Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal
Abstract
1. Introduction
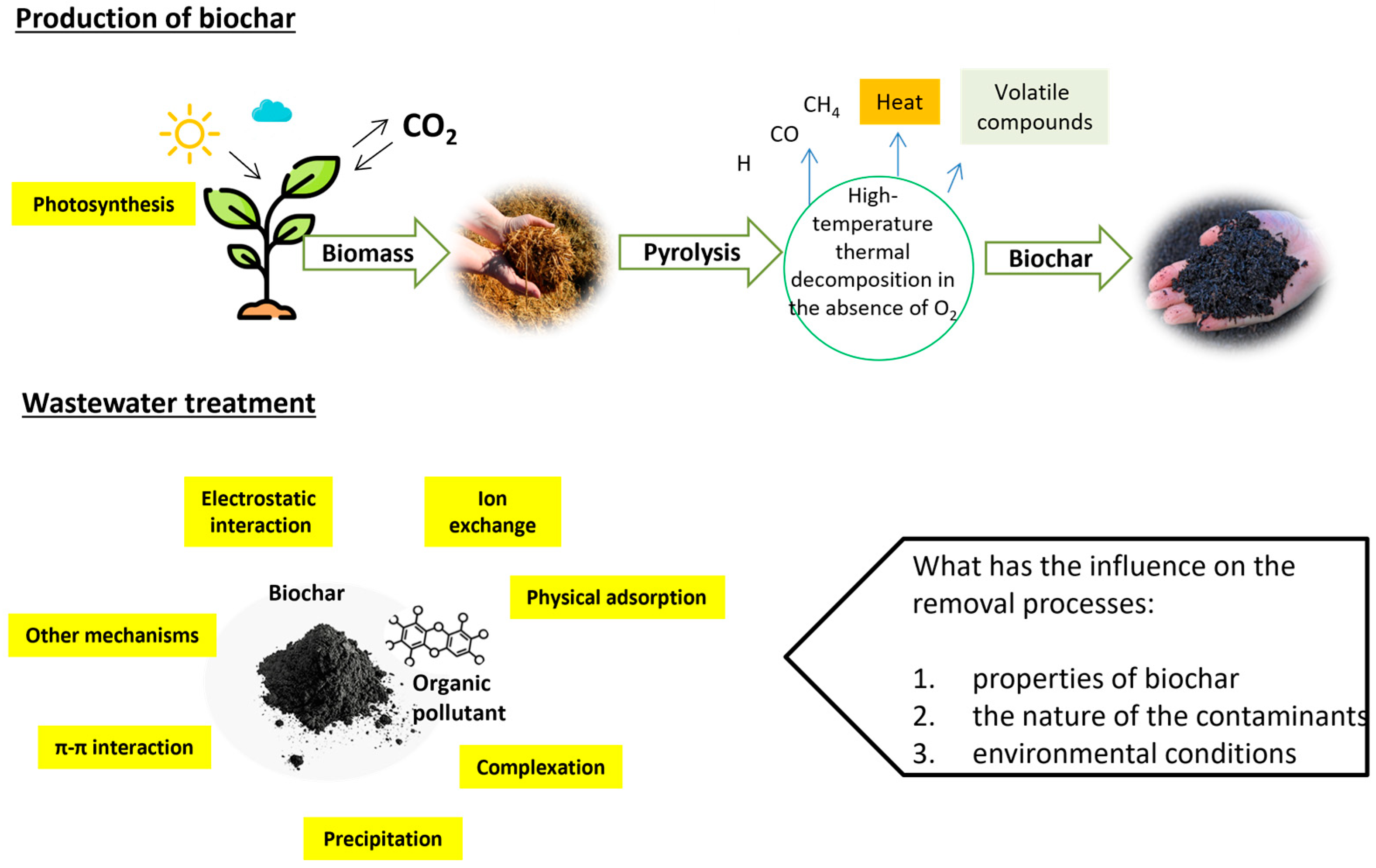
2. Production of Biochar and Application for PAH and Pesticide Removal from Water
| Biomass Types | Carbon Content (%) | Ash Content (%) | Porosity (%) | Surface Area (m2/g) | Targeted Pollutant | Removal Efficiency (%) | Production Conditions | Reference |
|---|---|---|---|---|---|---|---|---|
| Casuarina | 40–55 | 2–3 | 68–76 | 200–350 | Naphtalen | 63 | 25 °C to 900 °C; various heating rates (2.5, 5, and 15 °C min−1), followed by nitrogen purging at a rate of 40 mL min−1. | Nama et al. [34] |
| Pine sawdust | 55–65 | 2–6 | 2–6 | 180–380 | polystyrene microplastics | 84.8–96.2 | The Fe-impregnated pretreated pine sawdust, Fe/Mg modified biochar (Fe/MgBC), and Fe/ZnBC were pyrolyzed at 550 °C for 2 h in a tube furnace with a heating rate of 5 °C/min. | Zhang et al. [35] |
| Sugarcane bagasse 300 | 48.16 | 4.23 | / | 224.07 | 2,4,6-Trichlorophenol 2,4,6-Trichlorophenol 2,4,6-Trichlorophenol | 9.21–9.97 | The dried Sugarcane bagasse was charred in a muffle furnace at different temperatures (573, 673, and 773 K) for 20 min. | Mubarik et al. [36] |
| Sugarcane bagasse 400 | 50.47 | 4.17 | / | 361.77 | ||||
| Sugarcane bagasse 500 | 49.97 | 4.08 ± 0.21 | / | 291.37 | ||||
| Maize straw 350 | 58.9 | 15.4 | / | 6.71 | Carbaryl | 61.1–79.5 | The materials were pyrolyzed at 350 or 700 °C for 2 h in a closed container under an oxygen-limited condition in a preheated muffle furnace. | Ren et al. [37] |
| Maize straw 700 | 43.8 | 21.9 | / | 265 | 56.9–67.1 | |||
| Pig manure 350 | 31.6 | 45.3 | / | 23.8 | 60–75 | |||
| Pig manure 700 | 25.2 | 66.8 | / | 32.6 | 65.7–78.6 | |||
| Rice straw 350 | 44.5 | 29.1 | / | 9.01 | 71.8–73.2 | |||
| Rice straw 700 | 56.7 | 38.2 | / | 188 | 58.3–72.6 | |||
| Pine wood | 85.2 | 1.8 | / | / | Phenanthrene | <15 | Pyrolysis at 450 °C (15 min). | Jiménez et al. [38] |
| Olive pruning | 82.9 | 10 | ||||||
| Rice husk | 43.1 | 52 | ||||||
| Sewage sludge 400 | 32.7 | 53.5 | 11.4 | Phenol | ≤12.6 | Pyrolysis at 400 and 700 °C for 4 h. | Liang et al. [39] | |
| Sewage sludge 700 | 31.9 | 61.3 | 63.1 | ≤12.6 | ||||
| Sewage sludge 400—demineralized | 47 | 22.3 | 10.6 | 36.3 | ||||
| Sewage sludge 700—demineralized | 51.5 | 30.0 | 219 | 57.8 | ||||
| Willow | 11.4 (840.6) | PAHs | 41–84 | Pyrolysis at 350–650 °C. | Kotlowski et al. [40] | |||
| Coconut | 3.1 (626.8) | |||||||
| Wheat straw | 26.3 (246.2) |
3. Mechanisms of PAH and Pesticide Removal
- Chemical Activation: Impregnation of biochar with acids, bases, or metals can enhance porosity and introduce functional groups, increasing affinity for specific pollutants [50,51]. Chemical methods, particularly advanced oxidation processes (AOPs) like ozonation and UV/H₂O₂ treatment, utilize highly reactive species, such as hydroxyl radicals, to break down complex molecules into less harmful or more biodegradable forms [2].
| References | Interactions | Investigated Problem/Solution/Result |
|---|---|---|
