TiO2 Nanosphere/MoSe2 Nanosheet-Based Heterojunction Gas Sensor for High-Sensitivity Sulfur Dioxide Detection
Abstract
1. Introduction
2. Experiments
2.1. Materials
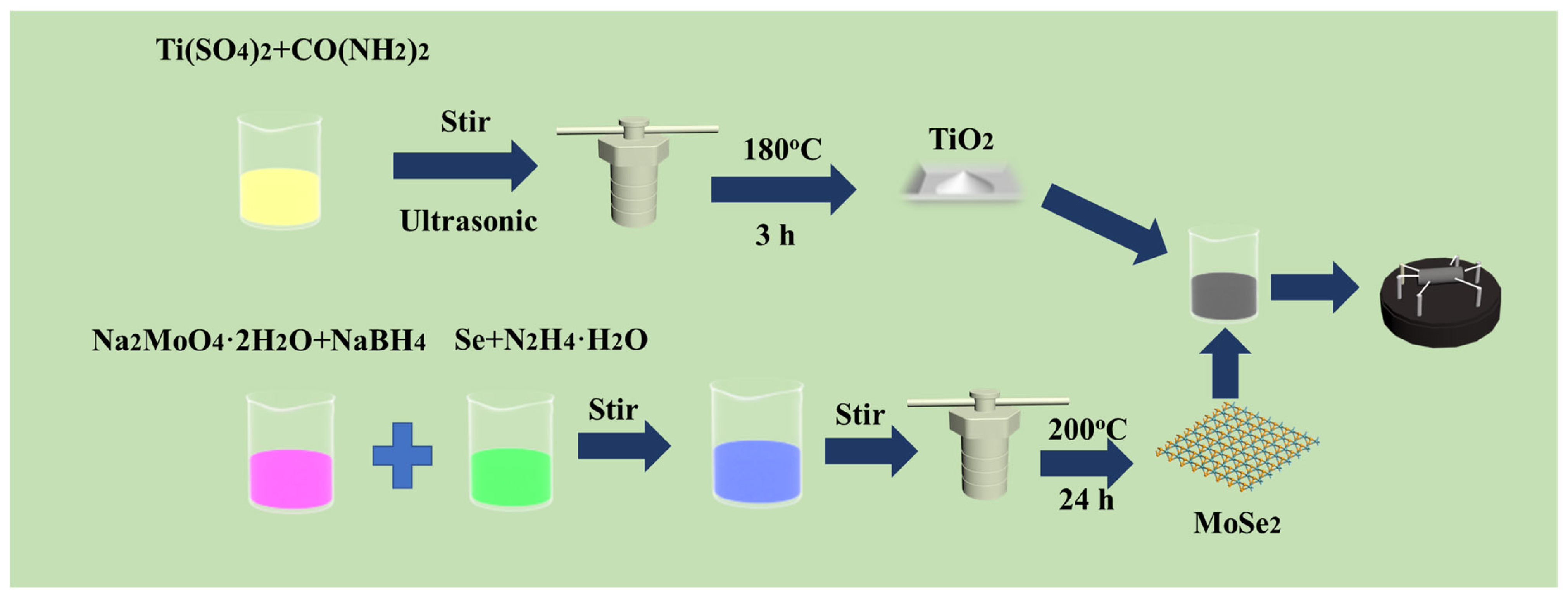
2.2. Sensor Fabrication
3. Results and Discussion
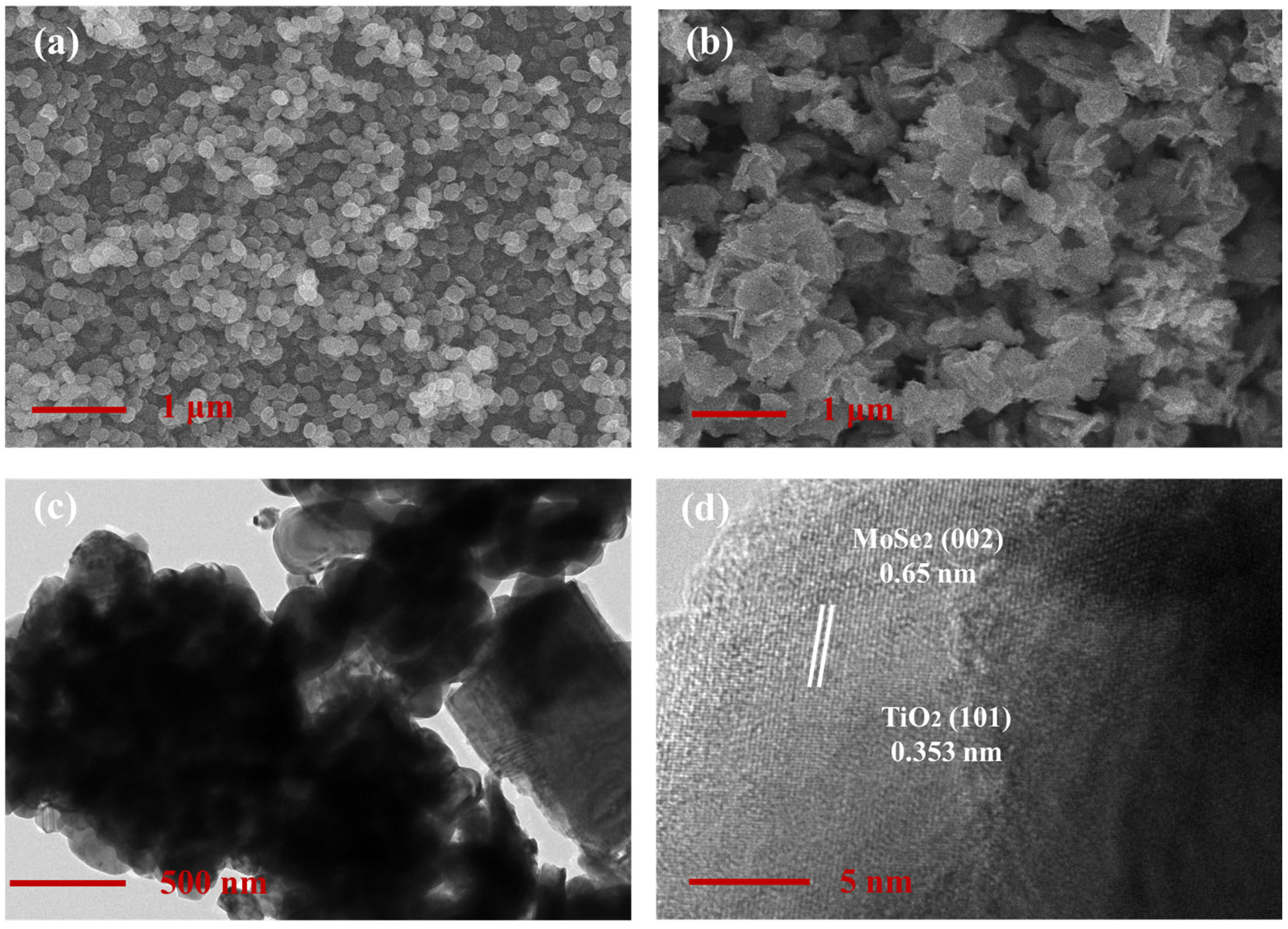
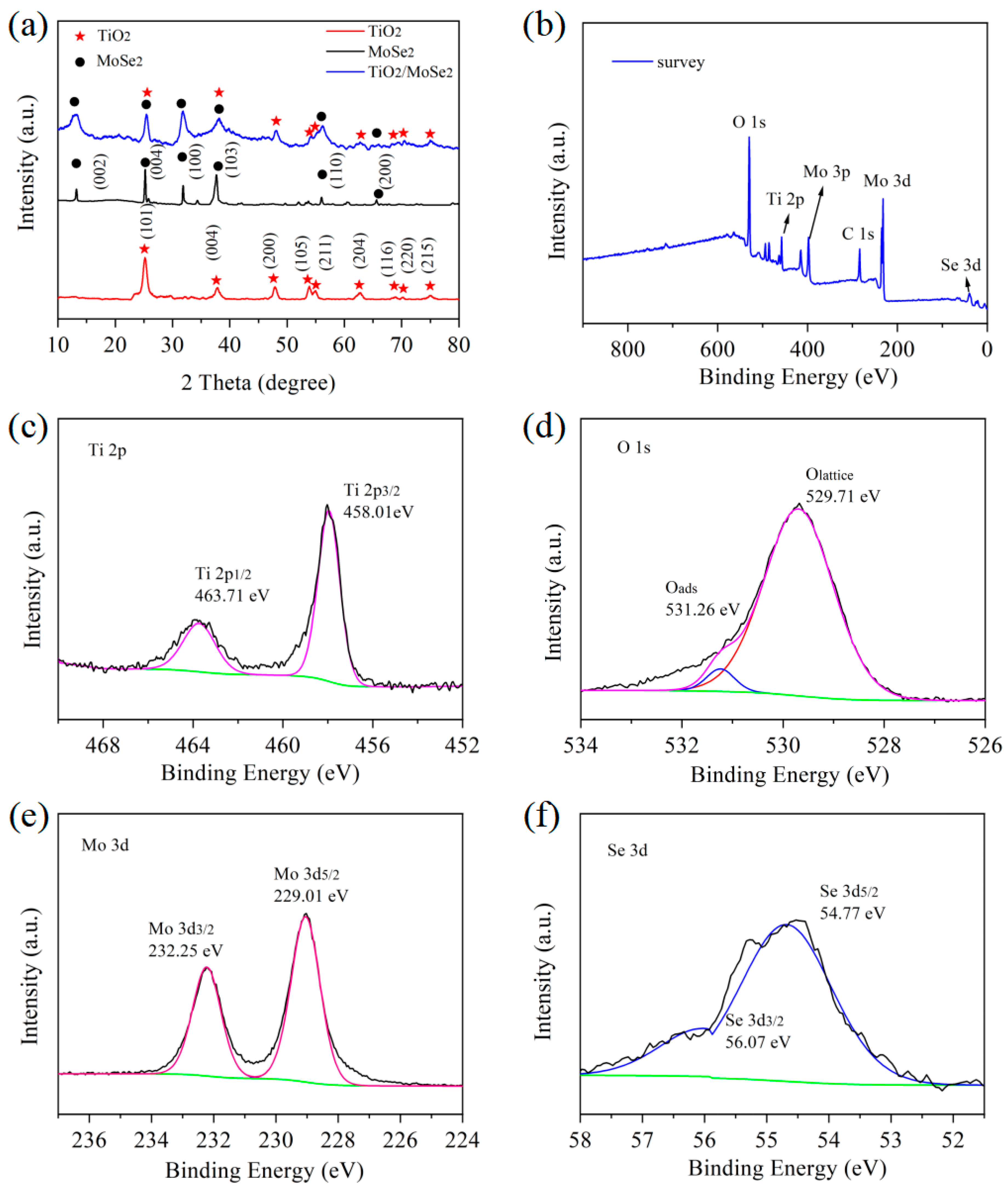
3.1. Characterization
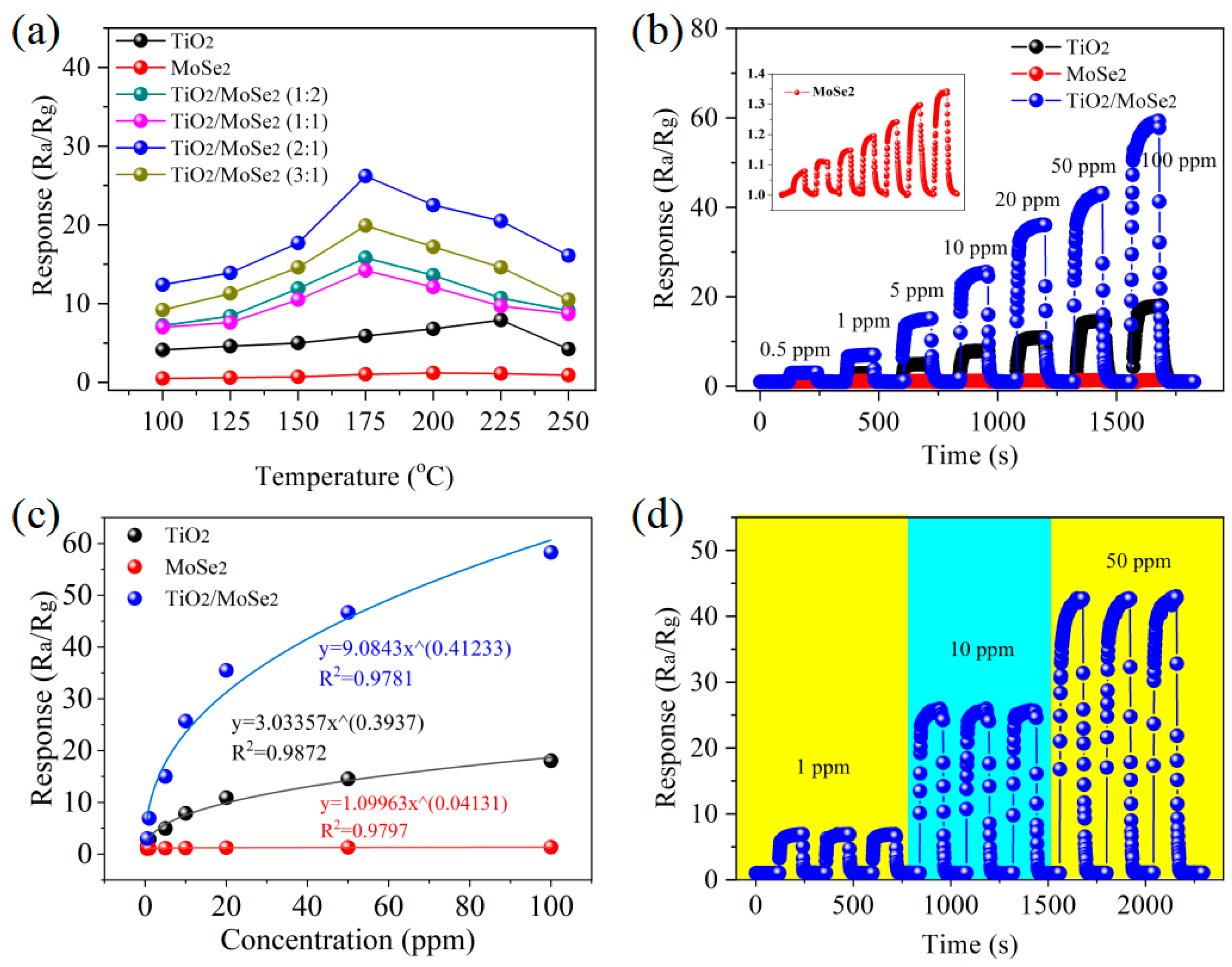
3.2. SO2 Sensing Properties
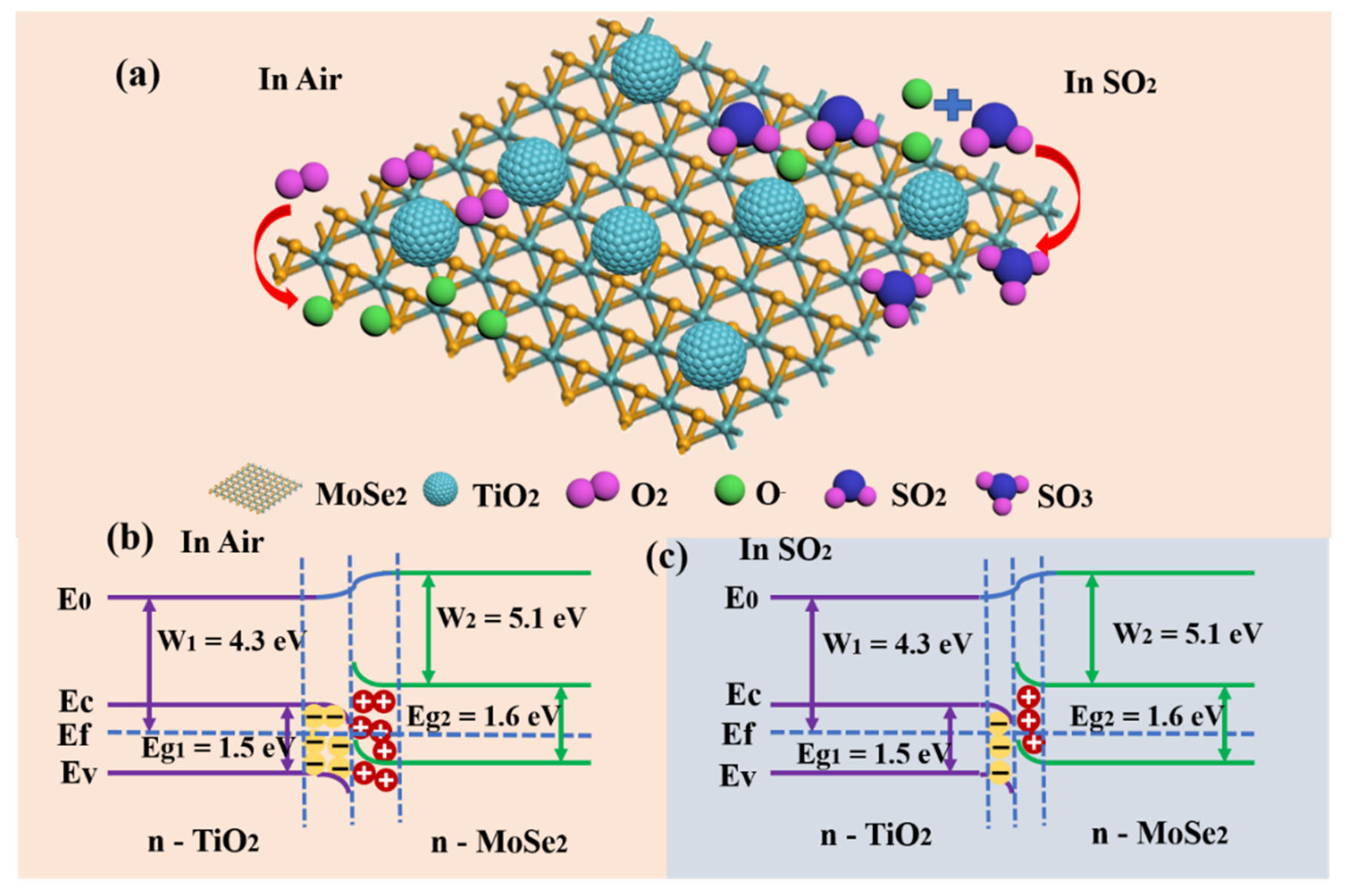
3.3. Sensing Mechanism of SO2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Velasco, R.P.; Jarosińska, D. Update of the WHO global air quality guidelines: Systematic reviews—An introduction. Environ. Int. 2022, 170, 107556. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.X.; Huang, B.Y.; Li, X.G. Graphene-Based heterostructure composite sensing materials for detection of nitrogen-containing. Adv. Funct. Mater. 2021, 31, 2104058. [Google Scholar] [CrossRef]
- Campbell-Lendrum, D.; Prüss-Ustün, A. Climate change, air pollution and noncommunicable diseases. Bull. World Health Organ. 2019, 97, 160–161. [Google Scholar] [CrossRef]
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef]
- Jia, X.; Qiao, P.; Wang, X.; Yan, M.; Chen, Y.; An, B.-L.; Hu, P.; Lu, B.; Xu, J.; Xue, Z.; et al. Building feedback-regulation system through atomic design for highly active SO2 sensing. Nano-Micro Lett. 2024, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, Y.; He, X.; Wang, F.; Cai, Q.; Shen, B. Insights into the adsorption of CO2, SO2 and NOx in flue gas by carbon materials: A critical review. Chem. Eng. J. 2024, 490, 151424. [Google Scholar] [CrossRef]
