Tunable Structure and Properties of Co-Evaporated Co–C60 Nanocomposite Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Electronic Structure of the Films
3.2. Microstructure of the Films
3.3. Magnetism of the Films
3.4. Electric and Magnetic Transport Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| θk | Kerr rotation |
| δw | Domain wall width |
| DSD | Critical size of single domain |
| DSP | Critical size of superparamagnetic |
| RMSR | Root-mean-square roughness |
| OSV | Organic spin valves |
| OMAR | Organic magnetoresistance |
References
- Singh, A.; Kulkarni, S.P.; Patel, R.S.; Narayanan, R.A.; Gopalan, B. Pore Engineering in γ-Fe2O3 Nanoparticles: Hierarchical Pores by Controlled Lixiviation Using Citrate Ligands. J. Phys. Chem. C 2025, 129, 627–637. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Ma, Y.; Wang, J.; Wang, Q. Nanomagnetic structure of composite films with cubic array distribution of FeNi nanoparticles. J. Colloid Interface Sci. 2021, 583, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dzidziguri, E.L.; Sidorova, E.N.; Inkar, M.; Yudin, A.G.; Kostitsyna, E.V.; Ozherelkov, D.Y.; Slusarsky, K.V.; Nalivaiko, A.Y.; Gromov, A.A. Cobalt nanoparticles synthesis by cobalt nitrate reduction. Mater. Res. Express 2019, 6, 105081. [Google Scholar] [CrossRef]
- Navarrete, B.; Stone, M.; Wang, P.; Guduru, R.; Luongo, K.; Hadjikhani, A.; Toledo, D.; Emirov, Y.; Arkook, B.; Liang, P.; et al. Nanomagnetic Particle-Based Information Processing. IEEE Trans. Nanotechnol. 2019, 18, 983–988. [Google Scholar] [CrossRef]
- Yakushiji, K.; Ernult, F.; Imamura, H.; Yamane, K.; Mitani, S.; Takanashi, K.; Takahashi, S.; Maekawa, S. Enhanced spin accumulation and novel magnetotransport in nanoparticles. Nat. Mater. 2005, 4, 57–61. [Google Scholar] [CrossRef]