| Yao et al. [65] | The mechanisms involved in pesticide removal using KOH-activated biochar include van der Waals forces, pore filling, hydrogen bonding, and π-π electron donor–acceptor interactions, indicating a complex interaction during the adsorption process for effective contaminant removal. | KOH-activated biochar effectively removes acetamiprid and triadimefon. The maximum adsorption capacity for acetamiprid is 40.41 mg g−1. |
| Chen et al. [56] | This paper discusses the removal of pesticides, specifically atrazine, using a green biochar iron composite (PC-ZVI) activated by peroxymonosulfate (PMS) and oxalic acid (OA). Mechanisms include reduction, oxidation, hydroxylation, substitution, and de-alkylation processes for effective degradation. | PMS enhances Cr(VI) and atrazine removal efficiency. PC-ZVI shows high recyclability and low toxicity. |
| Koippully Manikandan et al. [57] | This paper focuses on chlorpyrifos (CP) removal, highlighting a synergistic mechanism involving bacterial metabolism and adsorption. The immobilized Aeromonas veronii on rice husk biochar enhances pesticide degradation, effectively removing contaminants from water through these combined processes. | 96.25% chlorpyrifos removal in water within 24 h. 92.4% removal in soil within 42 days. |
| Eissa et al. [66] | The paper reveals that active adsorption groups and metal oxides in biochar play a significant role in pesticide sorption. | Rise husk biochar showed the highest pesticide removal percentages among biochar. Adsorption equilibrium achieved in 60–120 min for different biochar. |
| Cao et al. [67] | The mechanisms involved in pesticide removal using boric acid-modified walnut shell biochar (WAB4) include hydrogen bonding, pore filling, hydrophobic effects, and π–π interactions, contributing to its high adsorption capacity for various pesticides in water. | WAB4 effectively absorbs investigated pesticides over 70%. No significant acute toxicity to Daphnia magna. |
| Matos et al. [68] | This paper discusses adsorption as the primary mechanism for pesticide removal from water using biochar. The activated and magnetized biochar effectively adsorbed thiacloprid and thiamethoxam, with a pseudo-second-order model describing the kinetics of adsorption. | Activated biochar absorbs 1.02 mg thiacloprid and 0.97 mg thiamethoxam. Magnetized biochar adsorbs 0.73 mg thiacloprid and 0.40 mg thiamethoxam. |
| Yaashikaa et al. [24] | This review discusses various mechanisms for PAH removal using biochar, including adsorption due to its large surface area and functional groups, as well as surface modification methods like magnetization, which enhance biochar’s effectiveness in removing pollutants from water. | Biochar effectively remediates polycyclic aromatic hydrocarbons (PAHs). Surface modifications enhance biochar’s properties for pollutant removal. |
| Murtaza et al. [69] | The mechanisms involved in the removal of polycyclic aromatic hydrocarbons (PAHs) and pesticides from water using biochar include electrostatic interaction, partitioning, hydrophobic interaction, and pore filling, which enhance the adsorption efficiency of these organic pollutants. | Engineered biochar composites have a high adsorption capacity for removing aquatic pollutants. Clay-based biochar composites are efficient in removing antibiotics, dyes, metals, and nutrients. |
