New Strategy for Microbial Corrosion Protection: Photocatalytic Antimicrobial Quantum Dots
Abstract
1. Introduction
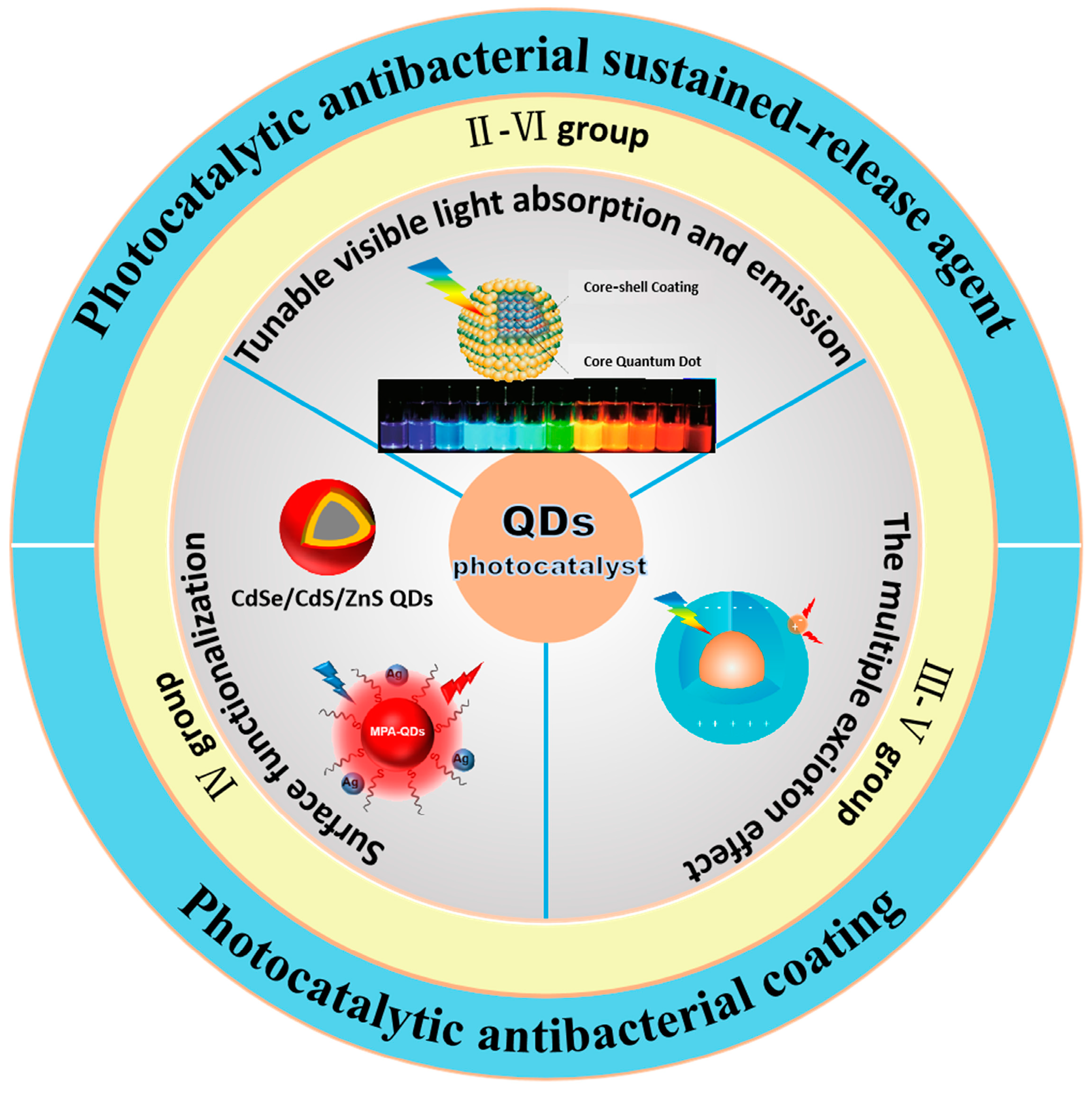
2. Classification and Characteristics of Quantum Dots
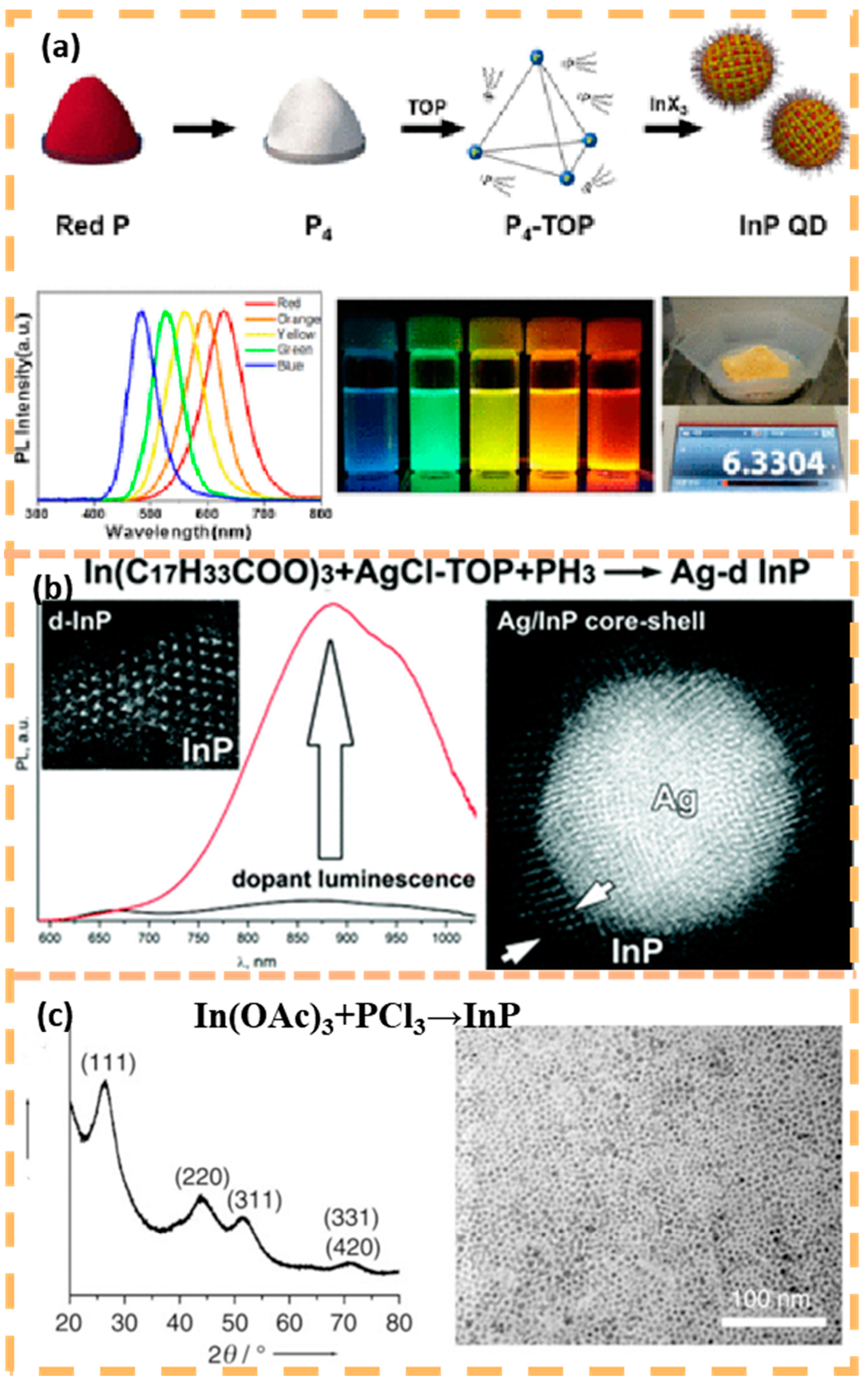
2.1. Group II–VI Quantum Dot Photocatalysts
2.2. Group IV Quantum Dot Photocatalysts
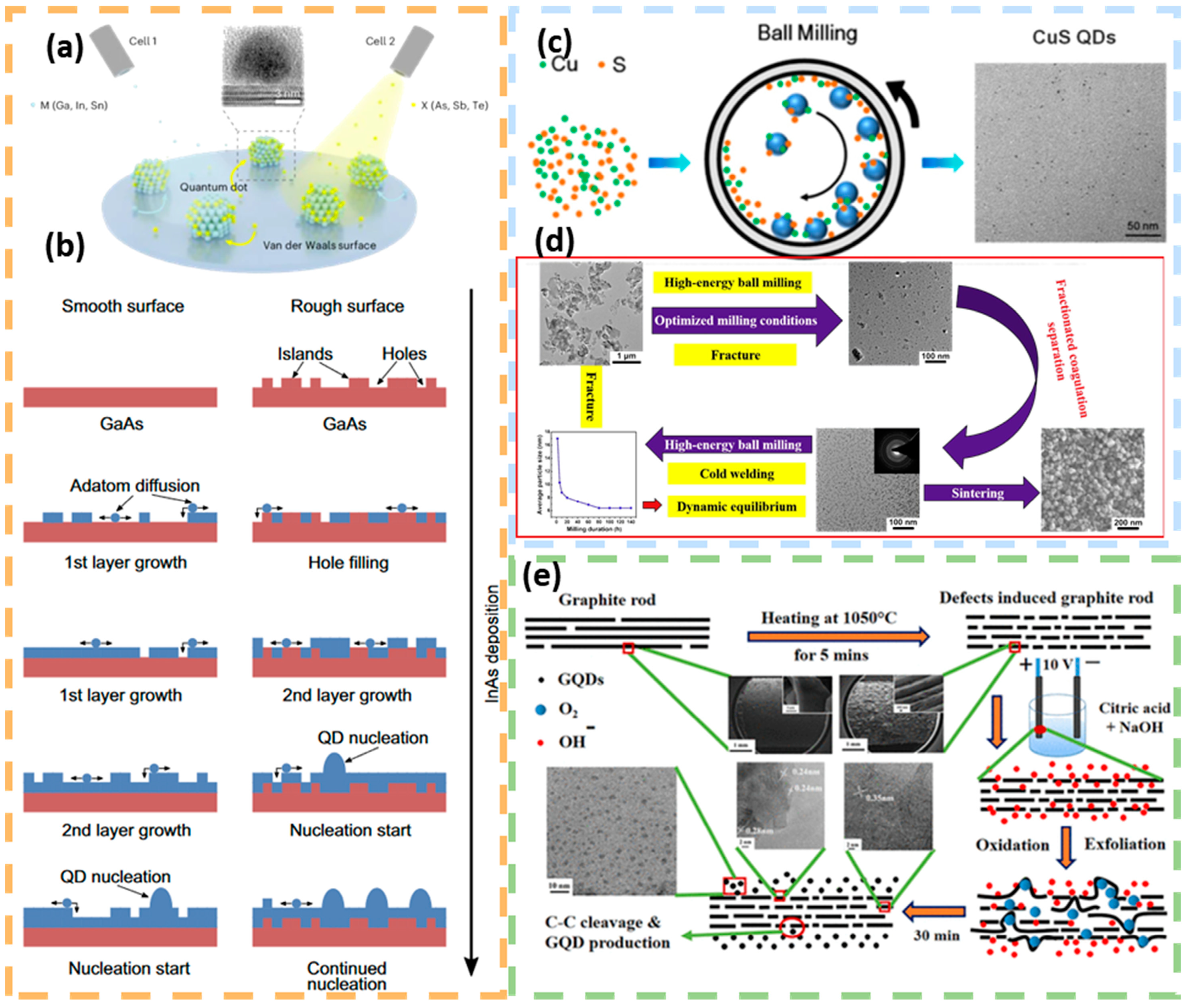
3. Synthesis and Preparation of Quantum Dots
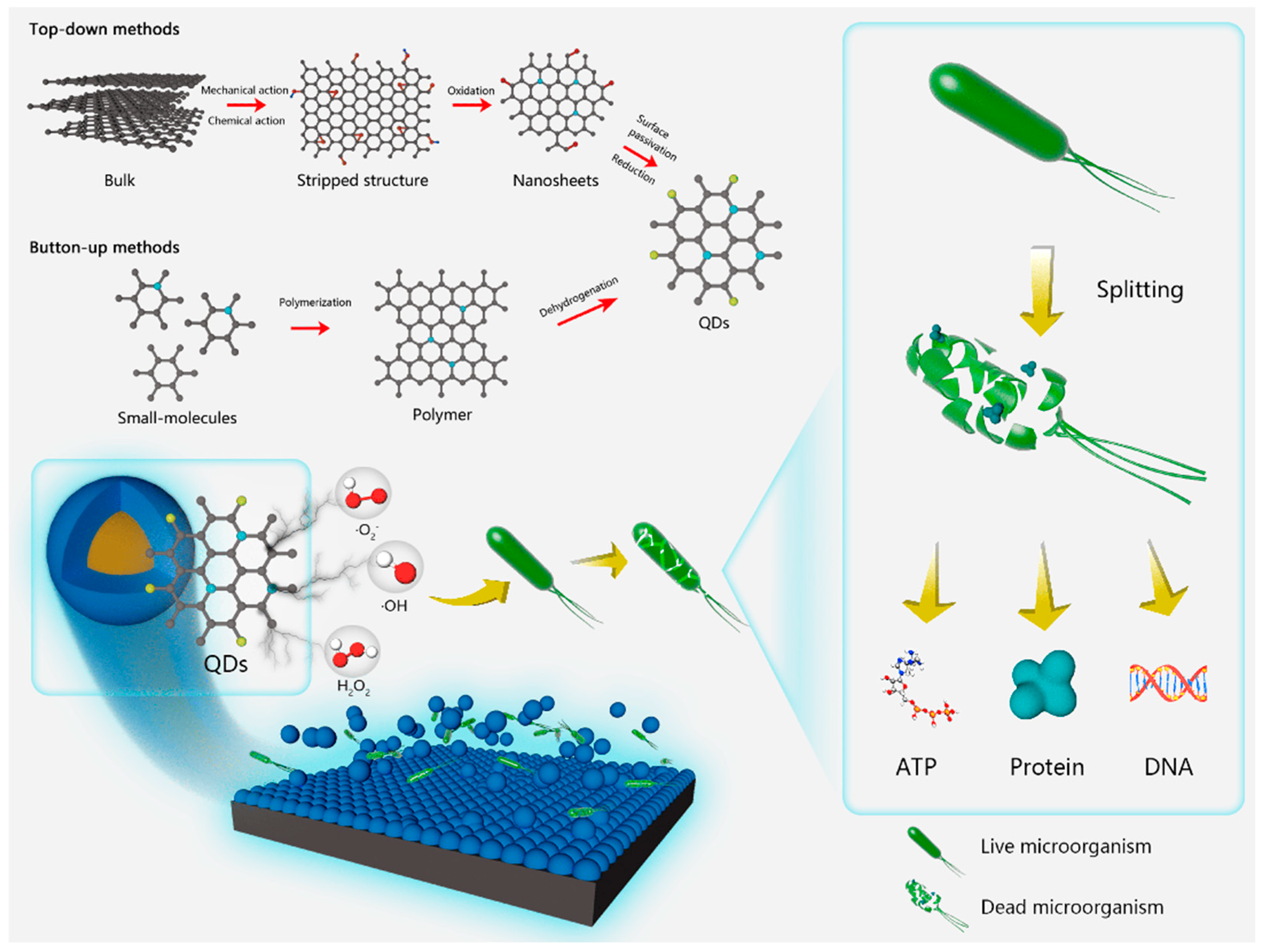
3.1. Top-Down Methods
3.2. Bottom-Up Methods

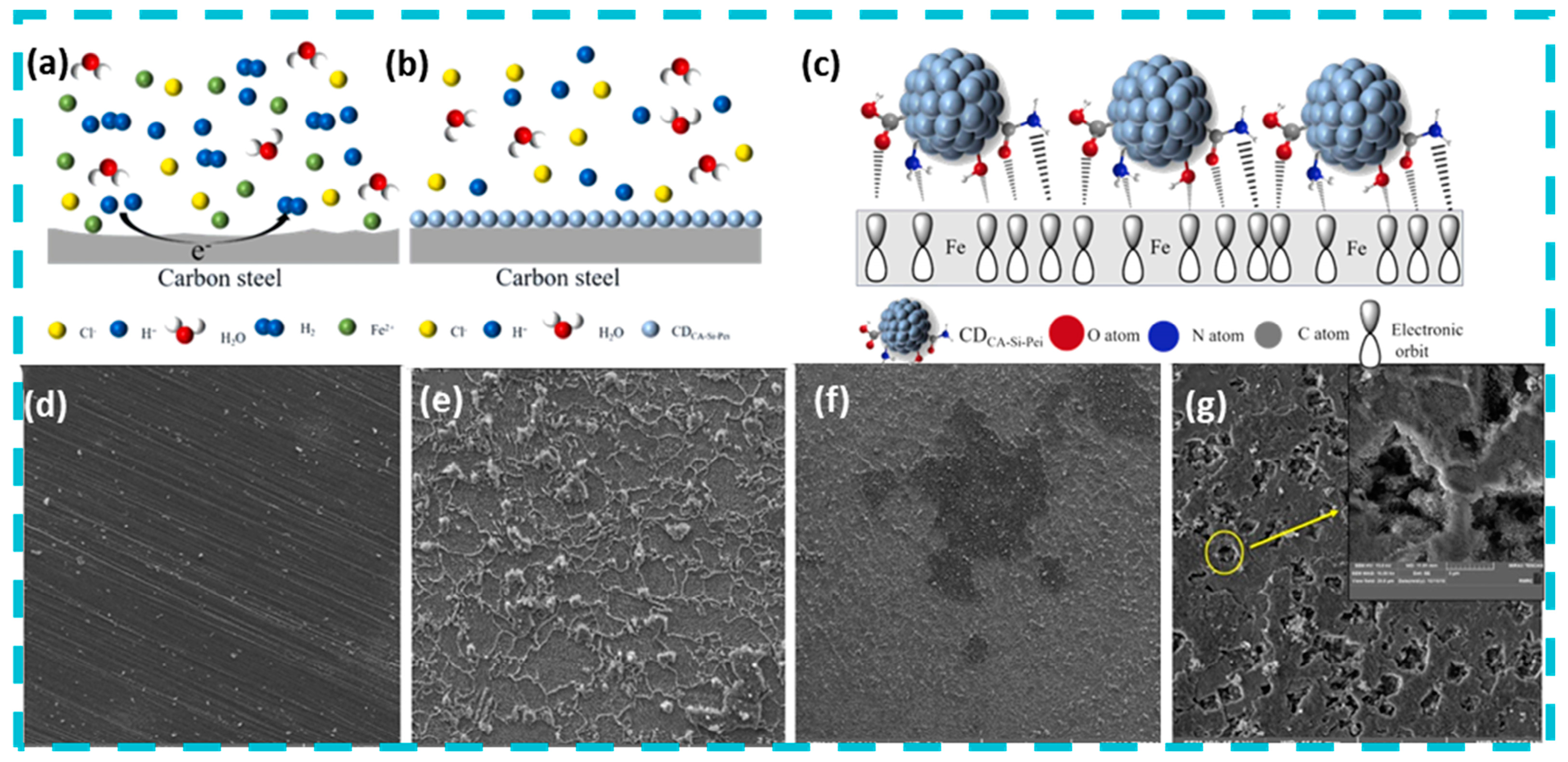
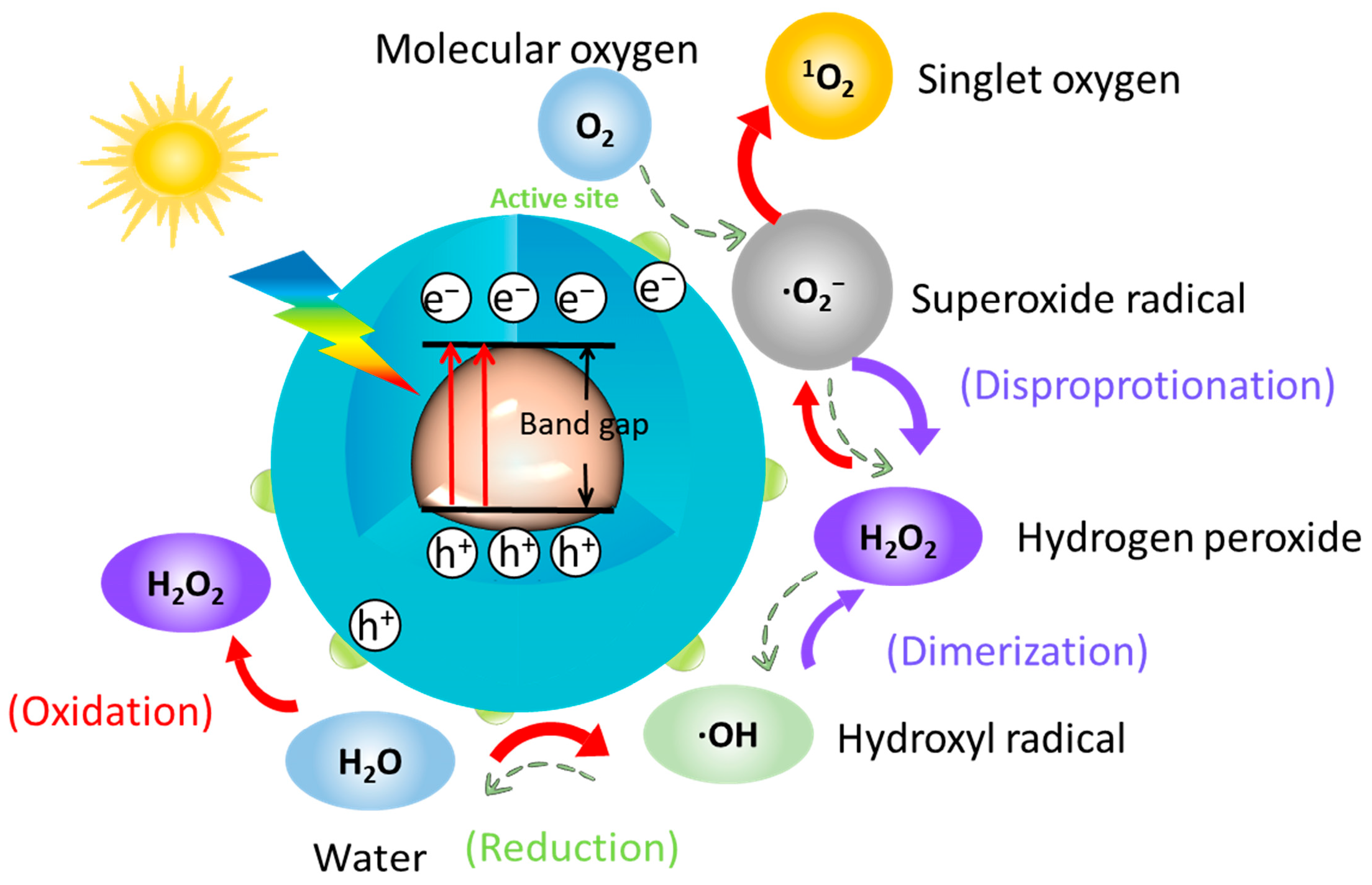
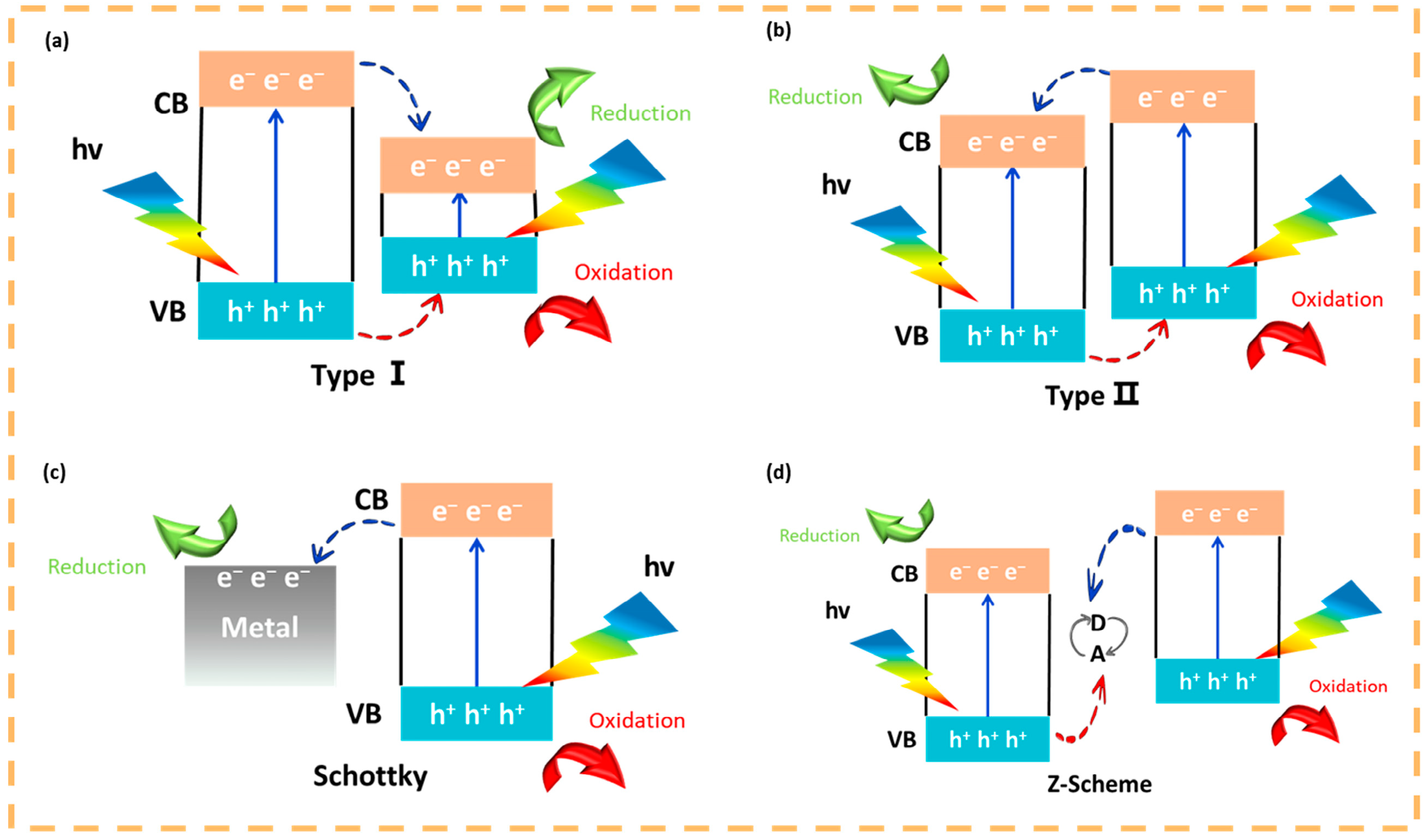
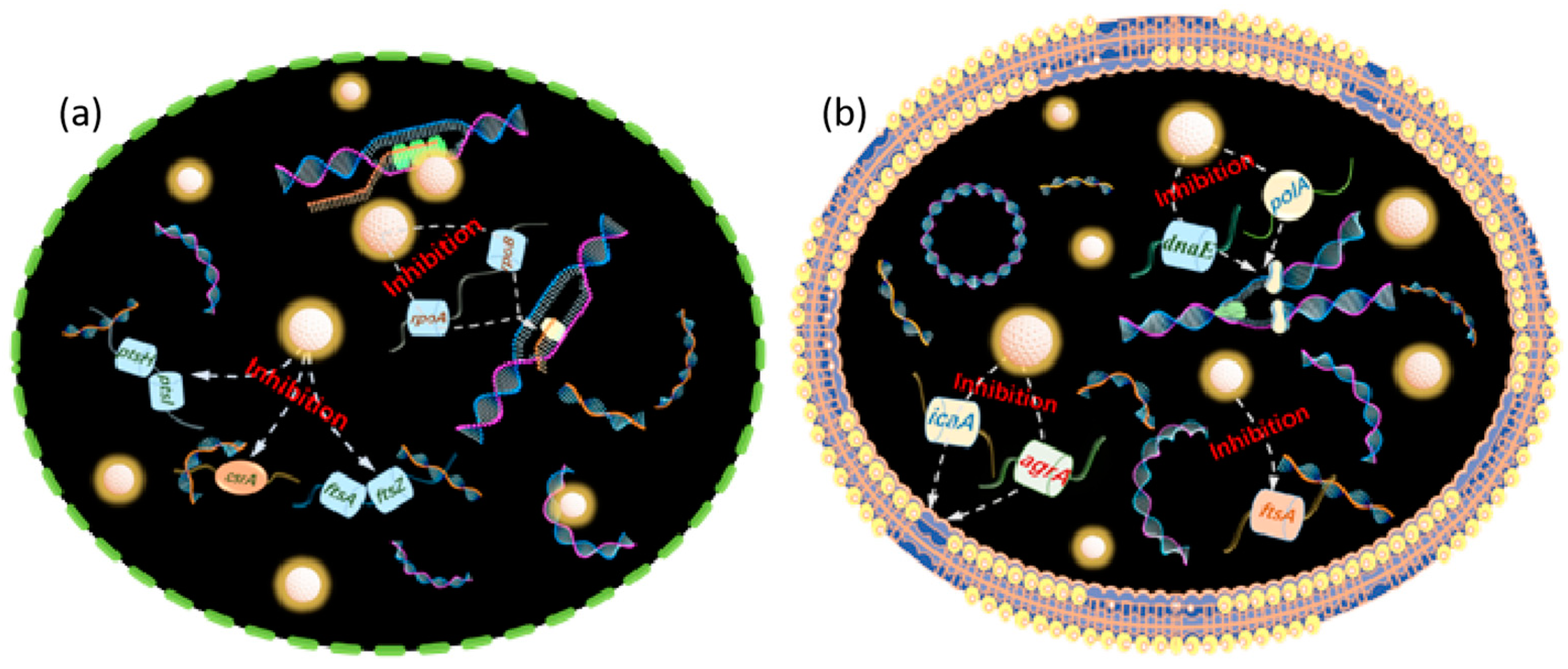
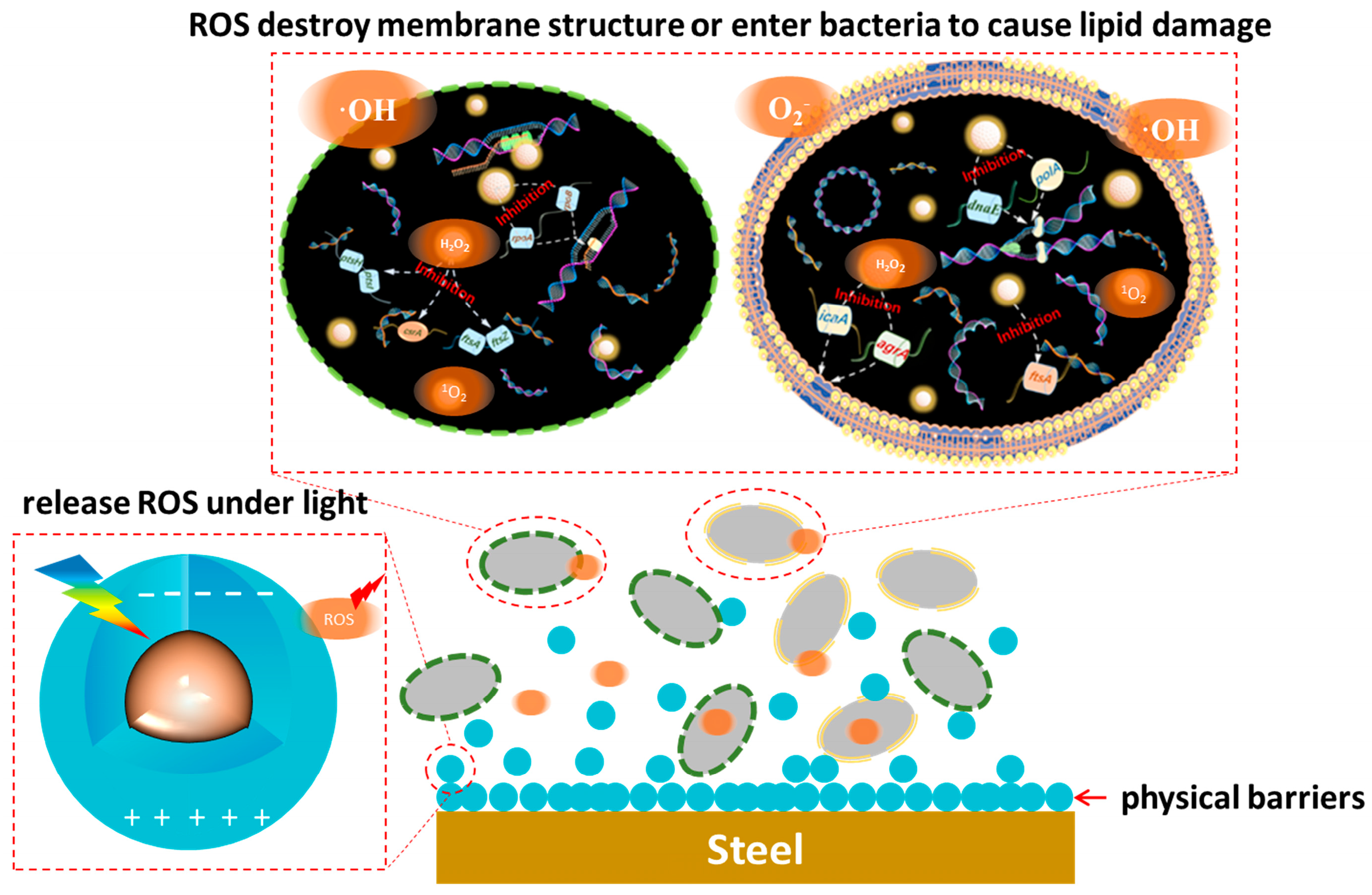
4. Mechanism of Microbial Corrosion Resistance of Photocatalytic Quantum Dots
5. Challenges and Future Prospects of Quantum Dots in Photocatalytic Antimicrobial Protection
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Du, K.; Yin, H.; Wang, D. Corrosion and Protection of Metallic Materials in Molten Carbonates for Concentrating Solar Power and Molten Carbonate Electrolysis Applications. Corros. Commun. 2023, 11, 58–71. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cheng, G.; Zhu, W. Localized Corrosion Behavior of 316L Stainless Steel in the Presence of Sulfate-Reducing and Iron-Oxidising Bacteria. Mater. Sci. Eng. A 2007, 443, 235–241. [Google Scholar] [CrossRef]
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; An Stepec, B.A.; Wade, S.A. Microbiologically Influenced Corrosion—More than Just Microorganisms. FEMS Microbiol. Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, G.; Zhou, Y.; Li, W. Behaviors and Mechanisms of Microbially-Induced Corrosion in Metal-Based Water Supply Pipelines: A Review. Sci. Total Environ. 2023, 895, 165034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, Y.; Cao, S.; Jiang, J.; Wu, C.; Zhao, K. Enhanced Anti-Microbial Corrosion of Nano-CuO-Loaded Ni Coatings on Pipeline Steels in Simulation Environment of Natural Gas Transportation Pipeline. Ceram. Int. 2023, 49, 5543–5549. [Google Scholar] [CrossRef]
- Njoku, C.N.; Enendu, B.N.; Okechukwu, S.J.; Igboko, N.; Anyikwa, S.O.; Ikeuba, A.I.; Onyeachu, I.B.; Etim, I.-I.N.; Njoku, D.I. Review on Anti-Corrosion Properties of Expired Antihypertensive Drugs as Benign Corrosion Inhibitors for Metallic Materials in Various Environments. Results Eng. 2023, 18, 101183. [Google Scholar] [CrossRef]
- Ayyamperumal, R.; Kumari, K.; Gandhi, M.S.; Huang, X.; Chengjun, Z.; Nazir, N.; Li, F.; Das, P. Environmental Hazard Assessment and Metal Contamination in Coastal Sediments. Chemosphere 2023, 338, 139434. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.V.P.; Sudhani, H.P.K.; Habila, M.A.; Rosaiah, P.; Shim, J. SnO2 Quantum Dot-Decorated g-C3N4 Ultrathin Nanosheets: A Dual-Function Photocatalyst for Pollutant Degradation and Hydrogen Evolution. Catalysts 2024, 14, 824. [Google Scholar] [CrossRef]