- Orellano, P.; Reynoso, J.; Quaranta, N. Short-term exposure to sulphur dioxide (SO2) and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2021, 150, 106434. [Google Scholar] [CrossRef] [PubMed]
- Gang, S.-Q.; Liu, Z.-Y.; Wu, S.-X.; Yang, S.; Wang, R.; Du, J.-L. A stable Zr(IV)-MOF for efficient removal of trace SO2 from flue gas in dry and humid conditions. J. Hazard. Mater. 2024, 470, 134180. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Chen, Y.; Zhang, Y.; Gao, X.G.; Xu, P.C.; Li, X.X.; Fang, J.H.; Wen, W.J. An integrated micro-chip with Ru/Al2O3/ZnO as sensing material for SO2 detection. Sens. Actuators B Chem. 2018, 262, 26–342. [Google Scholar] [CrossRef]
- Guan, L.; Chen, Z.; Liu, Y.; Wang, R.; Yan, K.; Xu, Z.; Li, J.; Liu, Z.; Li, J.; Liu, H. Engineering sulfur-rich MoS2 adsorbent with abundant unsaturated coordination sulfur sites for gaseous mercury capture from high-concentration SO2 smelting flue gas. Chem. Eng. J. 2024, 483, 149122. [Google Scholar] [CrossRef]
- Xu, H.Y.; Li, J.Z.; Li, P.D.; Shi, J.J.; Gao, X.W.; Luo, W.B. Highly efficient SO2 sensing by light-assisted Ag/PANI/SnO2 at room temperature and the sensing mechanism. ACS Appl. Mater. Interfaces 2021, 13, 49194–49205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, Y.; Wang, Z.; Liu, F.; Zhang, X. Switchable biomimetic nanochannels for on-demand SO2 detection by light-controlled photochromism. Nat. Commun. 