| Method | Mechanism | Efficiency | Key Notes | References |
|---|---|---|---|---|
| Biochar adsorption | π–π interactions, hydrophobic interactions, and pore filling | Up to 81% (100 mg/L, 120 min) | Effective for various PAHs, low-cost | Li et al. [71] Kong et al. [60] |
| AOPs | ROS-mediated oxidation (e.g., -OH, -O2−) | Over 90% (enhanced with biochar) | Sustainable, broad-spectrum removal | Miklos et al. [72] |
| Photocatalysis | UV-activated TiO2 | Up to 94.5% (5:1 biochar: TiO2 ratio) | Efficient for high-molecular PAHs | Boffito et al. [73] |
4. The Scalability and Long-Term Effectiveness of Biochar Uses for PAH and Pesticides Removal
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gahrouei, A.E.; Vakili, S.; Zandifar, A.; Pourebrahimi, S. From wastewater to clean water: Recent advances on the removal of metronidazole, ciprofloxacin, and sulfamethoxazole antibiotics from water through adsorption and advanced oxidation processes (AOPs). Environ. Res. 2024, 252 Pt 3, 119029. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Rezapour, A.; Pirooz, M.; Pourebrahimi, S. From classic to cutting-edge solutions: A comprehensive review of materials and methods for heavy metal removal from water environments. Desalin. Water Treat. 2024, 319, 100446. [Google Scholar] [CrossRef]
- Singh, J.; Verma, M. Waste derived modified biochar as promising functional material for enhanced water remediation potential. Environ. Res. 2024, 245, 117999. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Padhye, L.P. Fate of environmental pollutants. Water Environ. Res. 2015, 87, 1595–1610. [Google Scholar] [CrossRef]
- Ameur, W.B.; Annabi, A.; Mhadhbi, T.; Hassine, S.B.; Safouen, G.; Megdiche, Y.E.; Khadija, M.; Ennaceur, S.; Trabelsi, S.; Hammami, B.; et al. Polycyclic aromatic hydrocarbons in mullet (Chelon auratus) from two lagoons of great ecological and economic importance in Tunisia: Levels, sources and human health risk implications. J. Sea Res. 2023, 192, 102325. [Google Scholar] [CrossRef]
- Maletić, S.P.; Beljin, J.M.; Rončević, S.D.; Grgić, M.G.; Dalmacija, B.D. State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: Sources, fate, bioavailability and remediation techniques. J. Hazard. Mater. 2019, 365, 467–482. [Google Scholar] [CrossRef]
- Eissa, F.; Al-Sisi, M.; Ghanem, K. Occurrence and ecotoxicological risk assessment of pesticides in sediments of the Rosetta branch, Nile River, Egypt. J. Environ. Sci. 2022, 118, 21–31. [Google Scholar] [CrossRef]
- Bose, S.; Senthil Kumar, P.; Rangasamy, G.; Prasannamedha, G.; Kanmani, S. A review on the applicability of adsorption techniques for remediation of recalcitrant pesticides. Chemosphere 2023, 313, 137481. [Google Scholar] [CrossRef]
- Pereira, H.A.; da Boit Martinello, K.; Vieira, Y.; Diel, J.C.; Netto, M.S.; Reske, G.D.; Lorenzett, E.; Silva, L.F.O.; Burgo, T.A.L.; Dotto, G.L. Adsorptive behavior of multi-walled carbon nanotubes immobilized magnetic nanoparticles for removing selected pesticides from aqueous matrices. Chemosphere 2023, 325, 138384. [Google Scholar] [CrossRef]
- Water Framework Directive: Directive 2000/60/EC Official Journal L 327, 22/12/2000; 0001–0073. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 24 November 2024).
