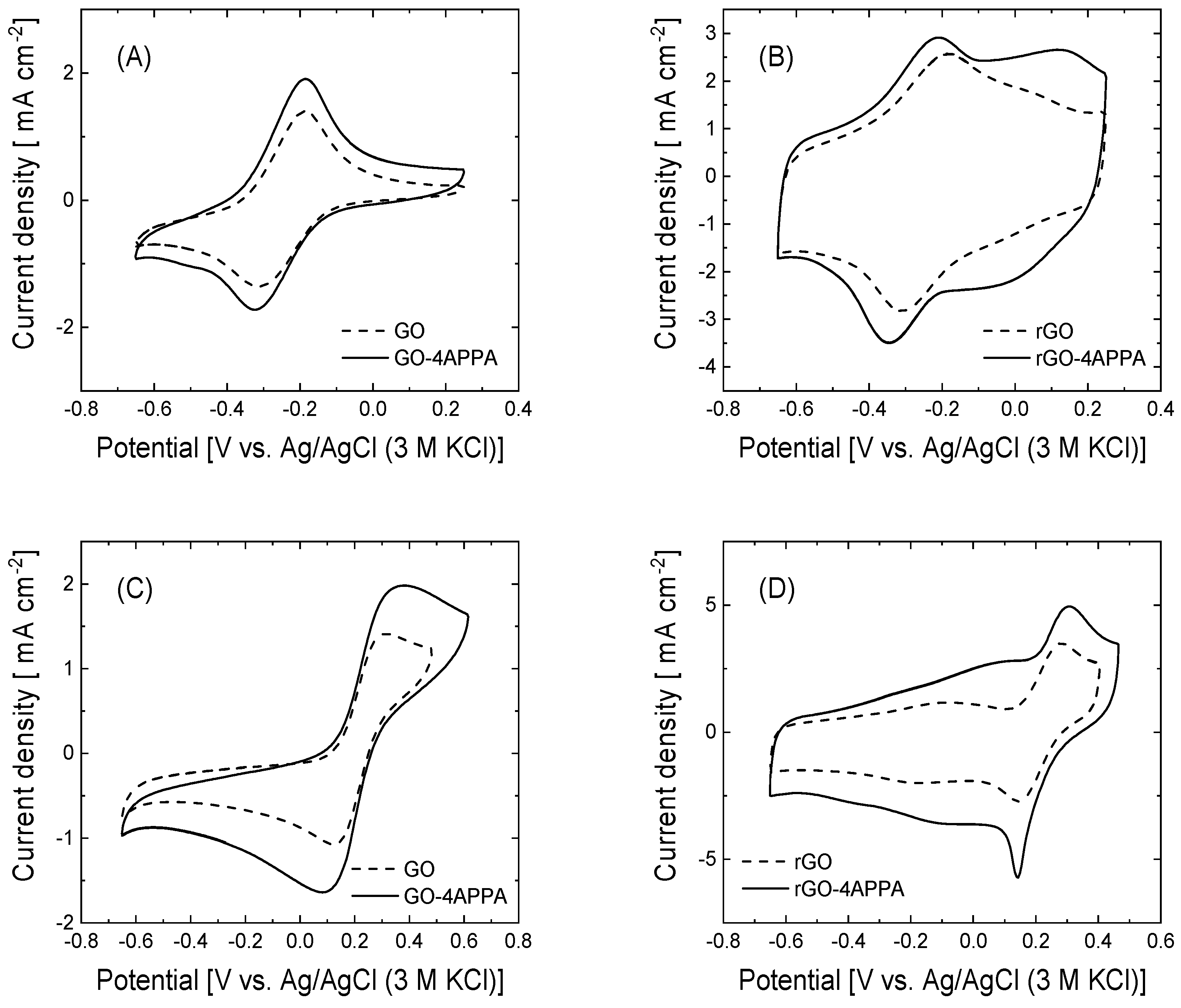
3.3. Electrochemical Behavior at Neutral pH
3.4. Electrocatalytic Activity of Cyt C Immobilized on Functionalized GO and rGO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruff, A.; Conzuelo, F.; Schuhmann, W. Bioelectrocatalysis as the basis for the design of enzyme-based biofuel cells and semi-artificial biophotoelectrodes. Nat. Catal. 2020, 3, 214–224. [Google Scholar] [CrossRef]
- Riedel, M.; Höfs, S.; Ruff, A.; Schuhmann, W.; Lisdat, F. A Tandem Solar Biofuel Cell: Harnessing Energy from Light and Biofuels. Angew. Chem. Int. Ed. 2021, 60, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Abdellaoui, S.; Khadka, N.; Dean, D.R.; Leech, D.; Seefeldt, L.C.; Minteer, S.D. Nitrogenase bioelectrocatalysis: Heterogeneous ammonia and hydrogen production by MoFe protein. Energy Environ. Sci. 2016, 9, 2550–2554. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ruff, A.; Cai, R.; Lim, K.; Schuhmann, W.; Minteer, S.D. Electroenzymatic Nitrogen Fixation Using a MoFe Protein System Immobilized in an Organic Redox Polymer. Angew. Chem. Int. Ed. 2020, 59, 16511–16516. [Google Scholar] [CrossRef]
- Schuhmann, W. Amperometric enzyme biosensors based on optimised electron-transfer pathways and non-manual immobilisation procedures. Rev. Mol. Biotechnol. 2002, 82, 425–441. [Google Scholar] [CrossRef]
- Bartlett, P.N. Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Page, C.C.; Moser, C.C.; Chen, X.; Dutton, P.L. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature 1999, 402, 47–52. [Google Scholar] [CrossRef]
- de Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New trends in enzyme immobilization at nanostructured interfaces for efficient electrocatalysis in biofuel cells. Electrochim. Acta 2014, 126, 104–114. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Conzuelo, F.; Schuhmann, W.; Cazorla-Amorós, D.; Morallón, E. Multi-wall carbon nanotubes electrochemically modified with phosphorus and nitrogen functionalities as a basis for bioelectrodes with improved performance. Electrochim. Acta 2021, 387, 138530. [Google Scholar] [CrossRef]
- Smutok, O.; Ngounou, B.; Pavlishko, H.; Gayda, G.; Gonchar, M.; Schuhmann, W. A reagentless bienzyme amperometric biosensor based on alcohol oxidase/peroxidase and an Os-complex modified electrodeposition paint. Sens. Actuators B: Chem. 2006, 113, 590–598. [Google Scholar] [CrossRef]
- Ferapontova, E.E.; Shleev, S.; Ruzgas, T.; Stoica, L.; Christenson, A.; Tkac, J.; Yaropolov, A.I.; Gorton, L. Direct Electrochemistry of Proteins and Enzymes. In Electrochemistry of Nucleic Acids and Proteins—Towards Electrochemical Sensors for Genomics and Proteomics; Paleček, E., Scheller, F., Wang, J.B.T.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 1, pp. 517–598. ISBN 1871-0069. [Google Scholar]
- Demurtas, D.; Alvarez-Malmagro, J.; Rathore, A.; Mandal, T.; Quintero-Jaime, A.F.; Belochapkine, S.; Lielpetere, A.; Jayakumar, K.; Leech, D.; Schuhmann, W.; et al. Development of flexible nanoporous gold electrodes for the detection of glucose. Bioelectrochemistry 2025, 165, 108949. [Google Scholar] [CrossRef]
- Reuillard, B.; Ly, K.H.; Hildebrandt, P.; Jeuken, L.J.C.; Butt, J.N.; Reisner, E. High Performance Reduction of H2O2 with an Electron Transport Decaheme Cytochrome on a Porous ITO Electrode. J. Am. Chem. Soc. 2017, 139, 3324–3327. [Google Scholar] [CrossRef] [PubMed]
- Cziegler, C.; Csarman, F.; Breslmeyer, E.; Ma, S.; Meinert, H.; Ludwig, R.; Rudroff, F.; Mihovilovic, M.D. Design and Synthesis of Artificial FAD Cofactors for the Light-Triggered Covalent Flavinylation of Flavoenzymes. ACS Catal. 2024, 14, 15988–15996. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Direct enzymatic bioelectrocatalysis: Differentiating between myth and reality. J. R. Soc. Interface 2017, 14, 20170253. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.K.; Carvalhal, R.F.; Kubota, L.T. Effects of different self-assembled monolayers on enzyme immobilization procedures in peroxidase-based biosensor development. J. Electroanal. Chem. 2008, 612, 164–172. [Google Scholar] [CrossRef]
