Research Progress on the Application of Nanoenzyme Electrochemical Sensors for Detecting Zearalenone in Food
Abstract
1. Introduction
1.1. Overview of Zearalenone
1.2. Physicochemical Properties of ZEN
1.3. Toxicity of ZEN
1.4. Relevant Regulations and Limits of ZEN
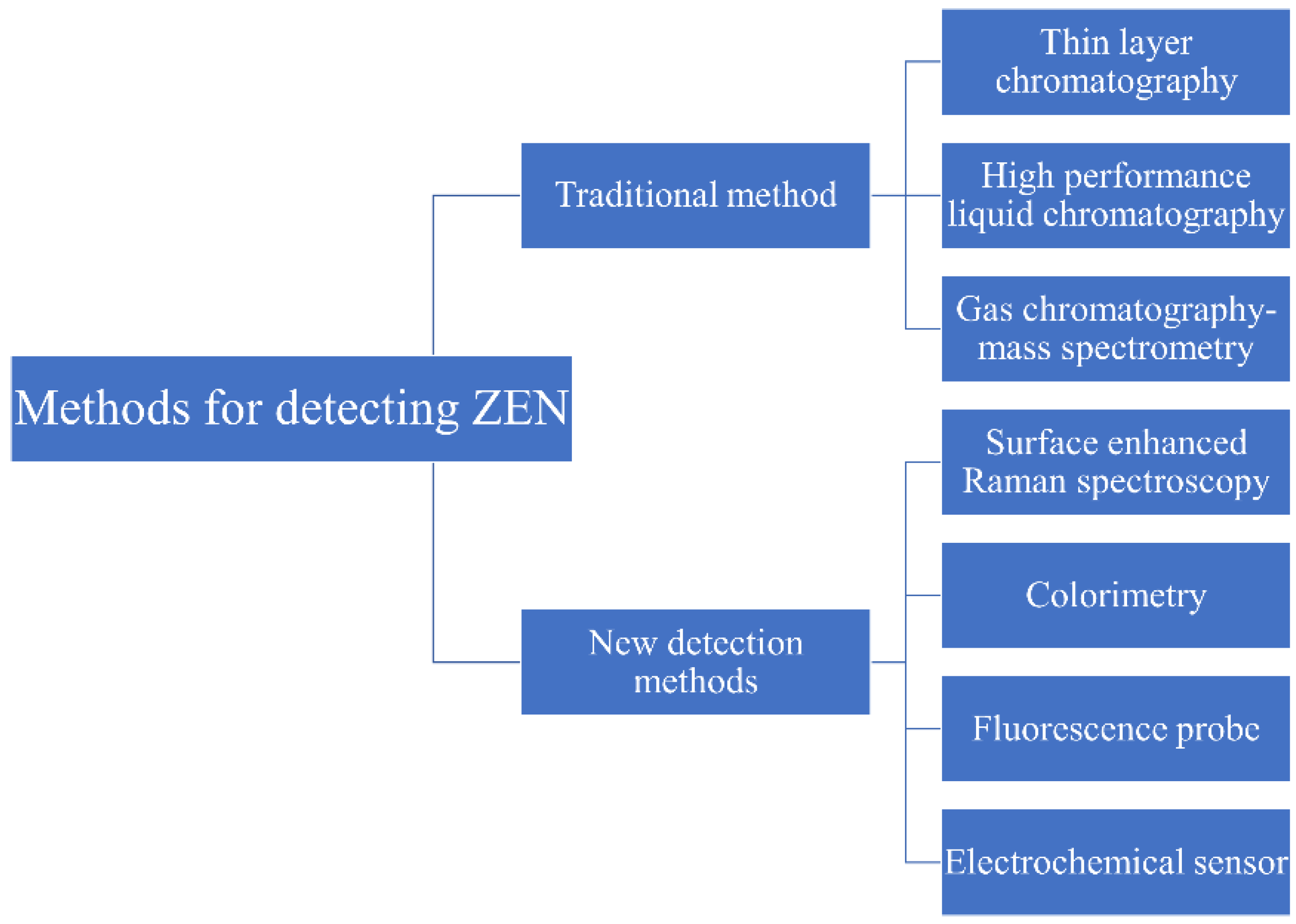
2. Overview of ZEN Detection Methods
2.1. Overview of Traditional Detection Methods of ZEN
2.2. Overview of New Detection Methods of ZEN
3. Research Progress of Electrochemical Sensors in ZEN Detection
3.1. Aptamer Electrochemical Sensor
3.2. Immuno-Electrochemical Sensor
3.3. Nanoenzyme Electrochemical Sensor
3.4. Comparison of Nanozyme Sensors for ZEN Detection with Other Sensors
4. The Existing Challenges of Electrochemical Sensors Based on Nanoenzymes
4.1. Production Costs and Large-Scale Applications
4.2. Control of Signal Amplification Effect
4.3. Safety Issues of Nanomaterials
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, C.; Yu, Y.; Han, J.; Hu, J.; He, D.; Zhang, H.; Shi, J.; Mohamed, S.R.; Dawood, D.H.; Wang, G.; et al. Isolation, Characterization, and Application of Clostridium sporogenes F39 to Degrade Zearalenone under Anaerobic Conditions. Foods 2022, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, M.; Wu, J.; Tao, F.; Chen, Q.; Wang, Q.; Ouyang, Q.; Shi, J.; Zou, X. Quantitative assessment of zearalenone in maize using multivariate algorithms coupled to Raman spectroscopy. Food Chem. 2019, 286, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hong, X.; Qiu, X.; Yang, C.; Mao, Y.; Li, Y.; Liu, Z.; Du, D. Ultrasensitive monitoring strategy of PCR-like levels for zearalenone contamination based DNA barcode. J. Sci. Food Agric. 2021, 101, 4490–4497. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Hu, J.; Chen, X.; Qiu, Y.; Shi, J.; Wang, G.; Xu, J. Isolation and characterization of Bacillus amyloliquefaciens MQ01, a bifunctional biocontrol bacterium with antagonistic activity against Fusarium graminearum and biodegradation capacity of zearalenone. Food Control. 2021, 130, 108259. [Google Scholar] [CrossRef]
- Zhen, H.; Hu, Y.; Xiong, K.; Li, M.; Jin, W. The occurrence and biological control of zearalenone in cereals and cereal-based feedstuffs: A review. Food Addit. Contam. Part A 2024, 41, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhang, W.; Xu, N.; Jiang, X.; Cheng, J.; Wang, R.; Wang, P. Efficient and simultaneous removal of aflatoxin B1, B2, G1, G2, and zearalenone from vegetable oil by use of a metal–organic framework absorbent. Food Chem. 2023, 418, 135881. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.; Li, X.; Hou, Z.; Mao, J.; Shi, J.; Battino, M.; Routledge, M.N.; Gong, Y.; Zou, X.; et al. Spatial-temporal distribution of deoxynivalenol, aflatoxin B1, and zearalenone in the solid-state fermentation basin of traditional vinegar and their potential correlation with microorganisms. Food Chem. 2023, 433, 137317. [Google Scholar] [CrossRef]
- Hu, J.; Wang, G.; Hou, M.; Du, S.; Han, J.; Yu, Y.; Gao, H.; He, D.; Shi, J.; Lee, Y.-W.; et al. New Hydrolase from Aeromicrobium sp. HA for the Biodegradation of Zearalenone: Identification, Mechanism, and Application. J. Agric. Food Chem. 2023, 71, 2411–2420. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, X.; Rong, Y.; Wei, W.; Wu, S.; Jiao, T.; Chen, Q. Label-free detection of trace level zearalenone in corn oil by surface-enhanced Raman spectroscopy (SERS) coupled with deep learning models. Food Chem. 2023, 414, 135705. [Google Scholar] [CrossRef]