- Sompornpailin, D.; Ratanatawanate, C.; Chantanavorakunchai, N.; Punyapalakul, P. Effects of electrolytes and fractionated dissolved organic matter on selective adsorption of pharmaceuticals on terephthalic acid-based metal-organic frameworks. Environ. Res. 2021, 196, 110335. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wen, Q.; Chen, Z. Effects of Fe/Fe–Mn oxides loaded biochar on anaerobic degradation of typical phenolic compounds in coal gasification wastewater: Performance and mechanism. Bioresour. Technol. 2024, 394, 130308. [Google Scholar] [CrossRef]
- Li, Q.; Chen, R.; Xu, Y.; Chen, C.; Xiong, J.; Tan, W.; Fang, L. Examining diverse remediation mechanisms of biochar in soil contaminated with polycyclic aromatic hydrocarbon (PAH) of various ring structures: A global meta-analysis. Sci. Total Environ. 2024, 921, 171178. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, Y.; Miranda-Aviles, R.; Zha, X.; Anguiano, J.H.H.; Moncada Sanchez, C.D.; Puy-Alquiza, M.J.; Gonzalez, V.P.; Garzon, L.F.R. In situ remediation and exsitu treatment practices of arsenic-contaminated soil: An overview on recent advances. J. Hazard. Mater. Adv. 2022, 8, 100157. [Google Scholar] [CrossRef]
- Hassan, M.; Wang, B.; Wu, P.; Wang, S. Engineered biochar for in-situ and ex-situ remediation of contaminants from soil and water. Sci. Total Environ. 2024, 957, 177384. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Karishma, S.; Kamalesh, R.; Vickram, A.S.; Anbarasu, K. A systematic review on enhancement strategies in biochar-based remediation of polycyclic aromatic hydrocarbons. Chemosphere 2024, 355, 141796. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M. Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J Soils Sediments 2011, 11, 62–71. [Google Scholar] [CrossRef]
- Dong, M.; He, L.; Jiang, M.; Zhu, Y.; Wang, J.; Gustave, W.; Wang, S.; Deng, Y.; Zhang, X.; Wang, Z. Biochar for the Removal of Emerging Pollutants from Aquatic Systems: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1679. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef]
- Bocșa, M.; Pintea, S.; Lung, I.; Opriș, O.; Stegarescu, A.; Humayun, M.; Bououdina, M.; Soran, M.L.; Bellucci, S. Biochar-Based Adsorbents for Pesticides, Drugs, Phosphorus, and Heavy Metal Removal from Polluted Water. Separations 2023, 10, 533. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedlacek, P.; Bielska, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Olugbenga, O.S.; Adeleye, P.G.; Oladipupo, S.B.; Adeleye, A.T.; John, K.I. Biomass-derived biochar in wastewater treatment- a circular economy approach. Waste Manag. Bull. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Díaz, B.; Sommer-Márquez, A.; Ordoñez, P.E.; Bastardo-González, E.; Ricaurte, M.; Navas-Cárdenas, C. Synthesis Methods, Properties, and Modifications of Biochar-Based Materials for Wastewater Treatment: A Review. Resources 2024, 13, 8. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Yadav, S.; Ghangrekar, M.M.; Surampalli, R.Y. The potential of biochar-based catalysts in advanced treatment technologies for efficacious removal of persistent organic pollutants from wastewater: A review. Chem. Eng. Res. Des. 2022, 187, 470–496. [Google Scholar] [CrossRef]
- Nama, M.; Satasiya, G.; Sahoo, T.P.; Moradeeya, P.G.; Sadukha, S.; Singhal, K.; Saravaia, H.T.; Dineshkumar, R.; Anil Kumar, M. Thermo-chemical behaviour of Dunaliella salina biomass and valorising their biochar for naphthalene removal from aqueous rural environment. Chemosphere 2024, 353, 141639. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Wang, Y.; Cui, X.; Liu, Y.; Ruan, R.; Wu, X.; Cao, L.; Zhao, L.; Zheng, H. Preparation and application of metal-modified biochar in the purification of micro-polystyrene polluted aqueous environment. J. Environ. Manag. 2023, 347, 119158. [Google Scholar] [CrossRef]