- Sun, D.; Yin, L.; Sun, C.; Guo, H.; Gai, Z.; Zhang, X.-G.; Ward, T.Z.; Cheng, Z.; Shen, J. Giant Magnetoresistance in Organic Spin Valves. Phys. Rev. Lett. 2010, 104, 236602. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.J.; Lin, L.; Chen, B.B.; Yue, F.J.; Du, J.; Ding, H.F.; Zhang, F.M.; Wu, D. Room-temperature spin valve effects in La0.67Sr0.33MnO3/Alq3/Co devices. Synth. Met. 2011, 161, 1738–1741. [Google Scholar] [CrossRef]
- Halder, A.; Bhandary, S.; O’Regan, D.D.; Sanvito, S.; Droghetti, A. Theoretical perspective on the modification of the magnetocrystalline anisotropy at molecule-cobalt interfaces. Phys. Rev. Mater. 2023, 7, 064409. [Google Scholar] [CrossRef]
- Sanvito, S. The rise of spinterface science. Nat. Phys. 2010, 6, 562–564. [Google Scholar] [CrossRef]
- Sun, D.; Fang, M.; Xu, X.; Jiang, L.; Guo, H.; Wang, Y.; Yang, W.; Yin, L.; Snijders, P.C.; Ward, T.Z.; et al. Active control of magnetoresistance of organic spin valves using ferroelectricity. Nat. Commun. 2014, 5, 4396. [Google Scholar] [CrossRef]
- Fang, M.; Wang, Y.; Wang, H.; Hou, Y.; Vetter, E.; Kou, Y.; Yang, W.; Yin, L.; Xiao, Z.; Li, Z.; et al. Tuning the interfacial spin-orbit coupling with ferroelectricity. Nat. Commun. 2020, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhang, W.; Wu, X.; Guo, W.; Xia, H.; Wang, Y.; Wang, W.; Shen, J. Recent advances in tunable spin–orbit coupling using ferroelectricity. APL Mater. 2021, 9, 060704. [Google Scholar] [CrossRef]
- Swain, B.; Hong, H.S.; Jung, H.C. Commercial process development for synthesis of spherical cobalt nanopowder by wet chemical reduction reaction. Chem. Eng. J. 2015, 264, 654–663. [Google Scholar] [CrossRef]
- Singh Gaur, R.P. Hydrogen reduction of heterogenite to make sub-micron cobalt metal powder for hard metal applications. Int. J. Refract. Met. Hard Mater. 2012, 35, 300–305. [Google Scholar] [CrossRef]
- Komogortsev, S.V.; Iskhakov, R.S.; Barnakov, C.N.; Momot, N.A.; Maltsev, V.K.; Kozlov, A.P. Study of the structure and magnetic properties of Co nanoparticles in the matrix of highly porous amorphous carbon. Phys. Met. Metallogr. 2010, 109, 130–134. [Google Scholar] [CrossRef]