- Hoogstra, S.J.; Hendricks, K.N.; McMullin, D.R.; Renaud, J.B.; Bora, J.; Sumarah, M.W.; Garnham, C.P. Enzymatic Hydrolysis of Resorcylic Acid Lactones by an Aeromicrobium sp. Toxins 2024, 16, 404. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Yin, L.; Arslan, M.; El-Seedi, H.R.; Zou, X. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2022, 403, 134384. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; You, T.; El-Seedi, H.R.; El-Garawani, I.M.; Guo, Z.; Zou, X.; Cai, J. Rapid and sensitive detection of zearalenone in corn using SERS-based lateral flow immunosensor. Food Chem. 2022, 396, 133707. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Lv, Q.; Xu, W.; Yang, F.; Wei, W.; Chen, X.; Zhang, Z.; Liu, Y. Reproductive Toxicity of Zearalenone and Its Molecular Mechanisms: A Review. Molecules 2025, 30, 505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Dong, F.; Li, Y.; Guo, Y.; Liu, X.; Xu, J.; Wu, X.; Zheng, Y. Ultrasensitive immunoassay for detection of zearalenone in agro-products using enzyme and antibody co-embedded zeolitic imidazolate framework as labels. J. Hazard. Mater. 2021, 412, 125276. [Google Scholar] [CrossRef]
- Faisal, Z.; Vörös, V.; Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Interactions of zearalanone, α-zearalanol, β-zearalanol, zearalenone-14-sulfate, and zearalenone-14-glucoside with serum albumin. Mycotoxin Res. 2020, 36, 389–397. [Google Scholar] [CrossRef]
- Ji, Y.-M.; Zhang, K.-H.; Pan, Z.-N.; Ju, J.-Q.; Zhang, H.-L.; Liu, J.-C.; Wang, Y.; Sun, S.-C. High-dose zearalenone exposure disturbs G2/M transition during mouse oocyte maturation. Reprod. Toxicol. 2022, 110, 172–179. [Google Scholar] [CrossRef]
- Xu, J.; Sun, L.; He, M.; Zhang, S.; Gao, J.; Wu, C.; Zhang, D.; Dai, J. Resveratrol Protects against Zearalenone-Induced Mitochondrial Defects during Porcine Oocyte Maturation via PINK1/Parkin-Mediated Mitophagy. Toxins 2022, 14, 641. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, G.X.; Liu, J.L.; Fan, J.J.; Cui, S. Toxic effects of zearalenone and its derivatives α-zearalenol on male reproductive system in mice. Reprod. Toxicol. 2007, 24, 381–387. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Tan, W.; Shi, J.; Li, Z.; Liu, H.; Yu, Y.; Yang, L.; Wang, X.; et al. A fluorescence resonance energy transfer probe based on functionalized graphene oxide and upconversion nanoparticles for sensitive and rapid detection of zearalenone. LWT 2021, 147, 111541. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, C.; Leung, L.K. Effect of zeranol on expression of apoptotic and cell cycle proteins in murine placentae. Toxicology 2013, 314, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, L.; Liu, X.; Luo, L.; Cheng, Z.; Sun, J.; Cai, Z.; Liu, J.; You, T. Inner filter effect-modulated ratiometric fluorescence aptasensor based on competition strategy for zearalenone detection in cereal crops: Using mitoxantrone as quencher of CdTe QDs@SiO2. Food Chem. 2021, 349, 129171. [Google Scholar] [CrossRef]
- Su, Y.; Sun, Y.; Ju, D.; Chang, S.; Shi, B.; Shan, A. The detoxification effect of vitamin C on zearalenone toxicity in piglets. Ecotoxicol. Environ. Saf. 2018, 158, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, H.; Li, Y.; Fu, X.; Du, D. A sensitive chemiluminescence immunoassay based on immunomagnetic beads for quantitative detection of zearalenone. Eur. Food Res. Technol. 2021, 247, 2171–2181. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Liu, Z.; Fu, X.; Du, D. High-performance liquid chromatography for the sensitive zearalenone determination by the automated immunomagnetic beads purifier for one-step sample pre-treatment. Eur. Food Res. Technol. 2021, 248, 109–117. [Google Scholar] [CrossRef]
- Harcarova, M.; Conkova, E.; Kolenicova, S.; Holeckova, B.; Proskovcova, M. In vitro evaluation of zearalenone toxicity by comet assay. Pol. J. Vet. Sci. 2022, 25, 475–477. [Google Scholar] [CrossRef]
- Feng, Y.-Q.; Zhao, A.-H.; Wang, J.-J.; Tian, Y.; Yan, Z.-H.; Dri, M.; Shen, W.; De Felici, M.; Li, L. Oxidative stress as a plausible mechanism for zearalenone to induce genome toxicity. Gene 2022, 829, 146511. [Google Scholar] [CrossRef]