- Mubarik, S.; Saeed, A.; Athar, M.M.; Iqbal, M. Characterization and mechanism of the adsorptive removal of 2, 4, 6-trichlorophenol by biochar prepared from sugarcane baggase. J. Ind. Eng. Chem. 2016, 33, 115–121. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, P.; Zhao, L.; Sun, H. Sorption and degradation of carbaryl in soils amended with biochars: Influence of biochar type and content. Environ. Sci. Pollut. Res. 2016, 23, 2724–2734. [Google Scholar] [CrossRef]
- Jiménez, E.M.; Aceña-Heras, S.; Frišták, V.; Heinze, S.; Marschner, B. The effect of biochar amendments on phenanthrene sorption, desorption and mineralisation in different soils. PeerJ 2018, 6, e5074. [Google Scholar] [CrossRef]
- Liang, J.; Xu, X.; Zhong, Q.; Xu, Z.; Zhao, L.; Qiu, H.; Cao, X. Roles of the mineral constituents in sludge-derived biochar in persulfate activation for phenol degradation. J. Hazard. Materials. 2020, 398, 122861. [Google Scholar] [CrossRef]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Effect of steam activated biochar application to industrially contaminated soils on bioavailability of polycyclic aromatic hydrocarbons and ecotoxicity of soils. Sci. Total Environ. 2016, 566–567, 1023–1031. [Google Scholar] [CrossRef]
- Babu, S.K.K.B.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of biochar from waste biomass using slow pyrolysis: Studies of the effect of pyrolysis temperature and holding time on biochar yield and properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Méndez, A.; Gascó, G.; Lado, M.; Paz-González, A. The Effects of Feedstock, Pyrolysis Temperature, and Residence Time on the Properties and Uses of Biochar from Broom and Gorse Wastes. Appl. Sci. 2024, 14, 4283. [Google Scholar] [CrossRef]
- Dira, A.; Elmouwahidi, A.; Khouja, S.; Boufetacha, M.; Bailón-garcía, E.; Barakat, A.; Tayibi, S.; Carrasco-Marin, F.; Gharibi, E. Feedstock type and pyrolysis temperature of rosemary wastes in a fixed-bed reactor affect the characteristics and application potentials of the bio-chars. J. Anal. Appl. Pyrolysis 2024, 182, 106697. [Google Scholar] [CrossRef]
- de Oliveira Paiva, I.; de Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. Waste Biomass Valorization 2024, 15, 3599–3619. [Google Scholar] [CrossRef]
- Nosratabad, N.A.; Yan, Q.; Cai, Z.; Wan, C. Exploring nanomaterial-modified biochar for environmental remediation applications. Heliyon 2024, 10, e37123. [Google Scholar] [CrossRef]
- Rajput, P.; Kumar, P.; Priya, A.K.; Kumari, S.; Shiade, S.R.G.; Rajput, V.D.; Fathi, A.; Pradhan, A.; Sarfraz, R.; Sushkova, S.; et al. Nanomaterials and biochar mediated remediation of emerging contaminants. Sci. Total Environ. 2024, 916, 170064. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pal, A.; Chen, C.W.; Pandey, A.; Dong, C.D. Advances on tailored biochar for bioremediation of antibiotics, pesticides and polycyclic aromatic hydrocarbon pollutants from aqueous and solid phases. Sci. Total Environ. 2022, 817, 153054. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Deng, L.; Wang, C.; Shah, Z.-u.-H.; Deng, L.; Li, Y.; Li, J.; Chachar, S.; Chachar, Z.; Hayat, F.; et al. Advancements in Biochar Modification for Enhanced Phosphorus Utilization in Agriculture. Land 2024, 13, 644. [Google Scholar] [CrossRef]
- Al Masud, M.A.; Shin, W.S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D.M.; Iqbal, M.A.; Islam, A.R.M.T.; Malafaia, G. A critical review of sustainable application of biochar for green remediation: Research uncertainty and future directions. Sci. Total Environ. 2023, 904, 166813. [Google Scholar] [CrossRef]
- Zhang, N.; Reguyal, F.; Praneeth, S.; Sarmah, A.K. A green approach of biochar-supported magnetic nanocomposites from white tea waste: Production, characterization and plausible synthesis mechanisms. Sci. Total Environ. 2023, 886, 163923. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Kumari, N.; Adnan, M.; Kumar, S.; Abdelgadir, A.; Saxena, J.; Badraoui, R.; Snoussi, M.; Khare, P.; Singh, R. Impregnation of Modified Magnetic Nanoparticles on Low-Cost Agro-Waste-Derived Biochar for Enhanced Removal of Pharmaceutically Active Compounds: Performance Evaluation and Optimization Using Response Surface Methodology. Water 2023, 15, 1688. [Google Scholar] [CrossRef]