- Tian, Z.; Li, S.; Chen, Y.; Li, L.; An, Z.; Zhang, Y.; Tong, A.; Zhang, H.; Liu, Z.; An, B. Self-Healing Coating with a Controllable Release of Corrosion Inhibitors by Using Multifunctional Zinc Oxide Quantum Dots as Valves. ACS Appl. Mater. Interfaces 2022, 14, 47188–47197. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, J.; Zhao, P.; Guo, S.; Han, S. One-Step Synthesis of Water-Soluble CdS Quantum Dots for Silver-Ion Detection. ACS Omega 2021, 6, 7139–7146. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Z.; Wang, J.; Li, Y.; Hou, X. Ultrasensitive Fluorescence Detection of Ascorbic Acid Using Silver Ion-Modulated High-Quality CdSe/CdS/ ZnS Quantum Dots. ACS Omega 2024, 9, 27127–27136. [Google Scholar] [CrossRef]
- Kroupa, D.M.; Hughes, B.K.; Miller, E.M.; Moore, D.T.; Anderson, N.C.; Chernomordik, B.D.; Nozik, A.J.; Beard, M.C. Synthesis and Spectroscopy of Silver-Doped PbSe Quantum Dots. J. Am. Chem. Soc. 2017, 139, 10382–10394. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence Mechanism in Graphene Quantum Dots: Quantum Confinement Effect and Surface/Edge State. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Bai, J.; Zhao, Z.; Fu, M.; Hu, X.; He, Y. Defected Mesoporous Carbon Nitride with Quantum Confinement Effect for NO Purification. J. Alloys Compd. 2023, 934, 167825. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, B.; Yang, H.; Xu, R.; Wang, X.; Xiao, M.; Cui, Y.; Zhang, J. Quantum-Confined Stark Effect in the Ensemble of Phase-Pure CdSe/CdS Quantum Dots. Nanoscale 2019, 11, 12619–12625. [Google Scholar] [CrossRef]
- Anandh Jesuraj, S.; Devadason, S.; Melvin David Kumar, M. Effect of Quantum Confinement in CdSe/Se Multilayer Thin Films Prepared by PVD Technique. Mater. Sci. Semicond. Process. 2017, 64, 109–114. [Google Scholar] [CrossRef]
- Le, P.; Vaidya, R.; Smith, L.D.; Han, Z.; Zahid, M.U.; Winter, J.; Sarkar, S.; Chung, H.J.; Perez-Pinera, P.; Selvin, P.R.; et al. Optimizing Quantum Dot Probe Size for Single-Receptor Imaging. ACS Nano 2020, 14, 8343–8358. [Google Scholar] [CrossRef]
- Joo, S.; Jeong, D.W.; Lee, C.; Kim, B.S.; Park, H.; Kim, W. Room-temperature processing of CdSe quantum dots with tunable sizes. J. Appl. Phys. 2017, 121, 223102. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Liu, Z.; Li, J. Research Progress in the Synthesis and Biological Application of Quantum Dots. New J. Chem. 2022, 46, 20515–20539. [Google Scholar] [CrossRef]
- Huang, C.Y.; Li, H.; Wu, Y.; Lin, C.H.; Guan, X.; Hu, L.; Kim, J.; Zhu, X.; Zeng, H.; Wu, T. Inorganic Halide Perovskite Quantum Dots: A Versatile Nanomaterial Platform for Electronic Applications. Nano-Micro Lett. 2023, 15, 16. [Google Scholar] [CrossRef]