2023, 14, 1901. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, C.; Zheng, C.; Fang, H.; Xu, D.; Zhao, H. Fabrication of a ppb-level NO2 gas sensor by sensitizing nanobundles assembled by In2O3 nanotubes with TiO2 quantum dots. Sens. Actuators B Chem. 2023, 387, 133833. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Tang, M.; Chen, Q.; Zhang, H.; Shao, X. Construction of ultra-fast hydrogen sensor for dissolved gas detection in oil-immersed transformers based on titanium dioxide quantum dots modified tin dioxide nanosheets. Sens. Actuators B Chem. 2023, 393, 134141. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Yang, Y.; Zhou, L.; Liu, Y.; Liu, W.; Sun, Y.; Guo, Y.; Ji, Y. Eco-friendly triboelectric nanogenerator for self-powering stacked In2O3 nanosheets/PPy nanoparticles-based NO2 gas sensor. Nano Energy 2024, 128, 109978. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Qiu, C.; Jia, P.; An, F.; Zhou, L.; Zhu, L.; Zhang, D. Polyaniline/ZnO heterostructure-based ammonia sensor self-powered by electrospinning of PTFE-PVDF/MXene piezo-tribo hybrid nanogenerator. Chem. Eng. J. 2024, 496, 154226. [Google Scholar] [CrossRef]
- Liu, C.; Zou, Q.-Q.; Liu, B.; Zhang, Y. A visible-light-assisted Pd/TiO2 gas sensor with carbon nanotubes electrodes for trace formaldehyde detection. Rare Met. 2024, 43, 257–266. [Google Scholar] [CrossRef]