- Li, D.; Fang, Y.; Lu, J.; Sun, J.; Zhao, X.; Hou, N.; Xing, J. Enhanced biodegradation of PAHs by biochar and a TiO2@biochar composite under light irradiation: Photocatalytic mechanism, toxicity evaluation and ecological response. Chem. Eng. J. 2023, 458, 141495. [Google Scholar] [CrossRef]
- Bratovčić, A.; Tomašić, V. Photocatalytic Composites Based on Biochar for Antibiotic and Dye Removal in Water Treatment. Processes 2024, 12, 2746. [Google Scholar] [CrossRef]
- Chen, M.L.; Yao, X.W.; Diao, Z.H.; Jin, J.C.; Qian, W.; Yi, Y.Q.; Chen, X.; Kong, L.J. Oxalic acid enhanced removal of heavy metal and pesticide by peroxymonosulphate activation with a green biochar iron composite: Reactivity and mechanism. Sep. Purif. Technol. 2023, 327, 125013. [Google Scholar] [CrossRef]
- Koippully Manikandan, S.; Mariyam, A.; Gowda, N.K.; Singh, A.; Nair, V. Mechanistic understanding of biochar-bacteria system for enhanced chlorpyrifos bioremediation in water and soil medium. Chem. Eng. J. 2024, 483, 149119. [Google Scholar] [CrossRef]
- Padilla-Garfias, F.; Araiza-Villanueva, M.; Calahorra, M.; Sánchez, N.S.; Peña, A. Advances in the Degradation of Polycyclic Aromatic Hydrocarbons by Yeasts: A Review. Microorganisms 2024, 12, 2484. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Maree, J.P.; Mokoena, L. Adsorption of Polycyclic Aromatic Hydrocarbons from Wastewater Using Iron Oxide Nanomaterials Recovered from Acid Mine Water: A Review. Minerals 2024, 14, 826. [Google Scholar] [CrossRef]
- Kong, F.; Liu, J.; Xiang, Z.; Fan, W.; Liu, J.; Wang, J.; Wang, Y.; Wang, L.; Xi, B. Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review. Water 2024, 16, 875. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Osman, A.M.; Sulieman, A. An Overview and Evaluation of Highly Porous Adsorbent Materials for Polycyclic Aromatic Hydrocarbons and Phenols Removal from Wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.; Ren, Y.; Zhu, X.; Si, S.; Kou, B.; Zhang, Z.; Wang, J.; Shen, B. Remediation of polycyclic aromatic hydrocarbons polluted soil by biochar loaded humic acid activating persulfate: Performance, process and mechanisms. Bioresour. Technol. 2024, 399, 130633. [Google Scholar] [CrossRef]
- Raczkiewicz, M.; Mašek, O.; Ok, Y.S.; Oleszczuk, P. Size reduction of biochar to nanoscale decrease polycyclic aromatic hydrocarbons (PAHs) and metals content and bioavailability in nanobiochar. Sci. Total Environ. 2024, 937, 173372. [Google Scholar] [CrossRef]
- Yao, X.W.; Chen, X.; Chen, M.L.; Feng, N.J.; Tong, L.Y.; Yi, Y.Q.; Qian, W.; Diao, Z.H. Removal of pesticide acetamiprid using KOH activated biochar derived from crayfish shell: Behavior and mechanism. Process Saf. Environ. Prot. 2024, 186, 808–818. [Google Scholar] [CrossRef]
- Eissa, F.; Alsherbeny, S.; El-Sawi, S.; Slaný, M.; Lee, S.S.; Shaheen, S.M.; Jamil, T.S. Remediation of pesticides contaminated water using biowastes-derived carbon rich biochar. Chemosphere 2023, 340, 139819. [Google Scholar] [CrossRef]
- Cao, N.; Ji, J.; Li, C.; Yuan, M.; Guo, X.; Zong, X.; Li, L.; Ma, Y.; Wang, C.; Pang, S. Rapid and efficient removal of multiple aqueous pesticides by one-step construction boric acid modified biochar. RSC Adv. 2023, 13, 8765–8778. [Google Scholar] [CrossRef]
- Matos, T.; Schultz, J.; Khan, M.; Zanoelo, E.; Mangrich, A.; Araújo, B.; Navickiene, S.; Romao, L. Using Magnetized (Fe3O4/Biochar Nanocomposites) and Activated Biochar as Adsorbents to Remove Two Neuro-Active Pesticides from Waters. J. Braz. Chem. Soc. 2017, 28, 1975–1987. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Dai, D.Q.; Iqbal, R.; Bawazeer, S.; Usman, M.; Rizwan, M.; Iqbal, J.; Akram, M.I.; Althubiani, A.S.; et al. A review of mechanism and adsorption capacities of biocharbased engineered composites for removing aquatic pollutants from contaminated water. Front. Environ. Sci. 2022, 10, 1035865. [Google Scholar] [CrossRef]
- Kang, Y.; Ma, H.; Jing, Z.; Zhu, C.; Li, Y.; Wu, H.; Dai, P.; Guo, Z.; Zhang, J. Enhanced benzofluoranthrene removal in constructed wetlands with iron- modified biochar: Mediated by dissolved organic matter and microbial response. J. Hazard. Mater. 2023, 443(B), 130322. [Google Scholar] [CrossRef]