- Dey, S.; Zhao, J. Plasmonic Effect on Exciton and Multiexciton Emission of Single Quantum Dots. J. Phys. Chem. Lett 2016, 7, 2921–2929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Zheng, W. Quantum Dots Compete at the Acme of MXene Family for the Optimal Catalysis. Nano-Micro Lett. 2022, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, L.; Rehberg, M.; Stoeger, T.; Zhang, J.; Chen, S. Applications and Immunological Effects of Quantum Dots on Respiratory System. Front. Immunol. 2022, 12, 795232. [Google Scholar] [CrossRef]
- Banerjee, S.; Zhang, P. Review of Recent Studies on Nanoscale Electrical Junctions and Contacts: Quantum Tunneling, Current Crowding, and Interface Engineering. J. Vac. Sci. Technol. A 2022, 40, 030802. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Zhao, J.; Wang, Y.; Zheng, J.; Liu, J.; Hong, W.; Tian, Z.-Q. The Fabrication, Characterization and Functionalization in Molecular Electronics. Int. J. Extrem. Manuf. 2022, 4, 022003. [Google Scholar] [CrossRef]
- Koshida, N.; Matsumoto, N. Fabrication and Quantum Properties of Nanostructured Silicon. Mater. Sci. Eng. R Rep. 2003, 40, 169–205. [Google Scholar] [CrossRef]
- Su, H.; Wang, W.; Shi, R.; Tang, H.; Sun, L.; Wang, L.; Liu, Q.; Zhang, T. Recent Advances in Quantum Dot Catalysts for Hydrogen Evolution: Synthesis, Characterization, and Photocatalytic Application. Carbon Energy 2023, 5, e280. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A Review on Bidirectional Analogies between the Photocatalysis and Antibacterial Properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, W.; Zheng, M.; Zhao, Y.; Wang, Y.; Chu, Y.; Du, S.; Shi, Z.; Gu, Q.; Chen, J. Ultraefficient OG-Mediated Photodynamic Inactivation Mechanism for Ablation of Bacteria and Biofilms in Water Augmented by Potassium Iodide under Blue Light Irradiation. J. Agric. Food Chem. 2023, 71, 13672–13687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, P.; Zhang, H.; Dong, S.; Huang, T. Preparation and Performance of a New Silylated Functional Carbon Quantum Dots Corrosion Inhibitor. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132164. [Google Scholar] [CrossRef]
- Dong, L.; Ma, Y.; Jin, X.; Feng, L.; Zhu, H.; Hu, Z.; Ma, X. High-Efficiency Corrosion Inhibitor of Biomass-Derived High-Yield Carbon Quantum Dots for Q235 Carbon Steel in 1 M HCl Solution. ACS Omega 2023, 8, 46934–46945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xiong, L.; He, Z.; Pu, J.; Guo, L. Synthesis and Structure of Water-Soluble Sb Quantum Dots and Enhanced Corrosion Inhibition Performance and Mechanisms. Inorg. Chem. 2021, 60, 16346–16356. [Google Scholar] [CrossRef]
- Kalajahi, S.T.; Rasekh, B.; Yazdian, F.; Neshati, J.; Taghavi, L. Green Mitigation of Microbial Corrosion by Copper Nanoparticles Doped Carbon Quantum Dots Nanohybrid. Environ. Sci. Pollut. Res. 2020, 27, 40537–40551. [Google Scholar] [CrossRef]