- Kościelecka, K.; Kuć, A.; Kubik-Machura, D.; Męcik-Kronenberg, T.; Włodarek, J.; Radko, L. Endocrine Effect of Some Mycotoxins on Humans: A Clinical Review of the Ways to Mitigate the Action of Mycotoxins. Toxins 2023, 15, 515. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ren, X.; Li, W.; Sun, J.; Wang, X.; Huang, Y.; Guo, Y.; Zeng, H. Novel fluorescence immunoassay for the detection of zearalenone using HRP-mediated fluorescence quenching of gold-silver bimetallic nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef]
- Stadnik, A.; Wojtowicz-Chomicz, K.; Borzecki, A. Influence of zearalenone on free radical reactions in rat liver cells. Bull. Vet. Inst. Pulawy 2010, 54, 611–615. [Google Scholar]
- Wang, S.; Niu, R.; Yang, Y.; Wang, Y. Development and evaluation of a rapid immunomagnetic extraction for effective detection of zearalenone in agricultural products. Food Control. 2020, 110. [Google Scholar] [CrossRef]
- Zheng, W.-L.; Wang, B.-J.; Wang, L.; Shan, Y.-P.; Zou, H.; Song, R.-L.; Wang, T.; Gu, J.-H.; Yuan, Y.; Liu, X.-Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Ma, S.; Li, L.; You, T. Quantification of zearalenone in mildewing cereal crops using an innovative photoelectrochemical aptamer sensing strategy based on ZnO-NGQDs composites. Food Chem. 2020, 322, 126778. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, Z.; Ding, K.; Liao, C. Research progress in microbial degradation of zearalenone. Acta Microbiol. Sin. 2023, 63, 3711–3726. [Google Scholar] [CrossRef]
- Luo, L.; Ma, S.; Li, L.; Liu, X.; Zhang, J.; Li, X.; Liu, D.; You, T. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef]
- Cai, G.; Sun, K.; Xia, S.; Feng, Z.; Zou, H.; Gu, J.; Yuan, Y.; Zhu, J.; Liu, Z.; Bian, J. Decrease in immune function and the role of mitogen-activated protein kinase (MAPK) overactivation in apoptosis during T lymphocytes activation induced by zearalenone, deoxynivalenol, and their combinations. Chemosphere 2020, 255, 126999. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Liu, C.-C.; Wang, S.-C.; Chen, K.-Y.; Lin, T.-C.; Liu, P.-L.; Chiu, C.-C.; Chen, I.-C.; Lai, Y.-H.; Cheng, W.-C.; et al. Mycotoxin Zearalenone Attenuates Innate Immune Responses and Suppresses NLRP3 Inflammasome Activation in LPS-Activated Macrophages. Toxins 2021, 13, 593. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Li, Q.; Zhou, Y.; Wang, Z. Aptamer-Based Lateral Flow Test Strip for Rapid Detection of Zearalenone in Corn Samples. J. Agric. Food Chem. 2018, 66, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; You, T.; Arslan, M.; El-Seedi, H.R.; Guo, Z.; Zou, X.; Cai, J. Dual-layers Raman reporter-tagged Au@Ag combined with core-satellite assemblies for SERS detection of Zearalenone. Food Chem. 2023, 429, 136834. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent trends in detecting, controlling, and detoxifying of patulin mycotoxin using biotechnology methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Kesici, E.; Yaralı, E.; Kanat, E. Single-use sensor technology for monitoring of zearalenone in foods: ZentoSens. Microchem. J. 2019, 147, 37–42. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Hassan, M.; Li, H.; Ahmad, W.; Zareef, M.; Wang, J.; Xie, S.; Wang, P.; Ouyang, Q.; Wang, S.; Chen, Q. Au@Ag nanostructure based SERS substrate for simultaneous determination of pesticides residue in tea via solid phase extraction coupled multivariate calibration. LWT 2019, 105, 290–297. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Ashiagbor, K.; Hu, Z.; Rostom, M. Mathematical modeling of thin-layer drying kinetics and moisture diffusivity study of apple slices using infrared conveyor-belt dryer. J. Food Sci. 2024, 89, 1658–1671. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, B.; Yu, M.; Han, J.; Wang, Y.; Tan, Z.; Yan, Y. Simultaneous separation/enrichment and detection of trace ciprofloxacin and lomefloxacin in food samples using thermosensitive smart polymers aqueous two-phase flotation system combined with HPLC. Food Chem. 2016, 210, 1–8. [Google Scholar] [CrossRef]