- Li, Y.; Pan, L.; Zhu, Y.; Yu, Y.; Wang, D.; Yang, G.; Yuan, X.; Liu, X.; Li, H.; Zhang, J. How does zero valent iron activating peroxydisulfate improve the dewatering of anaerobically digested sludge? Water Res. 2019, 163, 114912. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Boffito, D.; Crocellà, V.; Pirola, C.; Neppolian, B.; Cerrato, G.; Ashokkumar, M.; Bianchi, C. Ultrasonic enhancement of the acidity, surface area and free fatty acids esterification catalytic activity of sulphated ZrO2–TiO2 systems. J. Catal. 2013, 297, 17–26. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. J. Adv. Chem. Eng. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Zulfiqar, U.; Farooq, M.; Hussain, S.; Mehmood, T.; Naveed, M.; Li, Y.; Liqun, C.; Saeed, Q.; et al. Biochar application for remediation of organic toxic pollutants in contaminated soils; An update. Ecotoxicol. Environ. Saf. 2022, 248, 114322. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ali, S.; Zaman, W. Innovative Adsorbents for Pollutant Removal: Exploring the Latest Research and Applications. Molecules 2024, 29, 4317. [Google Scholar] [CrossRef]
- Motúzová, T.; Koutník, I.; Vráblová, M. Removal of Pesticides from Water by Adsorption on Activated Carbon Prepared from Invasive Plants. Water Air Soil Pollut. 2024, 235, 782. [Google Scholar] [CrossRef]
- de Souza, T.F.; Dias Ferreira, G.M. Biochars as Adsorbents of Pesticides: Laboratory-Scale Performances and Real-World Contexts, Challenges, and Prospects. ACS EST Water J. 2024, 4, 4264–4282. [Google Scholar] [CrossRef]
- Flafel, H.M.; Rafatullah, M.; Lalung, J.; Kapoor, R.T.; Siddiqui, M.R.; Qutob, M. A novel combination of wetland plants (Eichhornia crassipes) and biochar derived from palm kernel shells modified with melamine for the removal of paraquat from aqueous medium: A green and sustainable approach. Int. J. Phytoremediat. 2024, 26, 2378–2391. [Google Scholar] [CrossRef]
- Cui, S.; Lv, J.; Hough, R.; Fu, Q.; An, L.H.; Zhang, Z.; Ke, Y.; Liu, Z.; Li, Y.F. Recent advances and prospects of neonicotinoid insecticides removal from aquatic environments using biochar: Adsorption and degradation mechanisms. Sci. Total Environ. 2024, 939, 173509. [Google Scholar] [CrossRef]
- Kaur, J.; Chaudhary, S.; Bhalla, A. Recent Advances in Modification of Biochar for Removal of Emerging Contaminants from Water Bodies. In Occurrence, Distribution and Toxic Effects of Emerging Contaminants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; eBook; ISBN 9781003335757. [Google Scholar]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Iqbal, R.; Zulfiqar, F.; Tariq, A.; Ditta, A. Physicochemical properties and performance of non-woody derived biochars for the sustainable removal of aquatic pollutants: A systematic review. Chemosphere 2024, 359, 142368. [Google Scholar] [CrossRef]
- Devanand, D.; Khatkar, R.; Nahar, S.; Nagpal, S. Remediation of chlorpyrifos by utilizing waste packaging wood as magnetic biochar. Remediation 2024, 34, e21781. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, R.; Yue, C.; Wu, X.; Yan, Q.; Wang, H.; Zhang, H.; Ma, T. Enhanced charge transfer over sustainable biochar decorated Bi2WO6 composite photocatalyst for highly efficient water decontamination. Chin. J. Catal. 2024, 59, 169–184. [Google Scholar] [CrossRef]
- Gautam, M.K.; Mondal, T.; Nath, R.; Mahajon, B.; Chincholikar, M.; Bose, A.; Das, D.; Das, R.; Mondal, S. Harnessing Activated Hydrochars: A Novel Approach for Pharmaceutical Contaminant Removal. C 2024, 10, 8. [Google Scholar] [CrossRef]
- Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review. Toxics 2023, 11, 117. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Arsenov, D.; Beljin, J.; Jović, D.; Maletić, S.; Borišev, M.; Borišev, I. Nanomaterials as endorsed environmental remediation tools for the next generation: Eco-safety and sustainability. J. Geochem. Explor. 2023, 253, 107283. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, Y.; Wang, Z.; Tan, Z.; Zhou, T. Biochar application for the remediation of soil contaminated with potentially toxic elements: Current situation and challenges. J. Environ. Manag. 2024, 351, 119775. [Google Scholar] [CrossRef] [PubMed]