- Ekimov, A.; Onushchehko, A. Quantum Size Effect in Three-Dimensional Microscopic Semiconductor Crystals. ZhETF Pis ma Redaktsiiu 1981, 34, 363. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-Electron and Electron-hole Interactions in Small Semiconductor Crystallites: The Size Dependence of the Lowest Excited Electronic State. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Kaur, R.; Rana, A.; Singh, R.K.; Chhabra, V.A.; Kim, K.-H.; Deep, A. Efficient Photocatalytic and Photovoltaic Applications with Nanocomposites between CdTe QDs and an NTU-9 MOF. RSC Adv. 2017, 7, 29015–29024. [Google Scholar] [CrossRef]
- Lee, W.; Son, H.J.; Lee, D.-K.; Kim, B.; Kim, H.; Kim, K.; Ko, M.J. Suppression of Photocorrosion in CdS/CdSe Quantum Dot-Sensitised Solar Cells. Formation of a Thin Polymer Layer on the Photoelectrode Surface. Synth. Met. 2013, 165, 60–63. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Ye, X.; Guo, Z.; Liu, Y.; Song, Q.; Zheng, S.; Chen, X.; Wang, S.; Yang, S. Visible Photoactivity and Antiphotocorrosion Performance of CdS Photocatalysts by the Hybridisation of N-Substituted Carboxyl Group Polyaniline. Appl. Surf. Sci. 2019, 480, 557–564. [Google Scholar] [CrossRef]
- Castelli, A.; Dhanabalan, B.; Polovitsyn, A.; Caligiuri, V.; Di Stasio, F.; Scarpellini, A.; Brescia, R.; Palei, M.; Martín-García, B.; Prato, M.; et al. Core/Shell CdSe/CdS Bone-Shaped Nanocrystals with a Thick and Anisotropic Shell as Optical Emitters. Adv. Opt. Mater. 2020, 8, 1901463. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Torres, E.T.; Aoki, R.M.; de Jesus, J.P.A.; Duarte, J.L.; Lourenço, S.A.; da Silva, M.A.T. Synthesis and Characterisation of CdS/ZnS Heterostructures to Improve the Optical Properties of CdS Quantum Dots. J. Lumin. 2023, 257, 119706. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Meyer, J.R.; Ram-Mohan, L.R. Band Parameters for III–V Compound Semiconductors and Their Alloys. J. Appl. Phys. 2001, 89, 5815–5875. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, J.H.; Choi, H.; Kim, Y.-H.; Bae, W.K.; Jeong, S. III-V Colloidal Nanocrystals: Control of Covalent Surfaces. Chem. Sci. 2020, 11, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-B.; Bai, Y.-R.; Li, P.; Liu, W.-B.; He, H.; He, C.-H.; Zhao, X.-H. First-principles calculations of the migration mechanism of point defects in InP. Acta Phys. Sin. 2024, 73, 183101–183109. [Google Scholar] [CrossRef]
- Jalali, H.B.; Sadeghi, S.; Dogru Yuksel, I.B.; Onal, A.; Nizamoglu, S. Past, Present and Future of Indium Phosphide Quantum Dots. Nano Res. 2022, 15, 4468–4489. [Google Scholar] [CrossRef]
- Xie, R.; Battaglia, D.; Peng, X. Colloidal InP Nanocrystals as Efficient Emitters Covering Blue to Near-Infrared. J. Am. Chem. Soc. 2007, 129, 15432–15433. [Google Scholar] [CrossRef] [PubMed]
- Tessier, M.D.; Dupont, D.; De Nolf, K.; De Roo, J.; Hens, Z. Economic and Size-Tunable Synthesis of InP/ZnE (E = S, Se) Colloidal Quantum Dots. Chem. Mater. 2015, 27, 4893–4898. [Google Scholar] [CrossRef]