- Tahir, H.E.; Mariod, A.A.; Hashim, S.B.; Arslan, M.; Mahunu, G.K.; Xiaowei, H.; Zhihua, L.; Abdalla, I.I.; Xiaobo, Z. Classification of Black Mahlab seeds (Monechma ciliatum) using GC–MS and FT-NIR and simultaneous prediction of their major volatile compounds using chemometrics. Food Chem. 2022, 408, 134948. [Google Scholar] [CrossRef]
- Ghanati, K.; Basaran, B.; Abedini, A.; Akbari-Adergani, B.; Akbari, N.; Sadighara, P. Zearalenone, an estrogenic component, in bovine milk; amount and detection method; a systematic review and meta-analysis. Toxicol. Rep. 2024, 13, 101688. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fang, W.; Wang, S.; Shi, J.; Zou, X.; Huang, X. Identification of characteristic volatiles of Zhenjiang aromatic vinegar using HS-SPME-GC/MS coupled with multivariate analysis. Flavour Fragr. J. 2024, 39, 271–281. [Google Scholar] [CrossRef]
- He, P.; Hassan, M.; Tang, F.; Jiang, H.; Chen, M.; Liu, R.; Lin, H.; Chen, Q. Total Fungi Counts and Metabolic Dynamics of Volatile Organic Compounds in Paddy Contaminated by Aspergillus niger During Storage Employing Gas Chromatography-Ion Mobility Spectrometry. Food Anal. Methods 2022, 15, 1638–1651. [Google Scholar] [CrossRef]
- Švec, F. Neglected Applications of Monolithic Structures: The Beginnings of Planar Chromatography with Mass Spectrometric Detection. Chem. Listy 2023, 117, 308–318. [Google Scholar] [CrossRef]
- Hadiani, M.R.; Yazdanpanah, H.; Ghazi-Khansari, M.; Cheraghali, A.M.; Goodarzi, M. Survey of the natural occurrence of zearalenone in maize from northern Iran by thin-layer chromatography densitometry. Food Addit. Contam. 2003, 20, 380–385. [Google Scholar] [CrossRef]
- Mei, S.; Ding, J.; Chen, X. Identification of differential volatile and non-volatile compounds in coffee leaves prepared from different tea processing steps using HS-SPME/GC–MS and HPLC-Orbitrap-MS/MS and investigation of the binding mechanism of key phytochemicals with olfactory and taste receptors using molecular docking. Food Res. Int. 2023, 168, 112760. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, H.; Li, C.; Han, J.; Tan, Z.; Yan, Y. Separation, concentration and determination of trace chloramphenicol in shrimp from different waters by using polyoxyethylene lauryl ether-salt aqueous two-phase system coupled with high-performance liquid chromatography. Food Chem. 2016, 192, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Abedi, A.; Hemmati, F.; Abedini, A.H.; Mohammadi, A.; Moslemi, M. Application of thermal ultrasound-assisted liquid–liquid micro-extraction coupled with HPLC-UV for rapid determination of synthetic phenolic antioxidants in edible oils. J. Am. Oil Chem. Soc. 2021, 98, 969–978. [Google Scholar] [CrossRef]
- Bozkurt, S.S.; Işık, G. Ionic Liquid Based Dispersive Liquid–Liquid Microextraction for Preconcentration of Zearalenone and Its Determination in Beer and Cereal Samples by High-Performance Liquid Chromatography with Fluorescence Detection. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1601–1607. [Google Scholar] [CrossRef]
- Lindeque, J.Z. Targeted analysis of organic acids with GC-MS/MS: Challenges and prospects. Anal. Biochem. 2024, 694, 115620. [Google Scholar] [CrossRef]