- Tasyurek, L.B.; Isik, E.; Isik, I.; Kilinc, N. Enhancing the performance of TiO2 nanotube-based hydrogen sensors through crystal structure and metal electrode. Int. J. Hydrogen Energy 2024, 54, 678–690. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, D.; Fu, Y.; Feng, Q.; Mu, C.; Gao, K.; Ma, H.; Liu, M.; Zhang, M. Triazine-based multicomponent metallacages with tunable structures for SO2 selective capture and conversion. Aggregate 2023, 5, e408. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef]
- Zeng, S.M.; Zhang, Y.; Zhang, Y.; Li, Y.I.; Tang, C.G.; Li, K.; Sun, J.Y.; Deng, T. A novel room temperature SO2 gas sensor based on TiO2/rGO buried-gate FET. Microelectron. Eng. 2022, 263, 111841. [Google Scholar] [CrossRef]
- Thangamani, G.; Pasha, S. Titanium dioxide (TiO2) nanoparticles reinforced polyvinyl formal (PVF) nanocomposites as chemiresistive gas sensor for sulfur dioxide (SO2) monitoring. Chemosphere 2021, 25, 129960. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-K.; Zhao, J.; Li, K.; Zhao, J.; He, H.; Gao, Z.; Song, Y.-Y. Rational integration of SnMOF/SnO2 hybrid on TiO2 nanotube arrays: An effective strategy for accelerating formaldehyde sensing performance at room temperature. ACS Sens. 2023, 8, 4189–4197. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, T.; Li, F.; Li, D.; Yang, Y.; Yu, H.; Dong, X. Polyoxometalate as pivotal interface in SnO2@PW12@TiO2 coaxial nanofibers: From heterojunction design to photocatalytic and gas sensing applications. Sens. Actuators B Chem. 2023, 390, 133928. [Google Scholar] [CrossRef]