- Minnermann, M.; Grossmann, H.K.; Pokhrel, S.; Thiel, K.; Hagelin-Weaver, H.; Bäumer, M.; Mädler, L. Double flame spray pyrolysis as a novel technique to synthesize alumina-supported cobalt Fischer–Tropsch catalysts. Catal. Today 2013, 214, 90–99. [Google Scholar] [CrossRef]
- GRmen, S.; Stopić, S.; Friedrich, B. Synthesis of nanosized spherical cobalt powder by ultrasonic spray pyrolysis. Mater. Res. Bull. 2006, 41, 1882–1890. [Google Scholar] [CrossRef]
- Yao, L.; Xi, Y.; Xi, G.; Feng, Y. Synthesis of cobalt ferrite with enhanced magnetostriction properties by the sol−gel−hydrothermal route using spent Li-ion battery. J. Alloys Compd. 2016, 680, 73–79. [Google Scholar] [CrossRef]
- Sajjia, M.; Oubaha, M.; Hasanuzzaman, M.; Olabi, A.G. Developments of cobalt ferrite nanoparticles prepared by the sol–gel process. Ceram. Int. 2014, 40, 1147–1154. [Google Scholar] [CrossRef]
- Gubin, S.P.; Koksharov, Y.A. Preparation, Structure, and Properties of Magnetic Materials Based on Co-Containing Nanoparticles. ChemInform 2003, 34, 1085–1099. [Google Scholar] [CrossRef]
- Dong, X.L.; Choi, C.J.; Kim, B.K. Chemical synthesis of Co nanoparticles by chemical vapor condensation. Scr. Mater. 2002, 47, 857–861. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, F.; Zhang, Z. Preparation of cobalt nanoparticles by direct current arc plasma evaporation method. Int. J. Refract. Met. Hard Mater. 2012, 31, 224–229. [Google Scholar] [CrossRef]
- Iwasa, Y.; Kaneyasu, T. Optical study of electronic structures and phonons in alkali-metal-doped C60. Phys. Rev. B 1995, 51, 3678–3685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.A.; Lairson, B.M.; Barrera, E.V.; Shull, R.D. Formation of nanomagnetic thin films by dispersed fullerenes. Appl. Phys. Lett. 2000, 77, 3242–3244. [Google Scholar] [CrossRef]