- Lv, R.; Huang, X.; Aheto, J.H.; Mu, L.; Tian, X. Analysis of fish spoilage by gas chromatography–mass spectrometry and electronic olfaction bionic system. J. Food Saf. 2018, 38, e12557. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; You, T.; Wang, K. Using Magnetic Multiwalled Carbon Nanotubes as Modified QuEChERS Adsorbent for Simultaneous Determination of Multiple Mycotoxins in Grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Geng, W.; Haruna, S.A.; Zhou, C.; Wang, Y.; Ouyang, Q.; Chen, Q. Identification of characteristic volatiles and metabolomic pathway during pork storage using HS-SPME-GC/MS coupled with multivariate analysis. Food Chem. 2022, 373, 131431. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Yu, S.; Xiong, F.; Wang, Y.; Zhang, X.; Ren, Y. Characterization of the volatile flavor profiles of Zhenjiang aromatic vinegar combining a novel nanocomposite colorimetric sensor array with HS-SPME-GC/MS. Food Res. Int. 2022, 159, 111585. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, Y.; Guo, Q.; Wang, X.; Tian, Y.; Yang, W.; Li, J.; Chen, Y. Determination of Zearalenone and Its Derivatives in Feed by Gas Chromatography–Mass Spectrometry with Immunoaffinity Column Cleanup and Isotope Dilution. Toxins 2022, 14, 764. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2020, 52, 294–313. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Zhu, G.; Zhou, X.; Jia, L.; Zhang, T. Highly Sensitive Detection of Zearalenone in Feed Samples Using Competitive Surface-Enhanced Raman Scattering Immunoassay. J. Agric. Food Chem. 2014, 62, 8325–8332. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Belwal, T.; Lin, X.; Luo, Z. Insights into chemometric algorithms for quality attributes and hazards detection in foodstuffs using Raman/surface enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2476–2507. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jianbo, X.; Mahunu, G.K.; Jiyong, S.; Xu, J.-L.; Sun, D.-W. Recent Progress in Rapid Analyses of Vitamins, Phenolic, and Volatile Compounds in Foods Using Vibrational Spectroscopy Combined with Chemometrics: A Review. Food Anal. Methods 2019, 12, 2361–2382. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, D.; Yang, B.; Guo, S.; Chen, L.; Jung, Y.M. Noble metal-free SERS: Mechanisms and applications. Analyst 2023, 149, 11–28. [Google Scholar] [CrossRef]
- Zhai, W.; You, T.; Ouyang, X.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Hassan, M.; Zhu, J.; Ali, S.; Ahmad, W.; Wang, J.; Lv, C.; Chen, Q.; Li, H. Quantification of deltamethrin residues in wheat by Ag@ZnO NFs-based surface-enhanced Raman spectroscopy coupling chemometric models. Food Chem. 2021, 337, 127652. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2021, 39, 1440–1461. [Google Scholar] [CrossRef]
- Ge, K.; Hu, Y.; Li, G. Fabrication of branched gold copper nanoalloy doped mesoporous graphitic carbon nitride hybrid membrane for surface-enhanced Raman spectroscopy analysis of carcinogens. J. Hazard. Mater. 2022, 432, 128742. [Google Scholar] [CrossRef]
- Panferov, V.G.; Liu, J. Optical and Catalytic Properties of Nanozymes for Colorimetric Biosensors: Advantages, Limitations, and Perspectives. Adv. Opt. Mater. 2024, 12, 2401318. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Liu, G.; Xiao, Y.; Tian, Y.; Fang, S. Plasmonic colorimetry and G-quadruplex fluorescence-based aptasensor: A dual-mode, protein-free and label-free detection for OTA. Food Chem. 2024, 448, 139115. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Yang, Y.; Chen, Q. Upconversion Nanoprobes Based on a Horseradish Peroxidase-Regulated Dual-Mode Strategy for the Ultrasensitive Detection of Staphylococcus aureus in Meat. J. Agric. Food Chem. 2021, 69, 9947–9956. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Huang, X.; Hu, X.; Huang, X.; Yin, L.; Huang, Q.; Wen, Y.; Li, B.; Shi, J.; et al. Switchable aptamer-fueled colorimetric sensing toward agricultural fipronil exposure sensitized with affiliative metal-organic framework. Food Chem. 2022, 407, 135115. [Google Scholar] [CrossRef]
- Li, H.-T.; Kerr, E.D.; Schulz, B.L.; Gidley, M.J.; Dhital, S. Pasting properties of high-amylose wheat in conventional and high-temperature Rapid Visco Analyzer: Molecular contribution of starch and gluten proteins. Food Hydrocoll. 2022, 131, 107840. [Google Scholar] [CrossRef]