- Bang, E.; Choi, Y.; Cho, J.; Suh, Y.-H.; Ban, H.W.; Son, J.S.; Park, J. Large-Scale Synthesis of Highly Luminescent InP@ZnS Quantum Dots Using Elemental Phosphorus Precursor. Chem. Mater. 2017, 29, 4236–4243. [Google Scholar] [CrossRef]
- Vinokurov, A.; Chernysheva, G.; Mordvinova, N.; Dorofeev, S. Optical Properties and Structure of Ag-Doped InP Quantum Dots Prepared by a Phosphine Synthetic Route. Dalton Trans. 2018, 47, 12414–12419. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Shan, Y.; Cao, S.; Hu, Y.; Li, Q.; Zeng, R.; Zou, B.; Wang, Y.; Zhao, J. Inorganic Solid Phosphorus Precursor of Sodium Phosphaethynolate for Synthesis of Highly Luminescent InP-Based Quantum Dots. ACS Energy Lett. 2021, 6, 2697–2703. [Google Scholar] [CrossRef]
- Liu, Z.; Kumbhar, A.; Xu, D.; Zhang, J.; Sun, Z.; Fang, J. Coreduction Colloidal Synthesis of III-V Nanocrystals: The Case of InP. Angew. Chem. Int. Ed. Engl. 2008, 47, 3540–3542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, R.; Kuang, Y.; Bian, Y.; Chen, F.; Shen, H.; Chi, Z.; Ran, X.; Guo, L. Suppressed Auger Recombination and Enhanced Emission of InP/ZnSe/ZnS Quantum Dots through Inner Shell Manipulation. Nanoscale 2023, 15, 18920–18927. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, Y.; Kaledin, A.L.; He, S.; Jin, T.; McBride, J.R.; Lian, T. Surface Passivation Extends Single and Biexciton Lifetimes of InP Quantum Dots. Chem. Sci. 2020, 11, 5779–5789. [Google Scholar] [CrossRef]
- Pons, T.; Pic, E.; Lequeux, N.; Cassette, E.; Bezdetnaya, L.; Guillemin, F.; Marchal, F.; Dubertret, B. Cadmium-Free CuInS2/ZnS Quantum Dots for Sentinel Lymph Node Imaging with Reduced Toxicity. ACS Nano 2010, 4, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, S.; Wang, F.; Wu, Q.; Zhao, D.; Yang, X. A Layer-by-Layer Growth Strategy for Large-Size InP/ZnSe/ZnS Core–Shell Quantum Dots Enabling High-Efficiency Light-Emitting Diodes. Chem. Mater. 2018, 30, 8002–8007. [Google Scholar] [CrossRef]
- Rafipoor, M.; Tornatzky, H.; Dupont, D.; Maultzsch, J.; Tessier, M.D.; Hens, Z.; Lange, H. Strain in InP/ZnSe, S Core/Shell Quantum Dots from Lattice Mismatch and Shell Thickness—Material Stiffness Influence. J. Chem. Phys. 2019, 151, 154704. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, H.; Yang, H.; Shen, D.; Hu, H.; Dou, M. Formation Mechanism of Lignin-Derived Carbon Quantum Dots: From Chemical Structures to Fluorescent Behaviors. Bioresour. Technol. 2024, 413, 131490. [Google Scholar] [CrossRef]
- Li, J.-R.; Li, J.; Tang, X.-M.; Chu, W.-X.; An, B.-L.; Zhang, J.-M.; Wang, X.-H.; Bai, Y.-L.; Xu, J. Synthesis and Luminescence Properties of Carbon Quantum Dots with Core@shell Structures. Opt. Mater. 2024, 156, 115975. [Google Scholar] [CrossRef]
- Wang, L.; Weng, S.; Su, S.; Wang, W. Progress on the Luminescence Mechanism and Application of Carbon Quantum Dots Based on Biomass Synthesis. RSC Adv. 2023, 13, 19173–19194. [Google Scholar] [CrossRef]