- Kuzmany, H.; Pfeiffer, R.; Hulman, M.; Kramberger, C. Raman spectroscopy of fullerenes and fullerene–nanotube composites. Philos. Trans. R. Soc. Lond. Ser. A: Math. Phys. Eng. Sci. 2004, 362, 2375–2406. [Google Scholar] [CrossRef]
- Wang, Z.B.; Helander, M.G.; Greiner, M.T.; Qiu, J.; Lu, Z.H. Energy-level alignment and charge injection at metal/C60/organic interfaces. Appl. Phys. Lett. 2009, 95, 043302. [Google Scholar] [CrossRef]
- Zong, L.-N.; Wang, R.-S.; Peng, D.; Chen, X.-J. Superconductivity in Nonstoichiometric Rubidium-Doped C60. J. Phys. Chem. C 2022, 126, 2912–2919. [Google Scholar] [CrossRef]
- Penuelas, J.; Andreazza-VIgnolle, C.; Andreazza, P.; Ouerghi, A.; Bouet, N. Temperature effect on the ordering and morphology of CoPt nanoparticles. Surf. Sci. 2008, 602, 545–551. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Pakhomov, A.B.; Denardin, J.C.; de Lima, O.F.; Knobel, M.; Missell, F.P. Transport and magnetotransport properties of Co thin films on Si. J. Magn. Magn. Mater. 2001, 226, 1631–1632. [Google Scholar] [CrossRef]
- Baranovskii, S.D. Theoretical description of charge transport in disordered organic semiconductors. Phys. Status Solidi 2014, 251, 487–525. [Google Scholar] [CrossRef]
- Janssen, P.; Cox, M.; Wouters, S.H.W.; Kemerink, M.; Wienk, M.M. Tuning organic magnetoresistance in polymer-fullerene blends by controlling spin reaction pathways. Nat. Commun. 2013, 4, 2286. [Google Scholar] [CrossRef] [PubMed]
- Shumilin, A.V.; Kabanov, V.V.; Dediu, V.I. Magnetoresistance in organic semiconductors: Including pair correlations in the kinetic equations for hopping transport. Phys. Rev. B 2018, 97, 094201. [Google Scholar] [CrossRef]