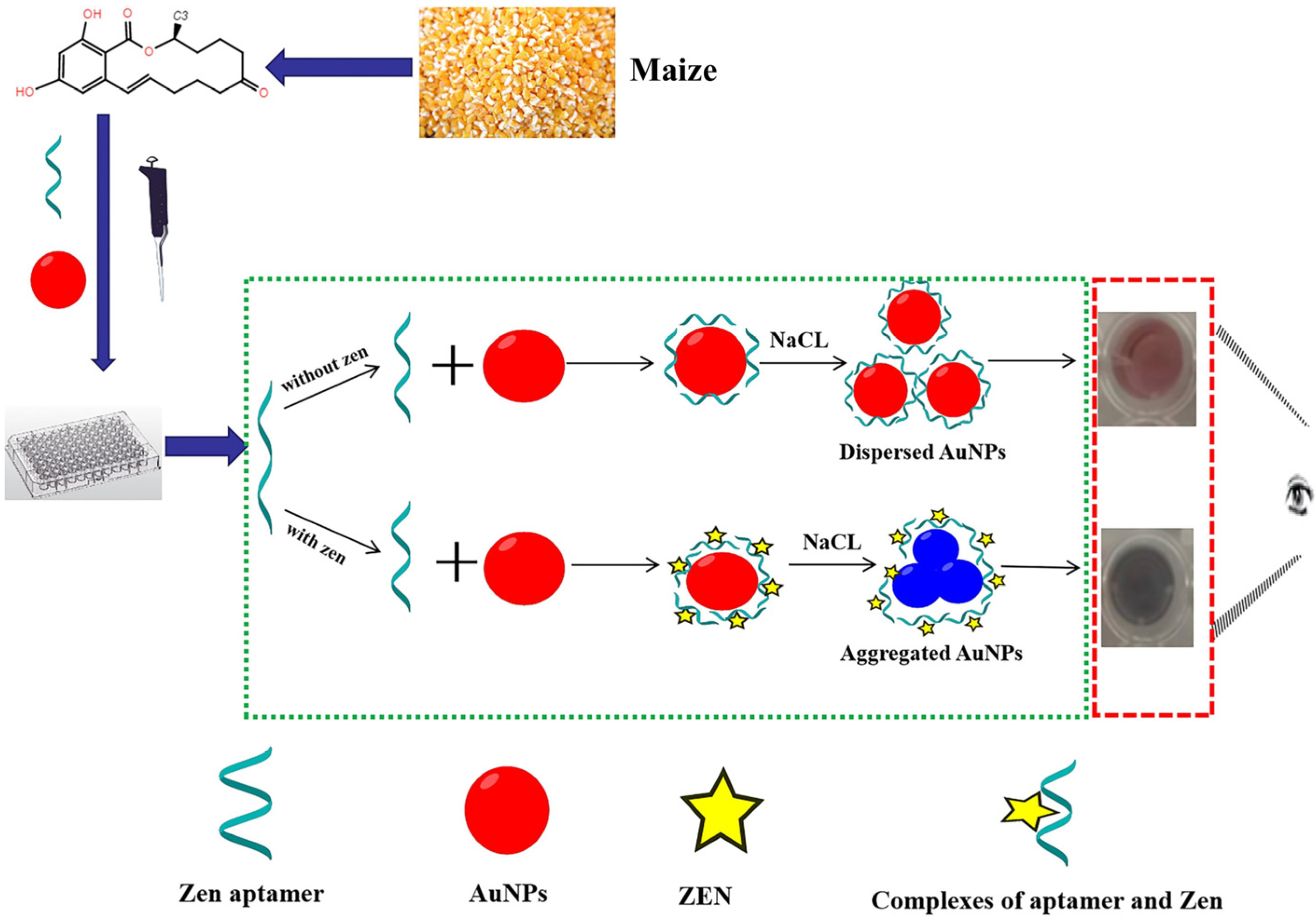
- Zhang, L.; Chen, J.; Lu, L.; Yu, R.; Zhang, D. A smartphone-assisted colorimetric aptasensor based on aptamer and gold nanoparticles for visual, fast and sensitive detection of ZEN in maize. Food Chem. X 2023, 19, 100792. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Yuan, C.; Yu, L.; Sun, M.; Huang, D.; Liu, S.; Wang, S. Metal-organic frameworks-based broad-spectrum sensor for total tetracycline antibiotics assay. Dye. Pigment. 2022, 208, 110887. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.M.; Blake, R.C.; Mayer, H.P.; Echevarria, A.A.; Nguyen, T.D.; Bostanian, L.A. A Fluorescent Biosensor for Detection of Zearalenone. Anal. Lett. 2000, 33, 405–412. [Google Scholar] [CrossRef]
- Mu, W.-Y.; Huang, P.-Z.; Chen, Q.-Y.; Wang, W. Determination of melamine and melamine–Cu(II) complexes in milk using a DNA-Ag hydrocolloid as the sensor. Food Chem. 2020, 311, 125889. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ahmad, W.; Rong, Y.; Chen, Q.; Zuo, M.; Ouyang, Q.; Guo, Z. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food. Food Control. 2020, 107, 106761. [Google Scholar] [CrossRef]
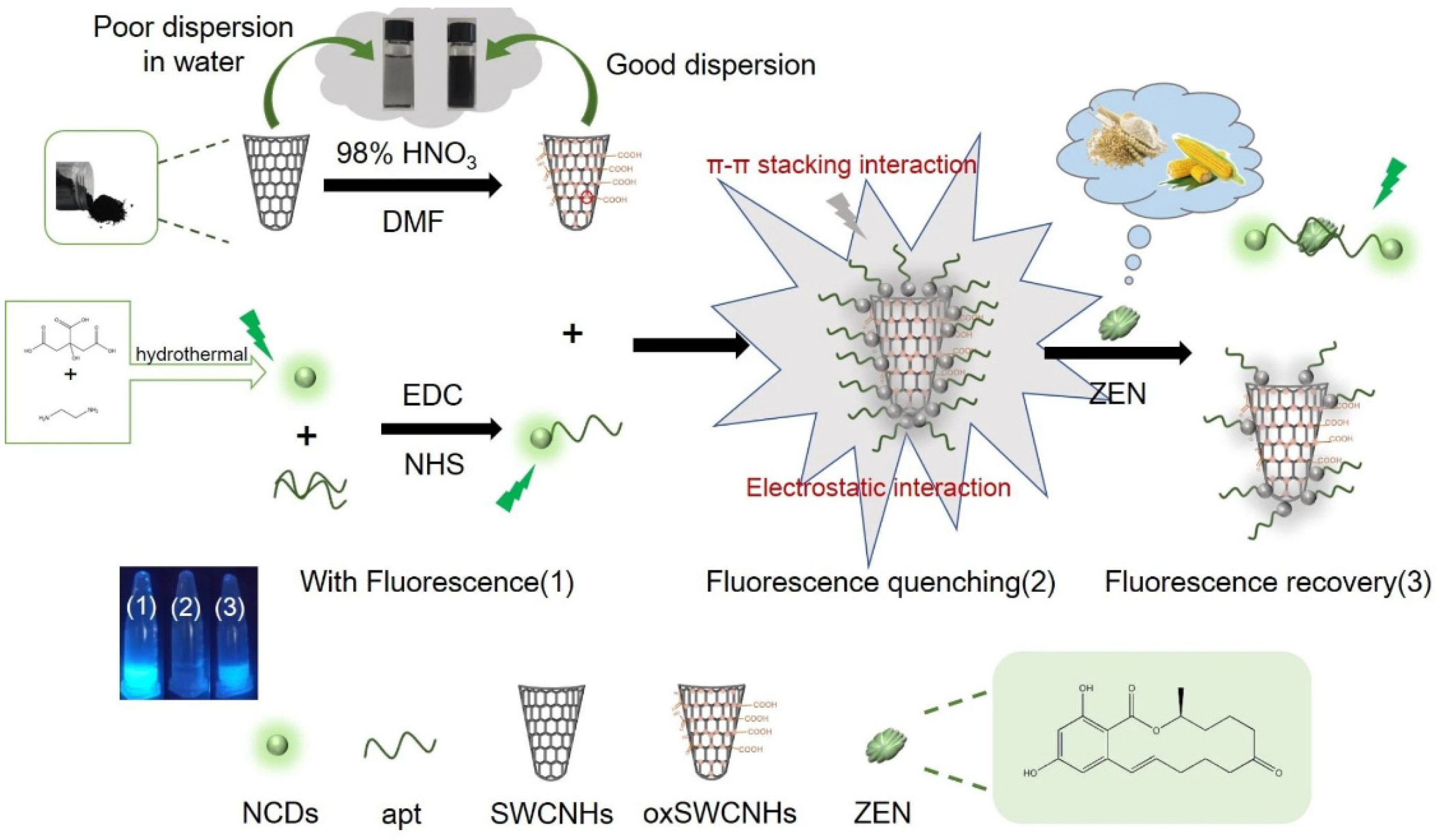
- Na, Y.; Zhang, J.; Zhang, S.; Liang, N.; Zhao, L. Fluorescence Sensor for Zearalenone Detection Based on Oxidized Single-walled Carbon Nanohorns/N-doped Carbon Quantum Dots-aptamer. J. Fluoresc. 2023, 34, 2557–2569. [Google Scholar] [CrossRef]
- De Rycke, E.; Foubert, A.; Dubruel, P.; Bol’Hakov, O.I.; De Saeger, S.; Beloglazova, N. Recent advances in electrochemical monitoring of zearalenone in diverse matrices. Food Chem. 2021, 353, 129342. [Google Scholar] [CrossRef]
- Tang, C.; He, Y.; Yuan, B.; Li, L.; Luo, L.; You, T. Simultaneous detection of multiple mycotoxins in agricultural products: Recent advances in optical and electrochemical sensing methods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70062. [Google Scholar] [CrossRef]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.; Zou, X.; Xu, Y.; Xu, X. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Chen, T.; You, T. Label-free ratiometric homogeneous electrochemical aptasensor based on hybridization chain reaction for facile and rapid detection of aflatoxin B1 in cereal crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, X.-X.; Gao, C.-B.; Gai, Z.; He, Y.-X.; Qian, Z.-J.; Liu, Y.; Lei, H.-T.; Sun, Y.-M.; Xu, Z.-L. Development of a highly sensitive and selective electrochemical immunosensor for controlling of rhodamine B abuse in food samples. Food Control. 2022, 133, 108662. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Du, W.; Sun, J.; Jiang, D. Self-powered photoelectrochemical sensor for chlorpyrifos detection in fruit and vegetables based on metal–ligand charge transfer effect by Ti3C2 based Schottky junction. Food Chem. 2022, 385, 132731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, N.; Tian, M.; Wen, Y.; Wang, J.; Mu, J. Research progress on electrochemical biosensor of heavy metal ions. J. Anal. Sci. 2024, 40, 98–104. [Google Scholar]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.; Feng, W.; Wu, X. Analytical detection methods for azo dyes: A focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection. J. Food Compos. Anal. 2022, 114, 104778. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Gu, C.; Xu, F.; Zhang, W.; Huang, X.; Qian, J. Fabrication of a Versatile Aptasensing Chip for Aflatoxin B1 in Photothermal and Electrochemical Dual Modes. Food Anal. Methods 2022, 15, 3390–3399. [Google Scholar] [CrossRef]
- Shkembi, X.; Svobodova, M.; Skouridou, V.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C.K. Aptasensors for mycotoxin detection: A review. Anal. Biochem. 2022, 644, 114156. [Google Scholar] [CrossRef]
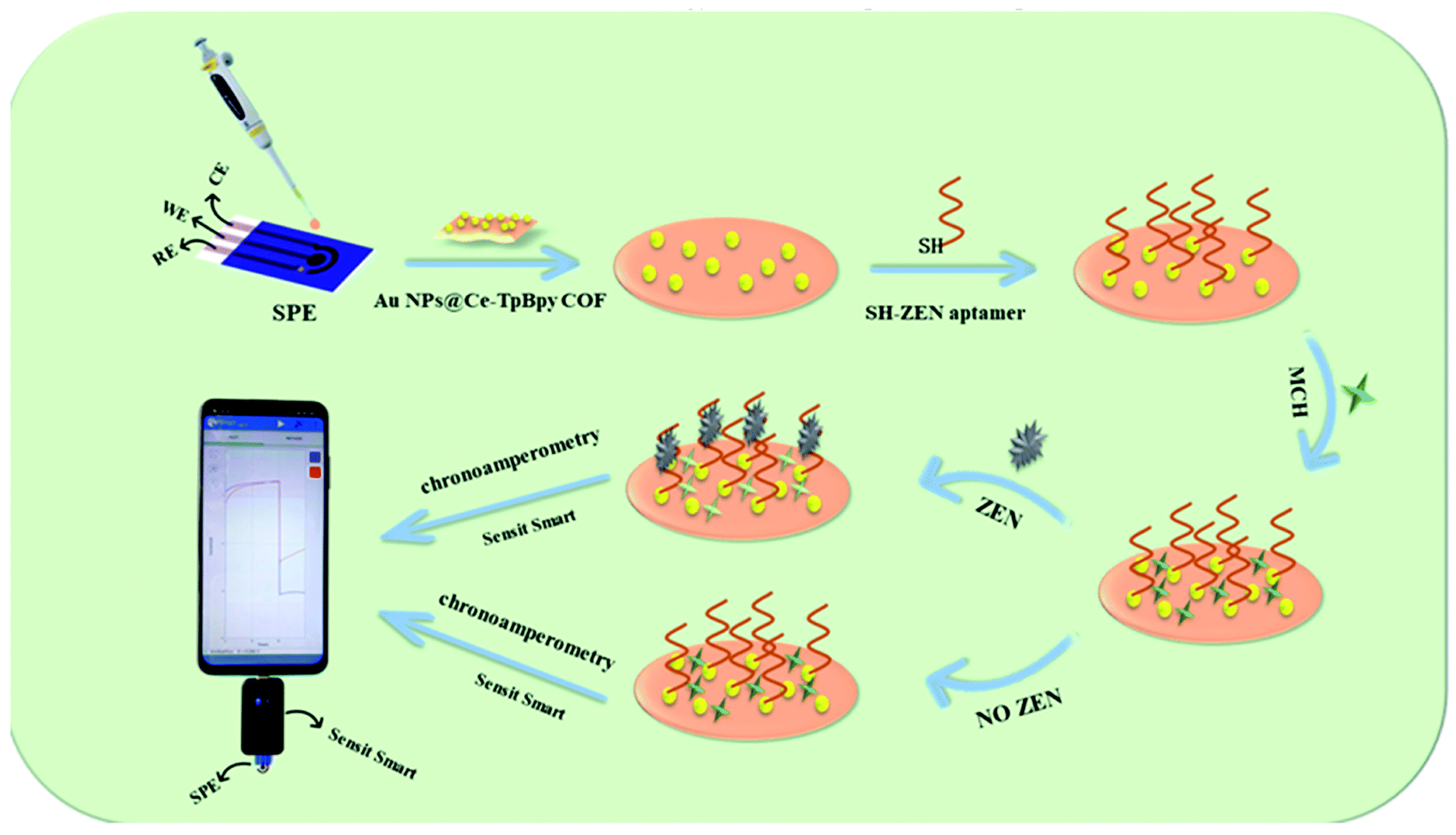