- Allen, P.M.; Allen, H.; Hill, O.; Walton, N.J. Surface modifiers for the promotion of direct electrochemistry of cytochrome c. J. Electroanal. Chem. Interfacial Electrochem. 1984, 178, 69–86. [Google Scholar] [CrossRef]
- Takeda, K.; Kusuoka, R.; Birrell, J.A.; Yoshida, M.; Igarashi, K.; Nakamura, N. Bioelectrocatalysis based on direct electron transfer of fungal pyrroloquinoline quinone-dependent dehydrogenase lacking the cytochrome domain. Electrochim. Acta 2020, 359, 136982. [Google Scholar] [CrossRef]
- Xu, S.; Minteer, S.D. Investigating the Impact of Multi-Heme Pyrroloquinoline Quinone-Aldehyde Dehydrogenase Orientation on Direct Bioelectrocatalysis via Site Specific Enzyme Immobilization. ACS Catal. 2013, 3, 1756–1763. [Google Scholar] [CrossRef]
- Xu, S.; Minteer, S.D. Pyrroloquinoline Quinone-Dependent Enzymatic Bioanode: Incorporation of the Substituted Polyaniline Conducting Polymer as a Mediator. ACS Catal. 2014, 4, 2241–2248. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Schuhmann, W.; Lammert, R.; Hämmerle, M.; Schmidt, H.-L. Electrocatalytic properties of polypyrrole in amperometric electrodes. Biosens. Bioelectron. 1991, 6, 689–697. [Google Scholar] [CrossRef]
- Trashin, S.A.; Haltrich, D.; Ludwig, R.; Gorton, L.; Karyakin, A.A. Improvement of direct bioelectrocatalysis by cellobiose dehydrogenase on screen printed graphite electrodes using polyaniline modification. Bioelectrochemistry 2009, 76, 87–92. [Google Scholar] [CrossRef] [PubMed]
- López-Bernabeu, S.; Huerta, F.; Morallón, E.; Montilla, F. Direct Electron Transfer to Cytochrome c Induced by a Conducting Polymer. J. Phys. Chem. C 2017, 121, 15870–15879. [Google Scholar] [CrossRef]
- López-Bernabeu, S.; Gamero-Quijano, A.; Huerta, F.; Morallón, E.; Montilla, F. Enhancement of the direct electron transfer to encapsulated cytochrome c by electrochemical functionalization with a conducting polymer. J. Electroanal. Chem. 2017, 793, 34–40. [Google Scholar] [CrossRef]
- Patel, J.; Cai, R.; Milton, R.; Chen, H.; Minteer, S.D. Pyrene-Based Noncovalent Immobilization of Nitrogenase on Carbon Surfaces. ChemBioChem 2020, 21, 1729–1732. [Google Scholar] [CrossRef]
- Hasan, K.; Grattieri, M.; Wang, T.; Milton, R.D.; Minteer, S.D. Enhanced Bioelectrocatalysis of Shewanella oneidensis MR-1 by a Naphthoquinone Redox Polymer. ACS Energy Lett. 2017, 2, 1947–1951. [Google Scholar] [CrossRef]
- Lavanya, V.; Pavithra, D.; Mohanapriya, A.; Santhakumar, K.; Senthil Kumar, A. A π–π Bonding-Assisted Molecular-Wiring of Folded-Cytochrome c and Naphthoquinone and Its Electron-Relay-Based Bioelectrocatalytic H2O2 Reduction Reaction Visualized by Redox-Competitive Scanning Electrochemical Microscopy. Langmuir 2023, 39, 11556–11570. [Google Scholar] [CrossRef]
- Ben Tahar, A.; Żelechowska, K.; Biernat, J.F.; Paluszkiewicz, E.; Cinquin, P.; Martin, D.; Zebda, A. High catalytic performance of laccase wired to naphthylated multiwall carbon nanotubes. Biosens. Bioelectron. 2020, 151, 111961. [Google Scholar] [CrossRef]
- Gandhi, M.; Rajagopal, D.; Senthil Kumar, A. Molecularly wiring of Cytochrome c with carboxylic acid functionalized hydroquinone on MWCNT surface and its bioelectrocatalytic reduction of H2O2 relevance to biomimetic electron-transport and redox signalling. Electrochim. Acta 2021, 368, 137596. [Google Scholar] [CrossRef]