- Bobbert, P.A.; Nguyen, T.D.; Van Oost, F.W.A.; Koopmans, B.; Wohlgenannt, M. Bipolaron Mechanism for Organic Magnetoresistance. Phys. Rev. Lett. 2007, 99, 216801. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhang, S.; Li, H.; Li, T.; Liu, F.; Zhang, W.; Guo, W.; Miao, T.; Hu, W.; Shen, J.; et al. Angular dependent magnetoresistance in organic spin valves. Results Phys. 2021, 22, 103963. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, S.; Zhang, W.; Jiang, L.; Vetter, E.; Lee, H.N.; Xu, X.; Sun, D.; Shen, J. Nonvolatile Multilevel States in Multiferroic Tunnel Junctions. Phys. Rev. Appl. 2019, 12, 044049. [Google Scholar] [CrossRef]
- Žutić, I.; Fabian, J.; Das Sarma, S. Spintronics: Fundamentals and applications. Rev. Mod. Phys. 2004, 76, 323–410. [Google Scholar] [CrossRef]
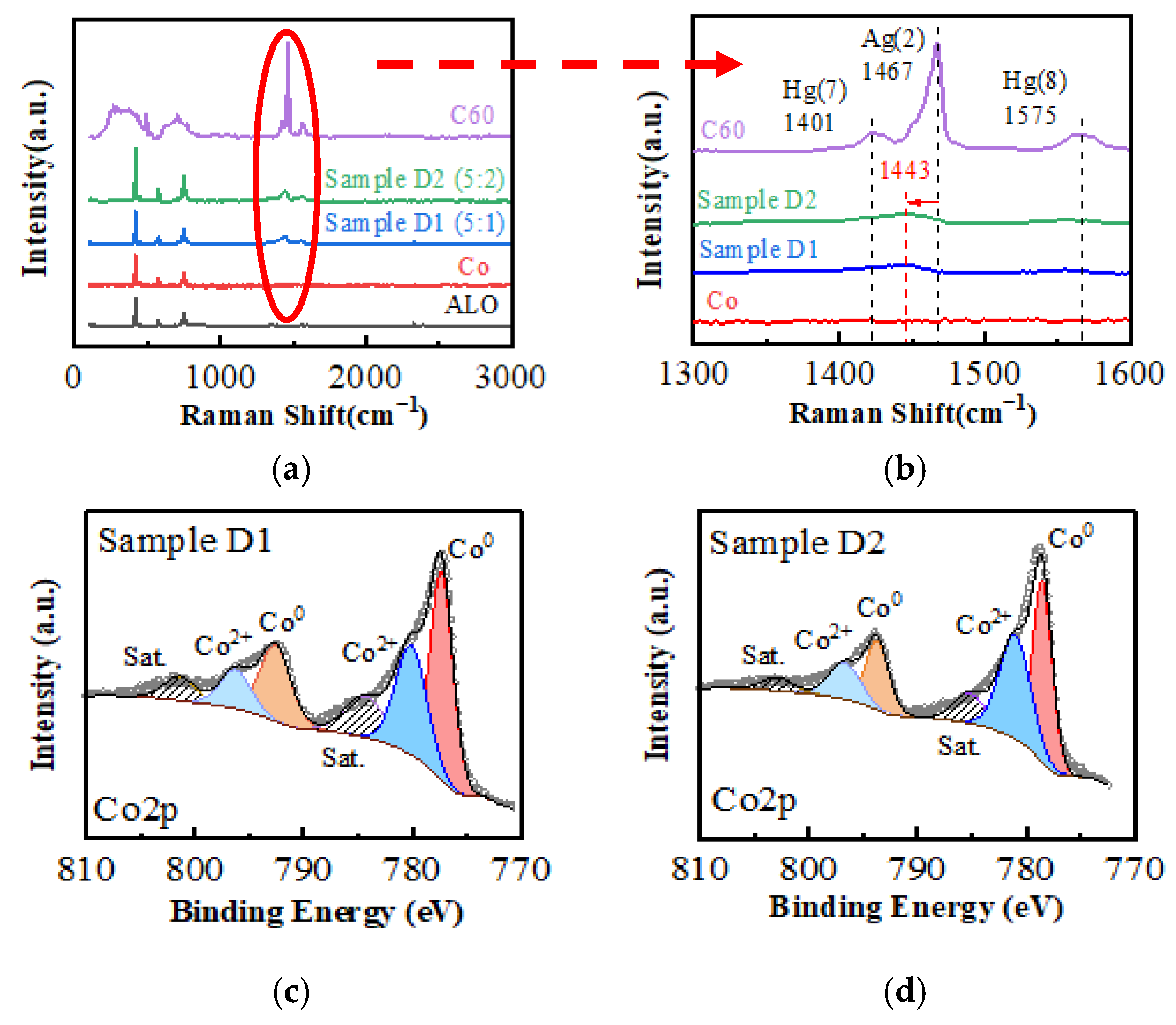



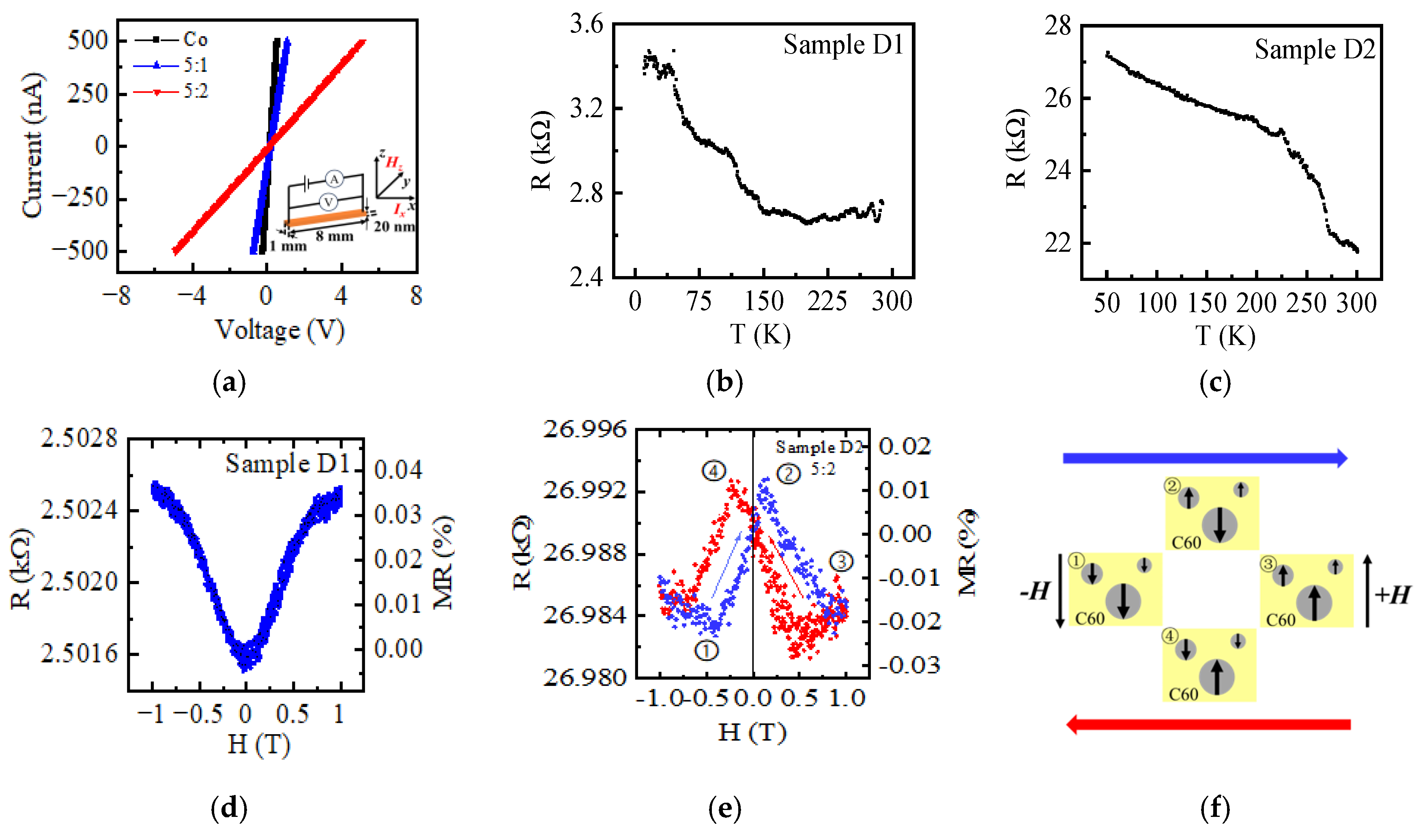
| Sample ID | Average Size (nm) | RMS Roughness (nm) | Peak-Valley Height (nm) | Coercivity 0° (Oe) | Coercivity 120° (Oe) | Conductivity 10 K (S/m) |
|---|---|---|---|---|---|---|
| Sample Co | -- | 1 | 4 | 30 | 90 | 5.3 × 105 |
| Sample D1 | 300 | 2 | 10 | 80 | 120 | 2.2 × 105 |
| Sample D2 | 130 | 12 | 72 | 20 | 100 | 4.0 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Gao, Y.; Li, Z.; Zou, W.; Li, K.; Xu, H.; Xiao, Z.; Fang, M. Tunable Structure and Properties of Co-Evaporated Co–C60 Nanocomposite Films. Nanomaterials 2025, 15, 715. https://doi.org/10.3390/nano15100715
Gu Z, Gao Y, Li Z, Zou W, Li K, Xu H, Xiao Z, Fang M. Tunable Structure and Properties of Co-Evaporated Co–C60 Nanocomposite Films. Nanomaterials. 2025; 15(10):715. https://doi.org/10.3390/nano15100715
Chicago/Turabian StyleGu, Ziyang, Yiting Gao, Zhou Li, Weihang Zou, Keming Li, Huan Xu, Zhu Xiao, and Mei Fang. 2025. "Tunable Structure and Properties of Co-Evaporated Co–C60 Nanocomposite Films" Nanomaterials 15, no. 10: 715. https://doi.org/10.3390/nano15100715
APA StyleGu, Z., Gao, Y., Li, Z., Zou, W., Li, K., Xu, H., Xiao, Z., & Fang, M. (2025). Tunable Structure and Properties of Co-Evaporated Co–C60 Nanocomposite Films. Nanomaterials, 15(10), 715. https://doi.org/10.3390/nano15100715