- Liu, X.; Song, H.; Miao, G.; Jiang, H.; Cao, L.; Sun, X.; Li, D.; Chen, Y.; Li, Z. Influence of Buffer Layer Thickness and Epilayer’s Growth Temperature on Crystalline Quality of InAs0.6P0.4/InP Grown by LP-MOCVD. Solid State Commun. 2011, 151, 904–907. [Google Scholar] [CrossRef]
- Bart, N.; Dangel, C.; Zajac, P.; Spitzer, N.; Ritzmann, J.; Schmidt, M.; Babin, H.G.; Schott, R.; Valentin, S.R.; Scholz, S.; et al. Wafer-Scale Epitaxial Modulation of Quantum Dot Density. Nat. Commun. 2022, 13, 1633. [Google Scholar] [CrossRef]
- Xin, K.; Li, L.; Zhou, Z.; Zhang, C.; Yang, J.; Deng, H.-X.; Zhang, J.; Liu, J.; Liu, K.; Liu, C.; et al. Epitaxial Growth of Quantum Dots on van Der Waals Surfaces. Nat. Synth. 2024, 3, 1176–1183. [Google Scholar] [CrossRef]
- Li, S.; Ge, Z.-H.; Zhang, B.-P.; Yao, Y.; Wang, H.-C.; Yang, J.; Li, Y.; Gao, C.; Lin, Y.-H. Mechanochemically Synthesized Sub-5 Nm Sized CuS Quantum Dots with High Visible-Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2016, 384, 272–278. [Google Scholar] [CrossRef]
- Li, L.; Pu, S.; Liu, Y.; Zhao, L.; Ma, J.; Li, J. High-Purity Disperse α-Al2O3 Nanoparticles Synthesized by High-Energy Ball Milling. Adv. Powder Technol. 2018, 29, 2194–2203. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Electrochemical Method To Prepare Graphene Quantum Dots and Graphene Oxide Quantum Dots. ACS Omega 2017, 2, 8343–8353. [Google Scholar] [CrossRef]
- Silva, S.E.; Freitas, D.V.; Navarro, M.; Azevedo, W.M. Green Bottom-up Synthesis of CdTe-MPA QDs Induced by Laser Ablation. Mater. Today Commun. 2022, 32, 104141. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Sun, Y.-P.; Zhou, B.; Lin, Y.; et al. Quantum-Sized Carbon Dots for Bright and Colourful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-Step Synthesis of Fluorescent Carbon Nanoparticles by Laser Irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Jalali, H.B.; Trizio, L.D.; Manna, L.; Stasio, F.D. Indium Arsenide Quantum Dots: An Alternative to Lead-Based Infrared Emitting Nanomaterials. Chem. Soc. Rev. 2022, 51, 9861–9881. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Kim, T.; Park, S.; Jeong, S. Diffusion Dynamics Controlled Colloidal Synthesis of Highly Monodisperse InAs Nanocrystals. Nat. Commun. 2021, 12, 3013. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Pu, C. Size Focusing of Colloidal Quantum Dots under High Monomer Concentration. Nano Res. 2022, 15, 7622–7630. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Liu, Y. A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles. Eur. J. Inorg. Chem. 2010, 28, 4411–4414. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, R.; Zhang, C.; Zhang, K.; Liu, W.; Shao, X.; Xue, Q.; Yue, Q. Fluorescence and Photocatalytic Activity of Metal-Free Nitrogen-Doped Carbon Quantum Dots with Varying Nitrogen Contents. Appl. Surf. Sci. 2020, 531, 147344. [Google Scholar] [CrossRef]