- Felipe Quintero-Jaime, A.; Conzuelo, F.; Cazorla-Amorós, D.; Morallón, E. Pyrroloquinoline quinone-dependent glucose dehydrogenase bioelectrodes based on one-step electrochemical entrapment over single-wall carbon nanotubes. Talanta 2021, 232, 122386. [Google Scholar] [CrossRef]
- Niu, J.; Jin, X.; Wang, X.; Ren, Z.; Li, B.; Liu, X.; Wong, D.K.Y. An Enzyme-Encapsulated MOF@MOF Nanocomposite for Detecting H2O2 Derived From Superoxide Anion Released by Mitochondria of HeLa Cells. Small Methods 2025, 9, 2401070. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M.; Le Goff, A. Recent Advances in Carbon Nanotube-Based Enzymatic Fuel Cells. Front. Bioeng. Biotechnol. 2014, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Kitazumi, Y.; Shirai, O.; Kano, K. Significance of Nanostructures of an Electrode Surface in Direct Electron Transfer-Type Bioelectrocatalysis of Redox Enzymes. In Novel Catalyst Materials for Bioelectrochemical Systems: Fundamentals and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1342, pp. 147–163. ISBN 978-0-8412-3668-4. [Google Scholar]
- Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Sousa, C.P.; Salazar-Banda, G.R.; de Lima-Neto, P.; Correia, A.N.; Morais, S. Current overview and perspectives on carbon-based (bio)sensors for carbamate pesticides electroanalysis. TrAC Trends Anal. Chem. 2020, 124, 115779. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Melle-Franco, M.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Towards understanding the active sites for the ORR in N-doped carbon materials through fine-tuning of nitrogen functionalities: An experimental and computational approach. J. Mater. Chem. A 2019, 7, 24239–24250. [Google Scholar] [CrossRef]
- Veizaga, N.S.; Mendow, G.; Quintero-Jaime, A.F.; Berenguer-Murcia, Á.; de Miguel, S.; Morallón, E.; Cazorla-Amorós, D. Controlled synthesis of mono- and bimetallic Pt-based catalysts for electrochemical ethanol oxidation. Mater. Chem. Phys. 2022, 275, 125282. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, G.; Huang, H.; Zhang, J.; Lv, Q.; Shuai, L.; Ni, Y.; Liao, G. Unraveling the dipole field in ultrathin, porous, and defective carbon nitride nanosheets for record-high piezo-photocatalytic H2O2 production. eScience 2024, 27, 100370. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, T.; Zhang, F.; Boury, B.; Huang, L.; Yang, Y.; Liao, G.; Xiao, H.; Chen, L. A Novel Dual-Channel Carbon Nitride Homojunction with Nanofibrous Carbon for Significantly Boosting Photocatalytic Hydrogen Peroxide Production. Adv. Fiber Mater. 2024, 6, 387–400. [Google Scholar] [CrossRef]
- Xiao, H.; Shan, Y.; Wu, S.; Shan, T.; Luo, H.; Zhang, F.; Huang, L.; Chen, L.; Liao, G. Enlarging the π-conjugation system of carbon nitride for boosting hydrogen peroxide generation. Chem. Eng. J. 2024, 492, 152228. [Google Scholar] [CrossRef]
- Miyake, T.; Haneda, K.; Yoshino, S.; Nishizawa, M. Flexible, layered biofuel cells. Biosens. Bioelectron. 2013, 40, 45–49. [Google Scholar] [CrossRef]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef]
- Zebda, A.; Gondran, C.; Le Goff, A.; Holzinger, M.; Cinquin, P.; Cosnier, S. Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube-enzyme electrodes. Nat. Commun. 2011, 2, 370. [Google Scholar] [CrossRef]
- Abreu, C.; Nedellec, Y.; Gross, A.J.; Ondel, O.; Buret, F.; Goff, A.L.; Holzinger, M.; Cosnier, S. Assembly and Stacking of Flow-through Enzymatic Bioelectrodes for High Power Glucose Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 23836–23842. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.J.; Tanaka, S.; Colomies, C.; Giroud, F.; Nishina, Y.; Cosnier, S.; Tsujimura, S.; Holzinger, M. Diazonium Electrografting vs. Physical Adsorption of Azure A at Carbon Nanotubes for Mediated Glucose Oxidation with FAD-GDH. ChemElectroChem 2020, 7, 4543–4549. [Google Scholar] [CrossRef]