- Chen, Z.; Yang, M.; Li, Z.; Liao, W.; Chen, B.; Yang, T.; Hu, R.; Yang, Y.; Meng, S. Highly sensitive and convenient aptasensor based on Au NPs@Ce-TpBpy COF for quantitative determination of zearalenone. RSC Adv. 2022, 12, 17312–17320. [Google Scholar] [CrossRef]
- Mu, Z.; Ma, L.; Wang, J.; Zhou, J.; Yuan, Y.; Bai, L. A target-induced amperometic aptasensor for sensitive zearalenone detection by CS@AB-MWCNTs nanocomposite as enhancers. Food Chem. 2021, 340, 128128. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kumar, V.S.; Hayat, A.; Gobi, K.V.; Song, H.; Kim, K.-H.; Marty, J.L. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Zeng, Y.; Camarada, M.B.; Lu, X.; Tang, K.; Li, W.; Qiu, D.; Wen, Y.; Wu, G.; Luo, Q.; Bai, L. Detection and electrocatalytic mechanism of zearalenone using nanohybrid sensor based on copper-based metal-organic framework/magnetic Fe3O4-graphene oxide modified electrode. Food Chem. 2022, 370, 131024. [Google Scholar] [CrossRef] [PubMed]
- Veenuttranon, K.; Lu, X.; Chen, J. Ultrasensitive electrochemical sensing for simultaneous rapid detection of zearalenone and ochratoxin A in feedstuffs and foodstuffs. Chem. Eng. J. 2024, 497, 154807. [Google Scholar] [CrossRef]
- Zhiyuan, L.; Fanzhuo, K.; Yuyang, Z.; Oussama, K.; Hongbo, S.; Rubing, H.; Bin, Z. Cu-doped ZiF-8 and calcined UIO-66-based biomimetic enzyme electrochemical sensor for rapid and sensitive detection of Zearalenone in vegetable oil. Food Chem. 2025, 483, 144260. [Google Scholar] [CrossRef] [PubMed]
- Zhiyuan, L.; Yuzhe, X.; Fanzhuo, K.; Jiaojiao, X.; Hongbo, S.; Rubing, H.; Bin, Z. Biomimetic enzyme ratiometric electrochemical sensor based on graphene, calcined UIO-66 and thionine for rapid and sensitive detection of zearalenone in vegetable oil. Food Biosci. 2025, 68, 106548. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Zhang, M.; Wang, Q.; Wang, Y.; Yu, S.; Dang, R.; Wang, X.; Yang, Z.; Fan, S.; et al. N/O co-doping porous biomass carbon constructed electrochemical sensor for universal and sensitive detection to mycotoxins. Food Chem. 2025, 475, 143397. [Google Scholar] [CrossRef]
- Zaman, G.S.; Waleed, I.; Obeid, R.A.; Khudair, S.A.; Al-Kahdum, S.A.A.; Al-Majdi, K.; Abed, A.S.; Alsalamy, A.; Qasim, M.T.; Alawadi, A.H.R. Electrochemical determination of zearalenone in agricultural food samples using a flower like nanocomposite-modified electrode. Mater. Chem. Phys. 2023, 305, 127986. [Google Scholar] [CrossRef]
- Huang, S.-J.; Gokulkumar, K.; Mani, G.; Lee, Y.-Y.; Kogularasu, S.; Chang-Chien, G.-P. Synthesis and characterization of Bi2S3-embedded carbon nanofibers as a novel electrochemical biosensor for the detection of mycotoxin zearalenone in food crops. FlatChem 2024, 45, 100652. [Google Scholar] [CrossRef]
- Huang, H.; Camarada, M.B.; Wang, D.; Liao, X.; Xiong, W.; Du, J.; Xiong, J.; Hong, Y. MoS2 quantum dots and titanium carbide co-modified carbon nanotube heterostructure as electrode for highly sensitive detection of zearalenone. Microchim. Acta 2021, 189, 1–13. [Google Scholar] [CrossRef]
- Girmatsion, M.; Tang, X.; Zhang, Q.; Jiang, J.; Li, P. Phycocyanin-based rapid fluorometric immunoassay for the determination of aflatoxin B1, deoxynivalenol, and zearalenone in food and feed matrices. Food Control. 2024, 164, 110585. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Liu, S.; Li, B. A label-free visual aptasensor for zearalenone detection based on target-responsive aptamer-cross-linked hydrogel and color change of gold nanoparticles. Food Chem. 2022, 389, 133078. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, K.; Du, Z.; Fang, B.; Zhan, J.; Zhu, L.; Xu, W. Magnetic aptamer copper nanoclusters fluorescent biosensor for the visual detection of zearalenone based on docking-aided rational tailoring. Food Chem. 2024, 448, 139127. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ma, J.; Neng, J.; Yang, B.; Wang, Y.; Xing, F. A Novel and Label-Free Chemiluminescence Detection of Zearalenone Based on a Truncated Aptamer Conjugated with a G-Quadruplex DNAzyme. Biosensors 2023, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liu, Y.; Cui, H.; Yang, H.; Hussain, M.; Wang, K.; Wei, J.; Long, L.; Ding, L.; Wang, C. Fabrication of a disposable aptasensing chip for simultaneous label-free detection of four common coexisting mycotoxins. Anal. Chim. Acta 2023, 1282, 341921. [Google Scholar] [CrossRef]