- Qiu, P.; Qin, Y.; Xia, Q. Ultrasensitive gas sensor developed from SnS/TiO2-based memristor for dilute methanol detection at room temperature. Sens. Actuators B Chem. 2023, 392, 134038. [Google Scholar] [CrossRef]
- Kumar, S.; Mirzaei, A.; Kumar, A.; Lee, M.H.; Ghahremani, Z.; Kim, T.-U.; Kim, J.-Y.; Kwoka, M.; Kumar, M.; Kim, S.S.; et al. Nanoparticles anchored strategy to develop 2D MoS2 and MoSe2 based room temperature chemiresistive gas sensors. Coord. Chem. Rev. 2024, 503, 215657. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, Y.; Yu, S.; Liu, X.; Zhang, D. Hydrogen sulfide gas sensing properties of metal organic framework-derived α-Fe2O3 hollow nanospheres decorated with MoSe2 nanoflowers. Sens. Actuators B Chem. 2021, 344, 130221. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhang, Y.; Zhang, D. Hydrogen sulfide gas sensing characteristics based on copper oxide/molybdenum diselenide heterojunction. J. Alloys Compd. 2023, 963, 171197. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.; Zhang, W.; Li, Q. Flexible humidity sensing and portable applications based on MoSe2 nanoflowers/copper tungstate nanoparticles. Sens. Actuators B Chem. 2020, 304, 127234. [Google Scholar] [CrossRef]
- Sun, X.; Lan, Q.; Geng, J.; Yu, M.; Li, Y.; Li, X.; Chen, L. Polyoxometalate as electron acceptor in dye/TiO2 films to accelerate room-temperature NO2 gas sensing. Sens. Actuators B Chem. 2023, 374, 132795. [Google Scholar] [CrossRef]