- Haddad, R.; Cosnier, S.; Maaref, A.; Holzinger, M. Non-covalent biofunctionalization of single-walled carbon nanotubesviabiotin attachment by π-stacking interactions and pyrrole polymerization. Analyst 2009, 134, 2412–2418. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.-S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.T.; Minson, M.; Hickey, D.; Artyushkova, K.; Glatzhofer, D.T.; Minteer, S.D. Anthracene-Modified Multi-Walled Carbon Nanotubes as Direct Electron Transfer Scaffolds for Enzymatic Oxygen Reduction. ACS Catal. 2011, 1, 1683–1690. [Google Scholar] [CrossRef]
- González-Gaitán, C.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Effects of the surface chemistry and structure of carbon nanotubes on the coating of glucose oxidase and electrochemical biosensors performance. RSC Adv. 2017, 7, 26867–26878. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. Electrochemical functionalization of single wall carbon nanotubes with phosphorus and nitrogen species. Electrochim. Acta 2020, 340, 135935. [Google Scholar] [CrossRef]
- Gentil, S.; Che Mansor, S.M.; Jamet, H.; Cosnier, S.; Cavazza, C.; Le Goff, A. Oriented Immobilization of [NiFeSe] Hydrogenases on Covalently and Noncovalently Functionalized Carbon Nanotubes for H2/Air Enzymatic Fuel Cells. ACS Catal. 2018, 8, 3957–3964. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Zhang, R.; Wang, Y.; He, P.; Fang, Y. Direct Electrochemistry and Electrocatalysis of the Hemoglobin Immobilized on Diazonium-Functionalized Aligned Carbon Nanotubes Electrode. Electroanalysis 2009, 21, 1672–1677. [Google Scholar] [CrossRef]
- Cosnier, S. Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review. Biosens. Bioelectron. 1999, 14, 443–456. [Google Scholar] [CrossRef]
- Yon-Hin, B.F.Y.; Lowe, C.R. An investigation of 3-functionalized pyrrole-modified glucose oxidase for the covalent electropolymerization of enzyme films. J. Electroanal. Chem. 1994, 374, 167–172. [Google Scholar] [CrossRef]
- Marrani, A.G.; Motta, A.; Schrebler, R.; Zanoni, R.; Dalchiele, E.A. Insights from experiment and theory into the electrochemical reduction mechanism of graphene oxide. Electrochim. Acta 2019, 304, 231–238. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallón, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. Effect of surface oxygen groups in the electrochemical modification of multi-walled carbon nanotubes by 4-amino phenyl phosphonic acid. Carbon 2020, 165, 328–339. [Google Scholar] [CrossRef]
- Probst, M.; Holze, R. A systematic spectroelectrochemical investigation of alkyl-substituted anilines and their polymers. Macromol. Chem. Phys. 1997, 198, 1499–1509. [Google Scholar] [CrossRef]
- Nateghi, M.R.; Zahedi, M.; Mosslemin, M.H.; Hashemian, S.; Behzad, S.; Minnai, A. Autoacceleration/degradation of electrochemical polymerization of substituted anilines. Polymer 2005, 46, 11476–11483. [Google Scholar] [CrossRef]
- Benyoucef, A.; Huerta, F.; Ferrahi, M.I.; Morallon, E. Voltammetric and in situ FT-IRS study of the electropolymerization of o-aminobenzoic acid at gold and graphite carbon electrodes: Influence of pH on the electrochemical behaviour of polymer films. J. Electroanal. Chem. 2008, 624, 245–250. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Tang, J.; Wang, F. Electrochemical polymerization of aniline. Synth. Met. 1987, 18, 323–328. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Ghisolfi, A.; Grau-Marín, D.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Post-synthetic efficient functionalization of polyaniline with phosphorus-containing groups. Effect of phosphorus on electrochemical properties. Eur. Polym. J. 2019, 119, 272–280. [Google Scholar] [CrossRef]