- Qian, J.; Yang, H.; Cui, H.; Ettayri, K.; You, F.; Wang, K.; Wei, J.; Wang, C. Integrating CdIn2S4 semiconductors with S-vacancy MoS2 nanosheets to fabricate a multi-channel aptasensing chip for photoelectrochemical detection of multiple mycotoxins. Anal. Chim. Acta 2024, 1319, 342982. [Google Scholar] [CrossRef]
- Zhiyuan, L.; Minqiao, X.; Jiaojiao, X.; Hongbo, S.; Rubing, H.; Bin, Z. Flower-like biomimetic enzyme for rapid and sensitive detection of zearalenone in vegetable oil deodorizer distillate. Anal. Biochem. 2025, 700, 115780. [Google Scholar] [CrossRef]



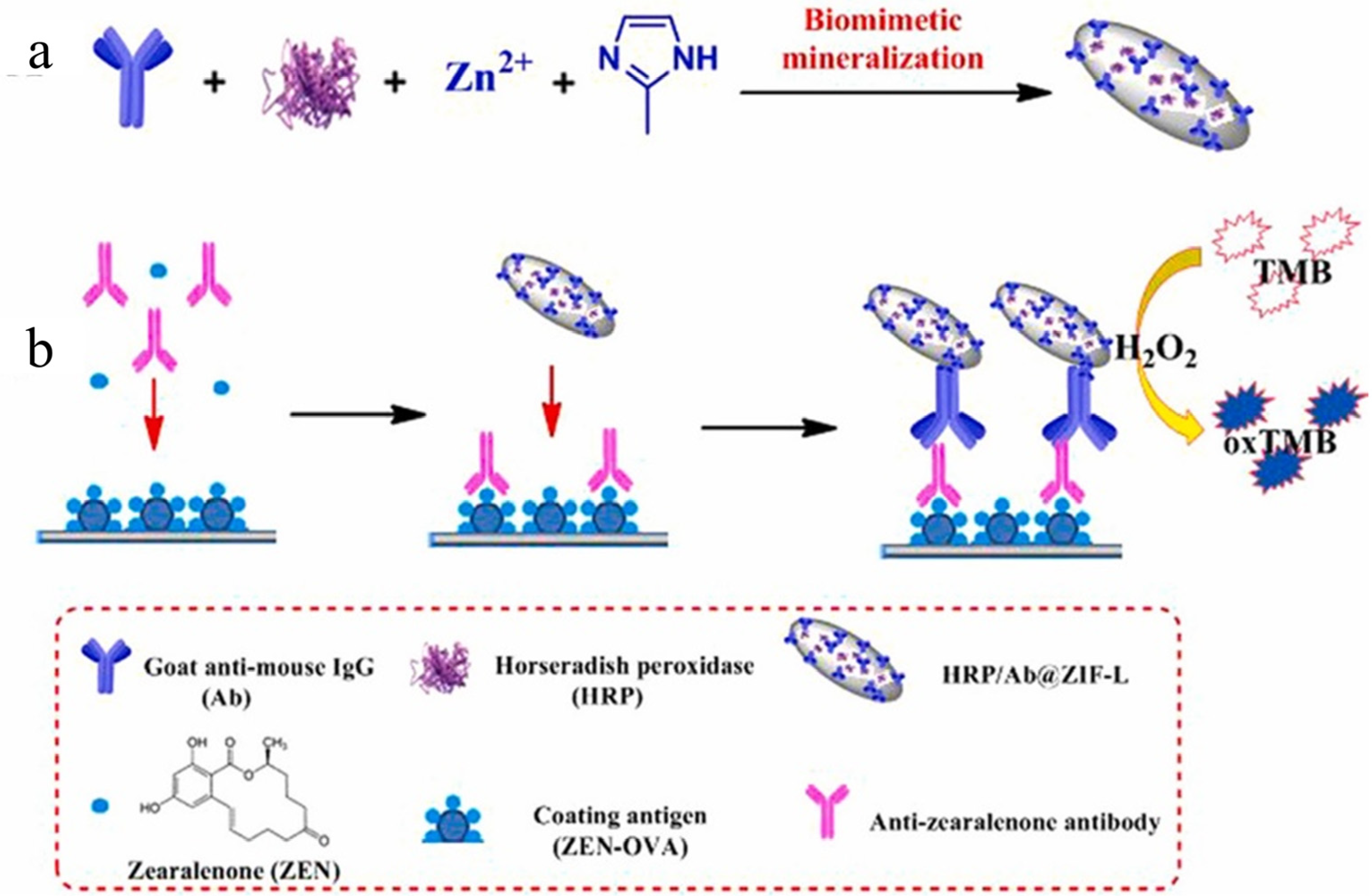
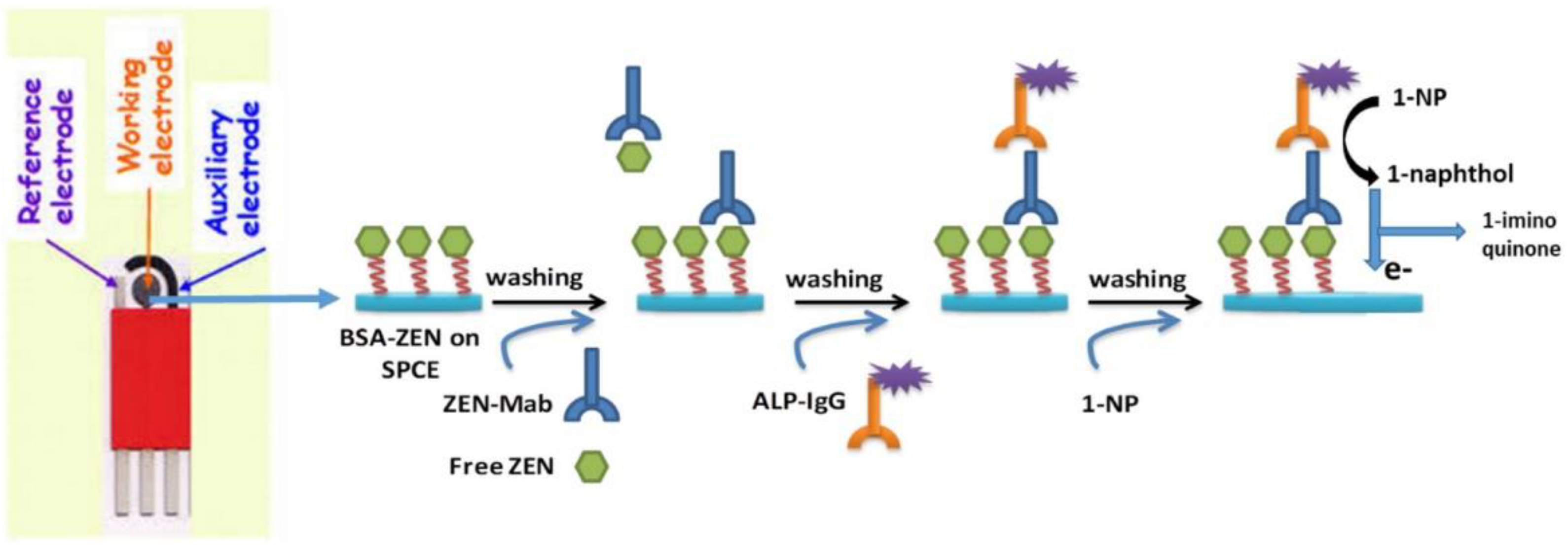
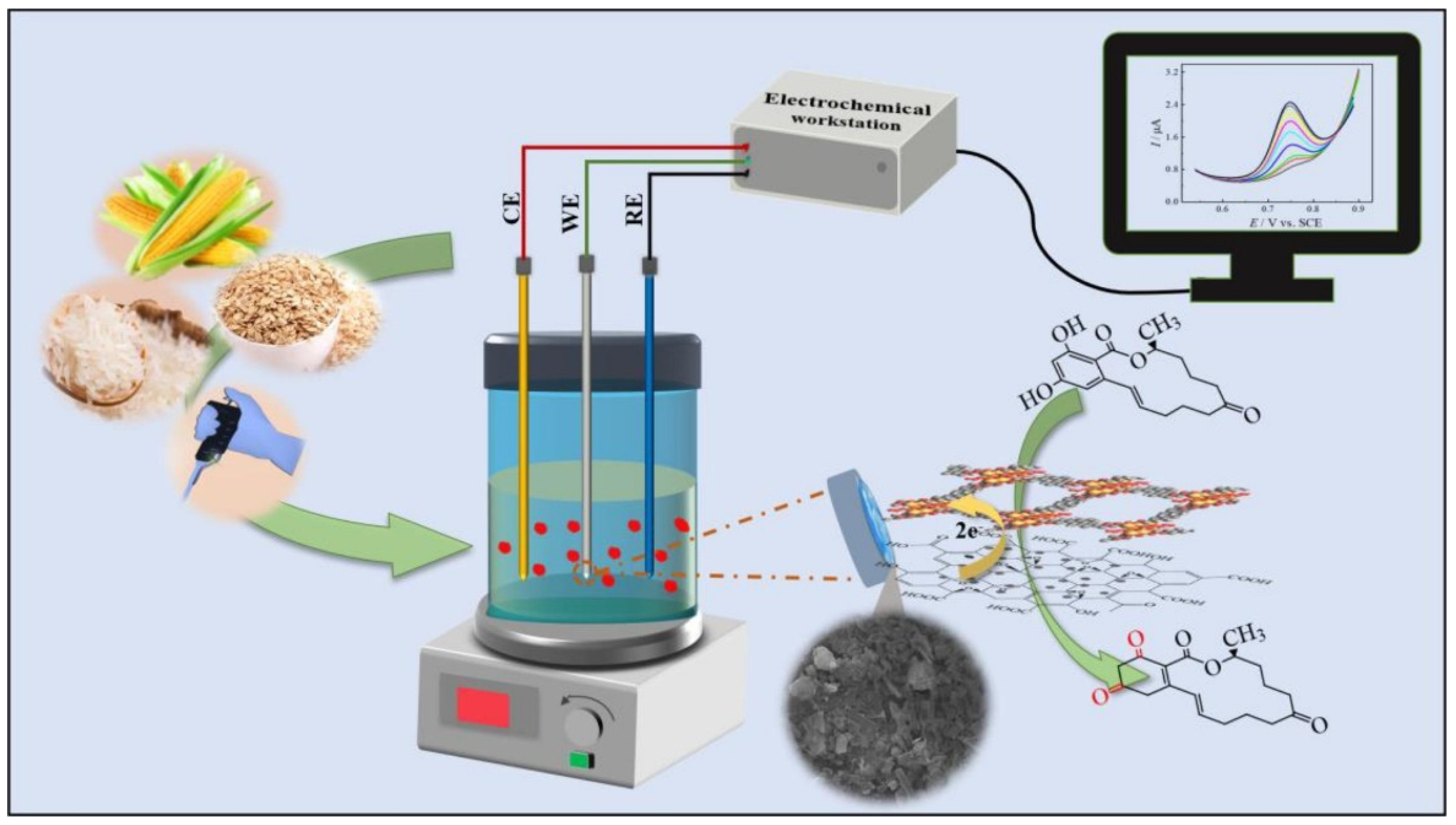
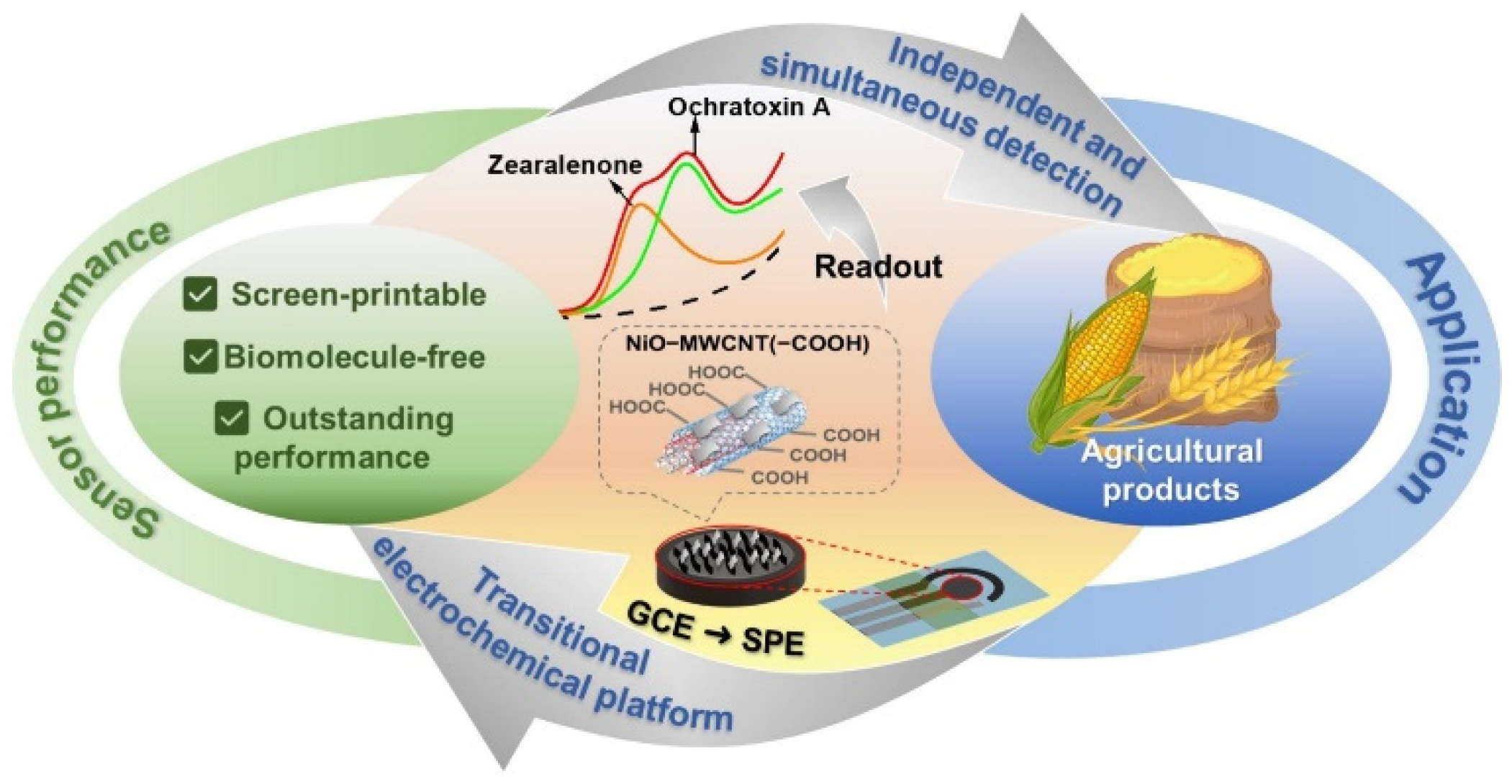

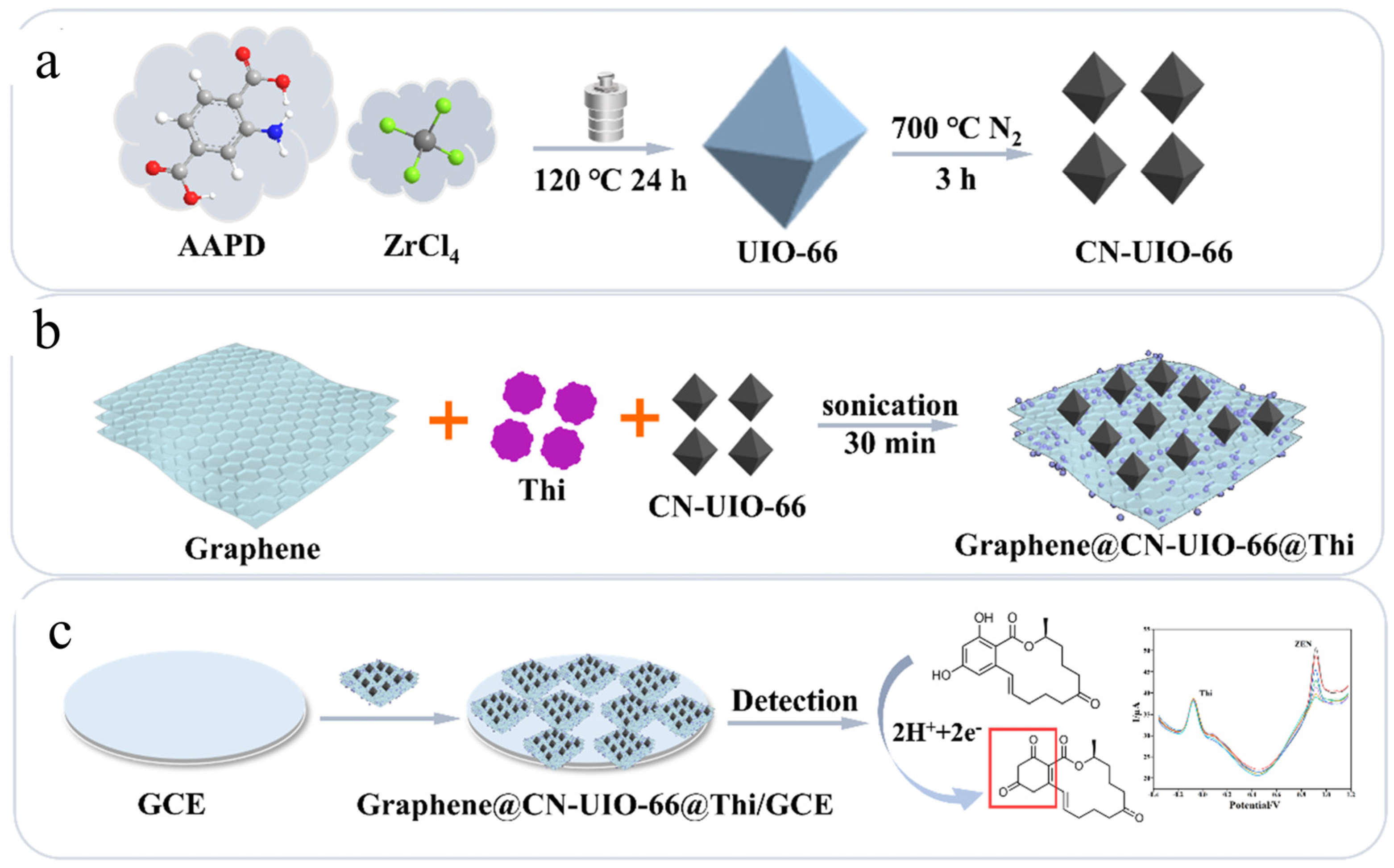
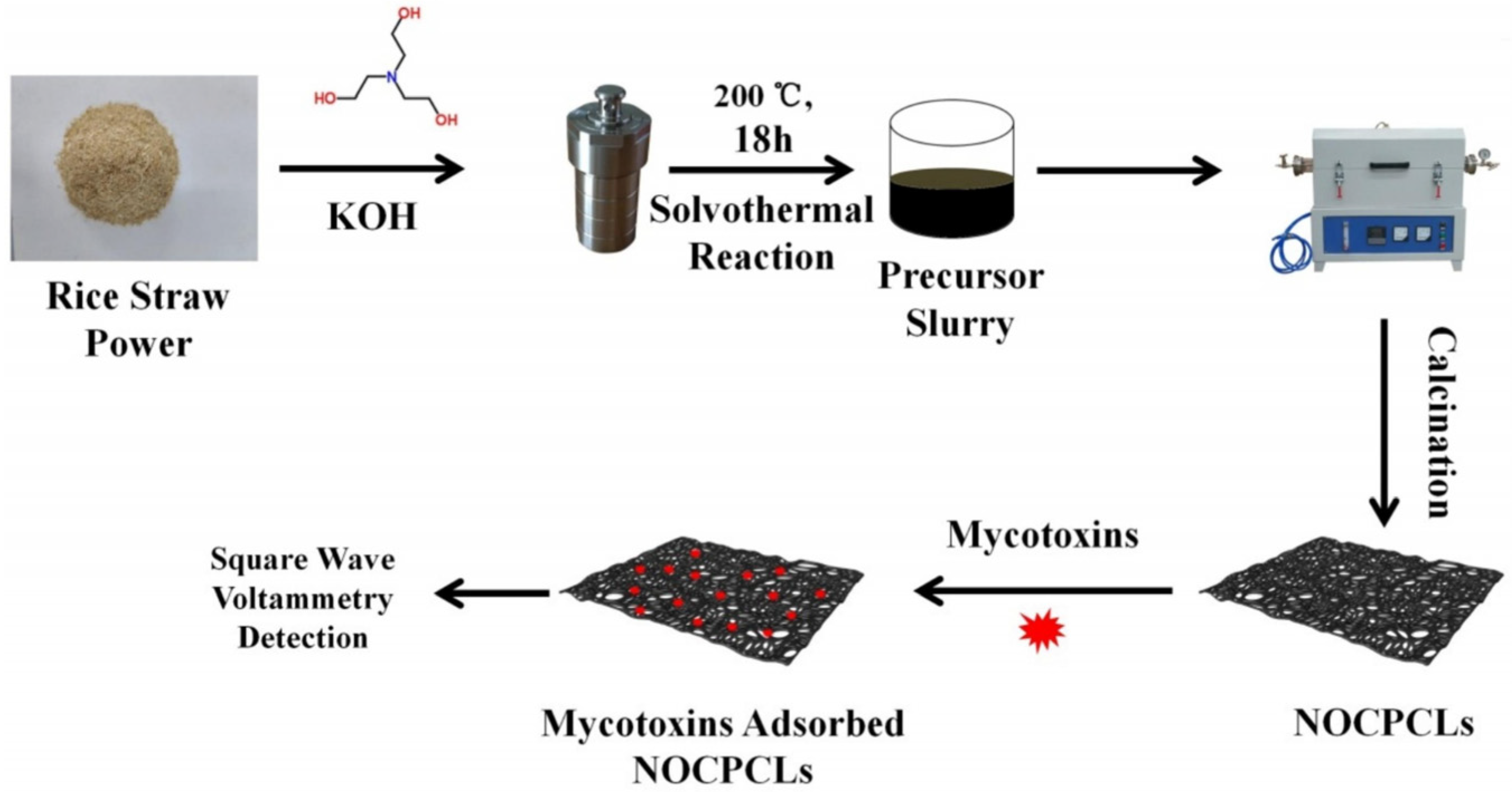
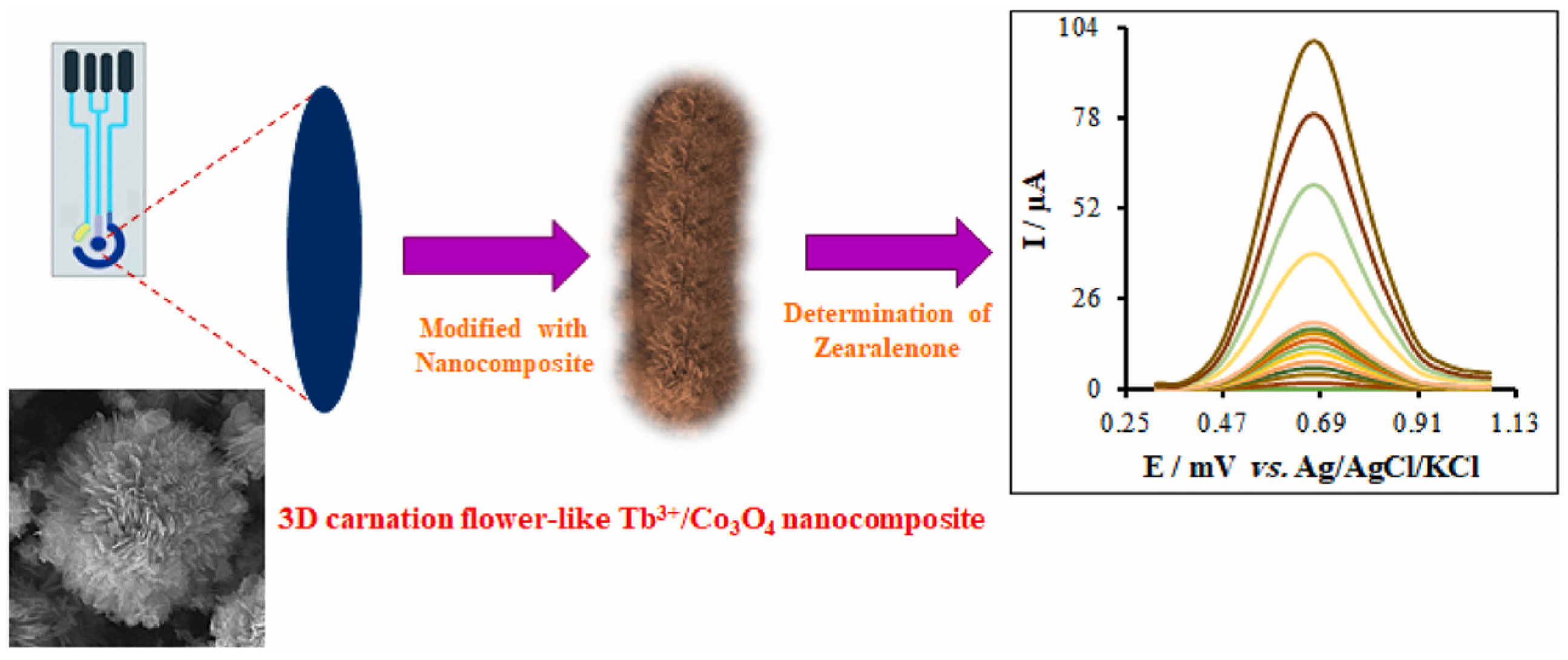


| Sensor | Sensor Type | Actual Samples | Preprocessing Time | LOD (ng mL−1) | Reference |
|---|---|---|---|---|---|
| oxSWCNHs/NCDs-apt fluorescence sensor | fluorescence sensor | corn, flour | 4 h | 18 ng mL−1 | [86] |
| SERS-based lateral flow immunosensor | SERS | corn | 24 h | 3.6 ng mL−1 | [12] |
| Multi-LFIA | fluorescence | wheat, corn, and feed | 2.5 h | 1.74 ng mL−1 | [110] |
| NiO-MWCNT(-COOH)/GCE | Nanozyme electrochemical sensor | feedstuffs and foodstuffs | 30 min | 6 ng mL−1 | [103] |
| Aptamer-Cross-Linked hydrogel | colorimetric | corn, beer | 2.75 h | 0.98 ng mL−1 | [111] |
| Fluorescent copper nanocluster sensor based on TdT amplification | fluorescence sensor | none | 2 h | 0.1 ng mL−1 | [112] |
| Chemiluminescence-based aptasensor | fluorescence sensor | corn, wheat | 50 min | 2.85 ng mL−1 | [113] |
| Disposable aptasensing chip | Non-competitive aptamer electrochemical sensor | corn starch | 14 h | 5.38 ng mL−1 | [114] |
| ITO#c-CdIn2S4/V–MoS2-Apt3 | fluorescence sensor | corn starch | 2.5 h | 0.033 ng mL−1 | [115] |
| ZnO@Ag/GCE | Nanozyme electrochemical sensor | vegetable oil deodorizer distillate | 10 min | 8 ng mL−1 | [116] |
| Cu-ZiF-8@CN-UIO-66/GCE | Nanozyme electrochemical sensor | vegetable oil deodorizer distillate, vegetable oil | 10 min | 0.6 ng mL−1 | [104] |
| Graphene@CN-UIO-66@Thi/GCE | Nanozyme electrochemical sensor | vegetable oil deodorizer distillate, vegetable oil | 10 min | 0.058 ng mL−1 | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, G.; Lin, Z.; Qian, J.; Wang, F.; Qu, L.; Zou, B. Research Progress on the Application of Nanoenzyme Electrochemical Sensors for Detecting Zearalenone in Food. Nanomaterials 2025, 15, 712. https://doi.org/10.3390/nano15100712
Guan G, Lin Z, Qian J, Wang F, Qu L, Zou B. Research Progress on the Application of Nanoenzyme Electrochemical Sensors for Detecting Zearalenone in Food. Nanomaterials. 2025; 15(10):712. https://doi.org/10.3390/nano15100712
Chicago/Turabian StyleGuan, Guoqiang, Zhiyuan Lin, Jingya Qian, Feng Wang, Liang Qu, and Bin Zou. 2025. "Research Progress on the Application of Nanoenzyme Electrochemical Sensors for Detecting Zearalenone in Food" Nanomaterials 15, no. 10: 712. https://doi.org/10.3390/nano15100712
APA StyleGuan, G., Lin, Z., Qian, J., Wang, F., Qu, L., & Zou, B. (2025). Research Progress on the Application of Nanoenzyme Electrochemical Sensors for Detecting Zearalenone in Food. Nanomaterials, 15(10), 712. https://doi.org/10.3390/nano15100712