- Hua, J.; Hua, P.; Qin, K. Highly Fluorescent N, F Co-Doped Carbon Dots with Tunable Light Emission for Multicolor Bio-Labelling and Antibacterial Applications. J. Hazard. Mater. 2023, 459, 132331. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Shao, J.; Jiang, C.; Zhang, F.; Lin, J.; Zhang, H.; Li, J.; Huang, P. Polydopamine-Functionalized Black Phosphorus Quantum Dots for Cancer Theranostics. Appl. Mater. Today 2019, 15, 297–304. [Google Scholar] [CrossRef]
- Wang, J.; Su, X.; Zhao, P.; Gao, D.; Chen, R.; Wang, L. Cancer Photothermal Therapy Based on near Infrared Fluorescent CdSeTe/ZnS Quantum Dots. Anal. Methods 2021, 13, 5509–5515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feng, L.; Zhao, R.; Liu, B.; Yang, P. Review of MXene-Derived Quantum Dots for Cancer Theranostics. ACS Appl. Nano Mater. 2024, 7, 2546–2574. [Google Scholar] [CrossRef]
- Vokhmintcev, K.V.; Samokhvalov, P.S.; Nabiev, I. Charge Transfer and Separation in Photoexcited Quantum Dot-Based Systems. Nano Today 2016, 11, 189–211. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ye, Z.; Cui, J.; Peng, X. Hot-Electron-Induced Photochemical Properties of CdSe/ZnSe Core/Shell Quantum Dots under an Ambient Environment. J. Am. Chem. Soc. 2023, 145, 13938–13949. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.M.; Bao, H.; Qiao, Y.; Toh, Y.; Yang, X. Mechanism of Antimicrobial Activity of CdTe Quantum Dots. Langmuir 2008, 24, 5445–5452. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum Dots: An Overview of Synthesis, Properties, and Applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar] [CrossRef]
- Jin, T.; Lian, T. Trap State Mediated Triplet Energy Transfer from CdSe Quantum Dots to Molecular Acceptors. J. Chem. Phys. 2020, 153, 074703. [Google Scholar] [CrossRef]
- Xiao, D.; Wu, C.; Liang, B.; Jiang, S.; Ma, J.; Li, Y. Outstanding ROS Generation Ability and the Mechanism of MXene Quantum Dots. J. Mater. Chem. A 2024, 12, 31655–31661. [Google Scholar] [CrossRef]
- Bao, H.; Lian, Y.; Qiao, Z.; Zhang, S.; Meng, X.; Ma, K.; Zhu, D.; Zhao, J.; Zhang, H. N-Doped Carbon Quantum Dots as Corrosion Inhibitor for Ultra-High Voltage Etched Al Foil. Surf. Coat. Technol. 2024, 476, 130240. [Google Scholar] [CrossRef]
- Zhang, J.; An, X.L.; Li, X.; Liao, X.; Nie, Y.; Fan, Z. Enhanced Antibacterial Properties of the Bracket under Natural Light via Decoration with ZnO/Carbon Quantum Dots Composite Coating. Chem. Phys. Lett. 2018, 706, 702–707. [Google Scholar] [CrossRef]
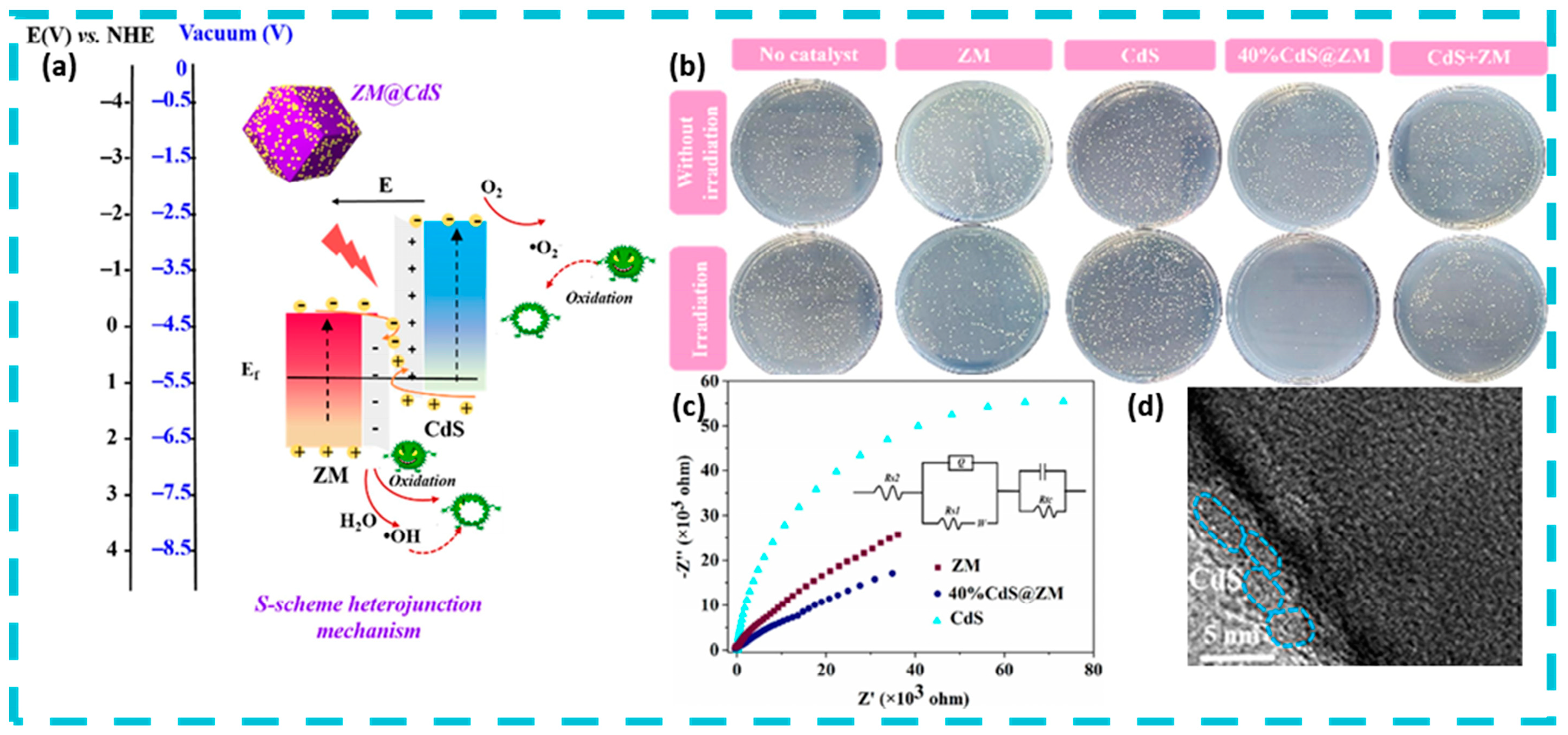