- Abidi, M.; López-Bernabeu, S.; Huerta, F.; Montilla, F.; Besbes-Hentati, S.; Morallón, E. Spectroelectrochemical study on the copolymerization of o-aminophenol and aminoterephthalic acid. Eur. Polym. J. 2017, 91, 386–395. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Li, H.; Zhao, Z.; Bin Wu, H.; Hao, C.; Liu, S.; Qiu, J.; Lou, X.W. (David) Enhancing lithium–sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002. [Google Scholar] [CrossRef] [PubMed]
- Valero-Romero, M.J.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T. Role of surface phosphorus complexes on the oxidation of porous carbons. Fuel Process. Technol. 2017, 157, 116–126. [Google Scholar] [CrossRef]
- Quesada-Plata, F.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Activated Carbons Prepared through H3PO4-Assisted Hydrothermal Carbonisation from Biomass Wastes: Porous Texture and Electrochemical Performance. ChemPlusChem 2016, 81, 1349–1359. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amorós, D.; Morallón, E.; Nishihara, H.; Kyotani, T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced electro-oxidation resistance of carbon electrodes induced by phosphorus surface groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef]
- Ruiz-Soria, G.; Susi, T.; Sauer, M.; Yanagi, K.; Pichler, T.; Ayala, P. On the bonding environment of phosphorus in purified doped single-walled carbon nanotubes. Carbon 2015, 81, 91–95. [Google Scholar] [CrossRef]
- Bard, A.; Faulkner, L. Electrochemical Methods: Fundamental and Applications; Harris, D., Swain, E., Aiello, E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 0-471-04372-9. [Google Scholar]
- Wang, J.; Zhang, S.; Zhang, Y. Fabrication of chronocoulometric DNA sensor based on gold nanoparticles/poly(l-lysine) modified glassy carbon electrode. Anal. Biochem. 2010, 396, 304–309. [Google Scholar] [CrossRef]
- Wang, Y.; Limon-Petersen, J.G.; Compton, R.G. Measurement of the diffusion coefficients of [Ru(NH3)6]3+ and [Ru(NH3)6]2+ in aqueous solution using microelectrode double potential step chronoamperometry. J. Electroanal. Chem. 2011, 652, 13–17. [Google Scholar] [CrossRef]
- Mahé, E.; Devilliers, D.; Comninellis, C. Electrochemical reactivity at graphitic micro-domains on polycrystalline boron doped diamond thin-films electrodes. Electrochim. Acta 2005, 50, 2263–2277. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Sezer, M.; Spricigo, R.; Utesch, T.; Millo, D.; Leimkuehler, S.; Mroginski, M.A.; Wollenberger, U.; Hildebrandt, P.; Weidinger, I.M. Redox properties and catalytic activity of surface-bound human sulfite oxidase studied by a combined surface enhanced resonance Raman spectroscopic and electrochemical approach. Phys. Chem. Chem. Phys. 2010, 12, 7894–7903. [Google Scholar] [CrossRef] [PubMed]
- Gabe, A.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Understanding of oxygen reduction reaction by examining carbon-oxygen gasification reaction and carbon active sites on metal and heteroatoms free carbon materials of different porosities and structures. Carbon 2019, 148, 430–440. [Google Scholar] [CrossRef]
- Han, G.-F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.-P.; Kim, S.-W.; Kim, S.-J.; Bu, Y.; Fu, Z.; Lu, Y.; et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Gorton, L.; Lindgren, A.; Larsson, T.; Munteanu, F.D.; Ruzgas, T.; Gazaryan, I. Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal. Chim. Acta 1999, 400, 91–108. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Rui, Q.; Tian, Y. Detection of Extracellular H2O2 Released from Human Liver Cancer Cells Based on TiO2 Nanoneedles with Enhanced Electron Transfer of Cytochrome c. Anal. Chem. 2009, 81, 3035–3041. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Thermo Scientific XPS Simplified [Online], Thermo Scientific XPS, Phosphorus (2020). Available online: https://xpssimplified.com/elements/phosphorus.php#appnotes (accessed on 3 March 2018).