- Singh, S.; Deb, J.; Singh, J.V.; Sarkar, U.; Sharma, S. Highly selective ethyl mercaptan sensing using a MoSe2/SnO2 composite at room temperature. ACS Appl. Mater. Interfaces 2022, 14, 23916–23927. [Google Scholar] [CrossRef]
- Singh, S.; Deb, J.; Kumar, S.; Sarkar, U.; Sharma, S. Selective N, N-dimethylformamide vapor sensing using MoSe2/Multiwalled carbon nanotube composites at room temperature. ACS Appl. Nano Mater. 2022, 5, 3913–3924. [Google Scholar] [CrossRef]
- Freddi, S.; Marzuoli, C.; Pagliara, S.; Drera, G.; Sangaletti, L. Targeting biomarkers in the gas phase through a chemoresistive electronic nose based on graphene functionalized with metal phthalocyanines. RSC Adv. 2023, 13, 251–263. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, P.; Su, X.; Liu, Y.; Sun, Y.; Yang, H.; Fu, H.; Qu, X.; Liu, S.; Zheng, S. Synergistic Ni single atoms and oxygen vacancies on SnO2 nanorods toward promoting SO2 gas sensing. Sens. Actuators B Chem. 2022, 351, 130983. [Google Scholar] [CrossRef]
- He, X.; Ying, Z.; Wen, F.; Li, L.; Zheng, X.; Zheng, P.; Wang, G. MoS2-doped spherical SnO2 for SO2 sensing under UV light at room temperature. Mater. Sci. Semicond. Process. 2021, 134, 105997. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Thomson, B.; Yu, J.; Debnath, R.; Motayed, A.; Rao, M.V. Scalable metal oxide functionalized GaN nanowire for precise SO2 detection. Sens. Actuators B Chem. 2020, 318, 128223. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Xu, H. SnO2 recycled from tin slime for enhanced SO2 sensing properties by NiO surface decoration. Mater. Sci. Semicond. Process. 2020, 114, 105073. [Google Scholar] [CrossRef]
- Li, A.; Zhao, S.; Bai, J.; Gao, S.; Wei, D.; Shen, Y.; Yuan, Z.; Meng, F. Optimal construction and gas sensing properties of SnO2@TiO2 heterostructured nanorods. Sens. Actuators B Chem. 2022, 355, 131261. [Google Scholar] [CrossRef]
- Wu, K.D.; Chai, H.F.; Xu, K.C.; Debliquy, M.; Zhang, C. Effect of {010} crystal facets of Bi2MoO6 and 1D/2D heterostructures for conductometric room temperature NH3 gas sensors. Sens. Actuators B Chem. 2023, 376, 132983. [Google Scholar] [CrossRef]
- Kumar, U.; Yang, Y.-H.; Deng, Z.-Y.; Lee, M.-W.; Huang, W.-M.; Wu, C.-H. In situ growth of ternary metal sulfide based quantum dots to detect dual gas at extremely low levels with theoretical investigations. Sens. Actuators B Chem. 2022, 353, 131192. [Google Scholar] [CrossRef]
- Fan, G.; Nie, L.; Wang, H.; Zhang, L.; Chai, S.; Wang, A.; Guan, J.; Han, N.; Chen, Y. Ce doped SnO/SnO2 heterojunctions for highly formaldehyde gas sensing at low temperature. Sens. Actuators B Chem. 2022, 373, 132640. [Google Scholar] [CrossRef]

| Materials | Method | Temp. (°C) | Res/Rec Time (s) | Response (%) | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| Ni-SnO2 | Drop coating | 250 | 52 s/45 s | 5.2@10 ppm | 0.1 ppm | [32] |