- Issaka, E.; Fapohunda, F.O.; Amu-Darko, J.N.O.; Yeboah, L.; Yakubu, S.; Varjani, S.; Ali, N.; Bilal, M. Biochar-based composites for remediation of polluted wastewater and soil environments: Challenges and prospects. Chemosphere 2022, 297, 134163. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management Science and Technology; Taylor & Francis Routledge: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Unravelling the Environmental Application of Biochar as Low-Cost Biosorbent: A Review. Appl. Sci. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An overview of biochar production techniques and application in iron and steel industries. Bioresour. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100059. [Google Scholar] [CrossRef]

| Adsorbent | Advantages | Disadvantages | References |
|---|---|---|---|
| Biochar | Sustainable, cost-effective, tunable surface properties, and versatile applications | Lower initial performance, variability in quality, and regeneration challenges | Qui et al. [61] Jha et al. [87] Srivatsav et al. [88] |
| Activated Carbon | High adsorption capacity, widely available, and well researched | High cost, environmental impact, and fouling issues | |
| Zeolites | Selective adsorption and thermal stability | High cost and limited effectiveness for hydrophobic pollutants | |
| Nanomaterials | Superior adsorption efficiency and customizable properties | Expensive, scalability issues, and potential toxicity |
| Factor | Explanation of Why This Is Important | References |
|---|---|---|
| Biochar properties affecting removal efficiency: surface area and porosity; chemical functional groups; surface charge; hydrophobicity and polarity | The performance of biochar in contaminant removal depends heavily on its intrinsic properties, which are shaped by the feedstock type, pyrolysis conditions, and post-production modifications. | Kong et al. [60] Dong et al. [29] |
| Environmental factors influencing biochar efficiency: pH of the environment; presence of natural organic matter (NOM); coexisting ions; temperature and humidity | Biochar’s performance in contaminant removal is also highly sensitive to environmental conditions, which can either enhance or hinder its efficacy. | Lechmann and Joseph [92] |
| Challenges in scalability: feedstock variability; environmental variability; regeneration and reusability; cost-effectiveness | Scaling up biochar-based water treatment systems for real-world applications introduces additional complexities: | Fdez-Sanromán et al. [93] Al Rumaihi et al. [94] |
| Strategies to enhance scalability and long-term effectiveness; tailored design; multi-functionality; adaptation to environmental conditions, life-cycle management | Addressing the challenges associated with biochar’s scalability and long-term effectiveness requires innovative approaches: | Ibitoye et al. [95] Guo et al. [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beljin, J.; Đukanović, N.; Anojčić, J.; Simetić, T.; Apostolović, T.; Mutić, S.; Maletić, S. Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal. Nanomaterials 2025, 15, 26. https://doi.org/10.3390/nano15010026
Beljin J, Đukanović N, Anojčić J, Simetić T, Apostolović T, Mutić S, Maletić S. Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal. Nanomaterials. 2025; 15(1):26. https://doi.org/10.3390/nano15010026
Chicago/Turabian StyleBeljin, Jelena, Nina Đukanović, Jasmina Anojčić, Tajana Simetić, Tamara Apostolović, Sanja Mutić, and Snežana Maletić. 2025. "Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal" Nanomaterials 15, no. 1: 26. https://doi.org/10.3390/nano15010026
APA StyleBeljin, J., Đukanović, N., Anojčić, J., Simetić, T., Apostolović, T., Mutić, S., & Maletić, S. (2025). Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal. Nanomaterials, 15(1), 26. https://doi.org/10.3390/nano15010026