- Zhang, L. Direct electrochemistry of cytochrome c at ordered macroporous active carbon electrode. Biosens. Bioelectron. 2008, 23, 1610–1615. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, J.; Zou, Y.; Zhang, W.; Lee, S.T. Direct Electrochemistry and Electrocatalytic Activity of Cytochrome c Covalently Immobilized on a Boron-Doped Nanocrystalline Diamond Electrode. Anal. Chem. 2008, 80, 4141–4146. [Google Scholar] [CrossRef]
- Lee, K.; Gopalan, A.; Komathi, S. Direct electrochemistry of cytochrome c and biosensing for hydrogen peroxide on polyaniline grafted multi-walled carbon nanotube electrode. Sens. Actuators B Chem. 2009, 141, 518–525. [Google Scholar] [CrossRef]
- Seo, H.; Kim, C.; Sohn, B.; Yeow, T.; Son, M.; Haskard, M. ISFET glucose sensor based on a new principle using the electrolysis of hydrogen peroxide. Sens. Actuators B Chem. 1997, 40, 1–5. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, Z.; Zhang, L.; Wei, X. Direct electrochemistry of cytochrome c on a multi-walled carbon nanotubes modified electrode and its electrocatalytic activity for the reduction of H2O2. Electrochem. Commun. 2005, 7, 256–260. [Google Scholar] [CrossRef]