| Ag-PANI/SnO2 | Drop coating | RT | 110 s/100 s | 20.1@50 ppm | 0.1 ppm | [34] |
| SnO2-MoS2 | Spin coating | RT | 217 s/633 s | 4.68@1 ppm | 0.5 ppm | [11] |
| ZnO/GaN | Sputtering | RT | 230 s/275 s | 12.1@10 ppm | 0.25 ppm | [35] |
| NiO-SnO2 | Drop coating | 240 | 25 s/35 s | 10.8@100 ppm | 0.5 ppm | [36] |
| TiO2-MoSe2 | Drop coating | 175 | 15 s/13 s | 59.3@100 ppm | 0.25 ppm | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Niu, C.; Wang, T.; Zhang, H.; Jiao, G.; Zhang, D. TiO2 Nanosphere/MoSe2 Nanosheet-Based Heterojunction Gas Sensor for High-Sensitivity Sulfur Dioxide Detection. Nanomaterials 2025, 15, 25. https://doi.org/10.3390/nano15010025
Zhou L, Niu C, Wang T, Zhang H, Jiao G, Zhang D. TiO2 Nanosphere/MoSe2 Nanosheet-Based Heterojunction Gas Sensor for High-Sensitivity Sulfur Dioxide Detection. Nanomaterials. 2025; 15(1):25. https://doi.org/10.3390/nano15010025
Chicago/Turabian StyleZhou, Lanjuan, Chang Niu, Tian Wang, Hao Zhang, Gongao Jiao, and Dongzhi Zhang. 2025. "TiO2 Nanosphere/MoSe2 Nanosheet-Based Heterojunction Gas Sensor for High-Sensitivity Sulfur Dioxide Detection" Nanomaterials 15, no. 1: 25. https://doi.org/10.3390/nano15010025
APA StyleZhou, L., Niu, C., Wang, T., Zhang, H., Jiao, G., & Zhang, D. (2025). TiO2 Nanosphere/MoSe2 Nanosheet-Based Heterojunction Gas Sensor for High-Sensitivity Sulfur Dioxide Detection. Nanomaterials, 15(1), 25. https://doi.org/10.3390/nano15010025