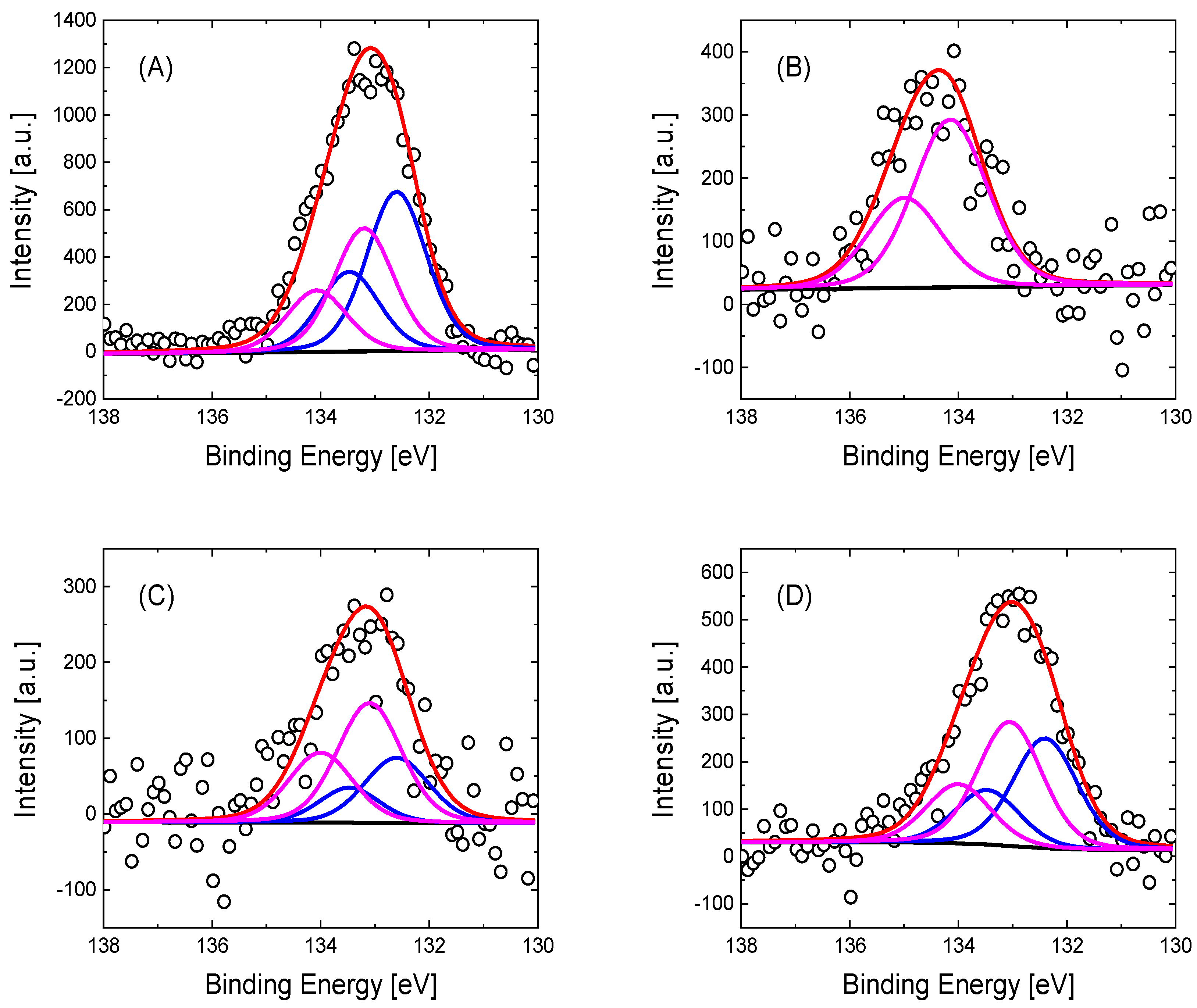

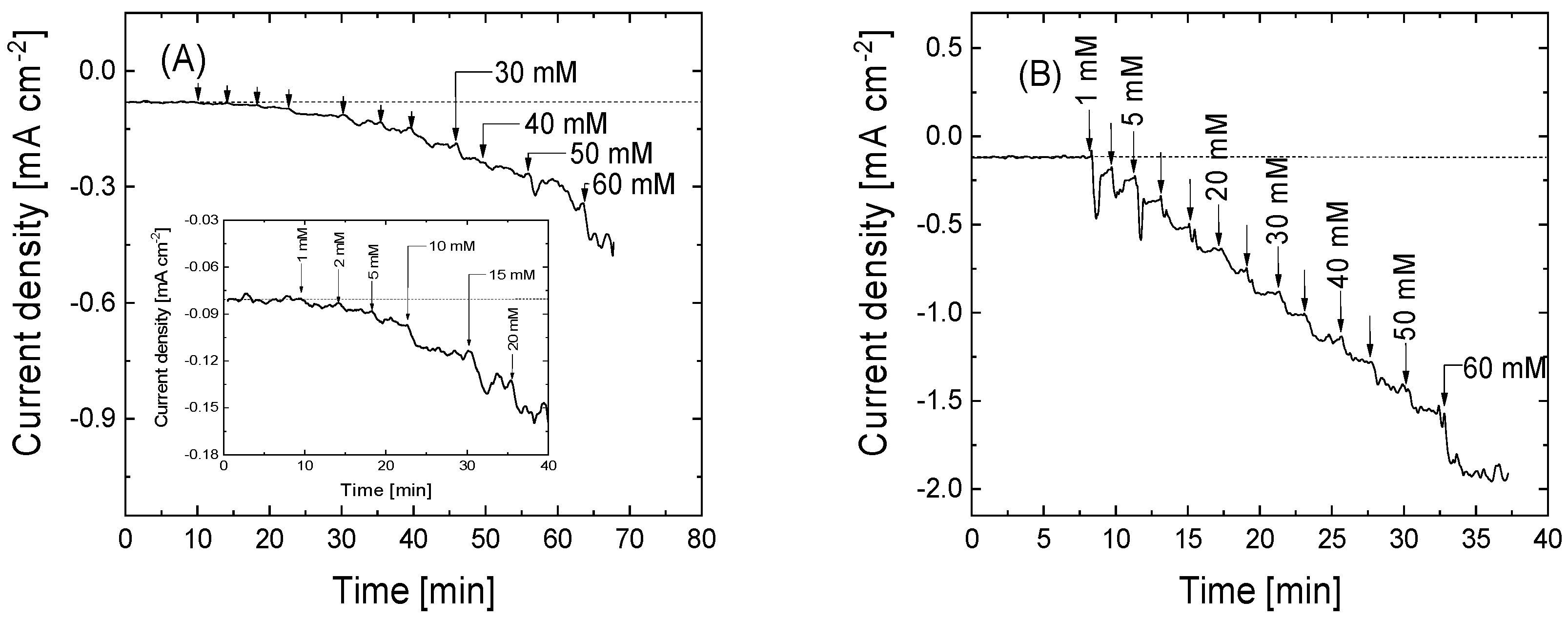

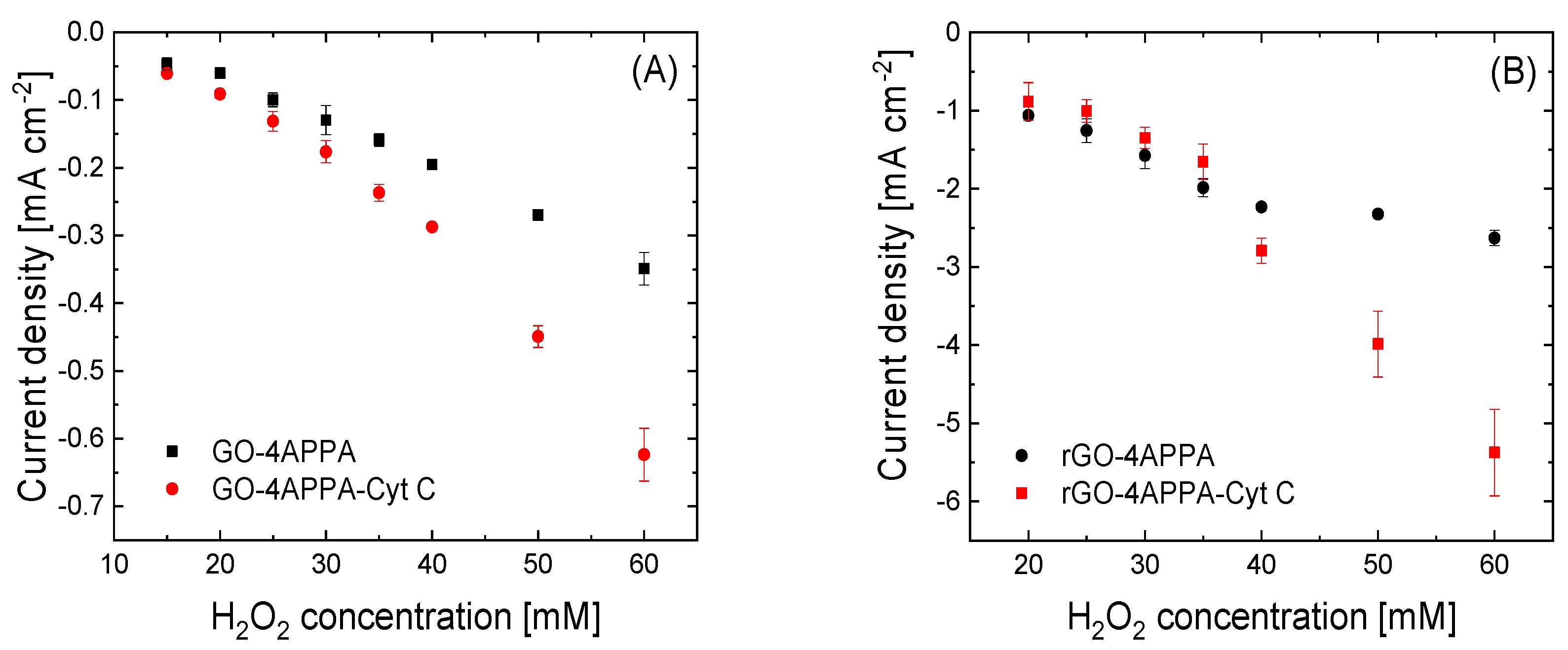
| Graphene-Based Material | Upper Potential Limit [V vs. RHE] | O (at%) | N (at%) | P (at%) | C (at%) |
|---|---|---|---|---|---|
| GO | Pristine | 34.8 | 0.28 | -- | 64.92 |
| 1.28 | 22.2 | 0.71 | 1.14 | 75.95 | |
| 1.58 | 29.7 | 1.54 | 0.61 | 68.15 | |
| rGO | Pristine | 18.7 | 0.39 | -- | 80.91 |
| 1.28 | 23.0 | 1.37 | 0.52 | 75.11 | |
| 1.58 | 21.5 | 1.03 | 0.65 | 76.82 |
| Graphene-Based Electrode Material | [cm2] | [cm2] | [mV] * | [mV] * | [V] * | [V] * | [×10−3 cm s−1] | [×10−3 cm s−1] |
|---|---|---|---|---|---|---|---|---|
| GO | 0.444 | 0.045 | 126 | 150 | −0.250 | 0.216 | 2.70 | 1.38 |
| GO-4APPA | 0.609 | 0.075 | 136 | 230 | −0.254 | 0.253 | 1.82 | 0.28 |
| rGO | 1.680 | 0.228 | 129 | 111 | −0.247 | 0.212 | 1.16 | 2.12 |
| rGO-4APPA | 2.003 | 0.399 | 137 | 128 | −0.279 | 0.221 | 0.98 | 1.34 |
| Graphene-Based Electrode Material | LOD [mM] * | LOQ [mM] ** | Sensitivity [μA mM−1 cm−2] |
|---|---|---|---|
| GO-4APPA | 0.1 | 0.33 | 6.65 |
| GO-4APPACyt C | 9.20 | ||
| rGO-4APPA | 44.30 | ||
| rGO-4APPA-Cyt C | 111.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. The Role of the Surface Functionalities in the Electrocatalytic Activity of Cytochrome C on Graphene-Based Materials. Nanomaterials 2025, 15, 722. https://doi.org/10.3390/nano15100722
Quintero-Jaime AF, Cazorla-Amorós D, Morallón E. The Role of the Surface Functionalities in the Electrocatalytic Activity of Cytochrome C on Graphene-Based Materials. Nanomaterials. 2025; 15(10):722. https://doi.org/10.3390/nano15100722
Chicago/Turabian StyleQuintero-Jaime, Andrés Felipe, Diego Cazorla-Amorós, and Emilia Morallón. 2025. "The Role of the Surface Functionalities in the Electrocatalytic Activity of Cytochrome C on Graphene-Based Materials" Nanomaterials 15, no. 10: 722. https://doi.org/10.3390/nano15100722
APA StyleQuintero-Jaime, A. F., Cazorla-Amorós, D., & Morallón, E. (2025). The Role of the Surface Functionalities in the Electrocatalytic Activity of Cytochrome C on Graphene-Based Materials. Nanomaterials, 15(10), 722. https://doi.org/10.3390/nano15100722