- Liang, R.; Yuan, H.; Wang, S.; Chen, F.; Si, R.; Wu, L.; Yan, G. Formation of CdS Quantum Dots on Zeolitic Imidazolate Framework-67 Dodecahedrons as S-Scheme Heterojunctions to Enhance Charge Separation and Antibacterial Activity. Sep. Purif. Technol. 2022, 303, 122291. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.-H.; Gao, G.; Chen, X.; Jia, H.-R.; Li, Y.-H.; Chen, Z.; Wu, F.-G. Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-S.; Shao, Y.-T.; Huang, K.-S.; Chou, T.-M.; Yang, C.-H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Ku, J.W.K.; Gan, Y.-H. New Roles for Glutathione: Modulators of Bacterial Virulence and Pathogenesis. Redox Biol. 2021, 44, 102012. [Google Scholar] [CrossRef]
- Yu, W.; Hu, C.; Bai, L.; Tian, N.; Zhang, Y.; Huang, H. Photocatalytic hydrogen peroxide evolution: What is the most effective strategy? Nano Energy 2022, 98, 107906. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, R.; Ravi, M.; Ravi, S.; Ramasamy, M.; Maruthapillai, A. Electrochemical and Morphological Investigations of Elettaria Cardamomum Pod Extract as a Green Corrosion Inhibitor for Mild Steel Corrosion in 1 N HCl. Inorg. Chem. Commun. 2023, 154, 110958. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Xu, C.; Tan, B.; Li, W.; Zheng, X.; Brahmia, A. Honeysuckle Extract as an Environment-Friendly Corrosion Inhibitor for Copper in Sulfuric Acid Medium. Ind. Crops Prod. 2023, 197, 116551. [Google Scholar] [CrossRef]
- Zhou, Q.; Yuan, G.; Lin, M.; Wang, P.; Li, S.; Tang, J.; Lin, J.; Huang, Y.; Zhang, Y. Large-scale electrochemical fabrication of nitrogen-doped carbon quantum dots and their application as corrosion inhibitor for copper. J. Mater. Sci. 2021, 56, 12909–12919. [Google Scholar] [CrossRef]
- Fowle, D.A.; Fein, J.B. Competitive Adsorption of Metal Cations onto Two Gram Positive Bacteria: Testing the Chemical Equilibrium Model. Geochim. Cosmochim. Acta 1999, 63, 3059–3067. [Google Scholar] [CrossRef]
- Shabbir, H.; Csapó, E.; Wojnicki, M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Sensors 2024, 11, 262. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High Adsorption Capability and Selectivity of ZnO Nanoparticles for Dye Removal. colloids and Surfaces A. Physicochem. Eng. Asp. 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Holmberg, J.P.; Ahlberg, E.; Bergenholtz, J.; Hassellöv, M.; Abbas, Z. Surface Charge and Interfacial Potential of Titanium Dioxide Nanoparticles. Experimental and Theoretical Investigations. J. Colloid Interface Sci. 2013, 407, 168–176. [Google Scholar] [CrossRef]
- Wen, S.; Wu, T.; Long, H.; Ke, L.; Deng, S.; Huang, L.; Zhang, J.; Tan, S. Mechanism Insight into Rapid Photodriven Sterilization Based on Silver Bismuth Sulfide Quantum Dots. ACS Appl. Mater. Interfaces 2021, 13, 21979–21993. [Google Scholar] [CrossRef] [PubMed]

| Half Reaction | Electrode Potential/V |
|---|---|
| ·OH + e− + H+ → H2O | +2.31 |
| O3 + 2e− + 2H+ → H2O + O2 | +2.075 |
| H2O2 + 2e− + 2H+ → 2H2O | +1.76 |
| ·RO + e−+ H+ → ROH | +1.6 |
| ·HO2 + e− + 2H+ → H2O2 | +1.06 |
| ·ROO + e− + H+ → ROOH | +1.0 |
| 1O2(g) + e− → ·O2− | +0.64 |
| H2O2 + e− + H+ → H2O +·OH | +0.32 |
| ·O2− + e− + 2H+ → H2O2 | +0.36 |
| O2(aq) + e−→ ·O2− | −0.33 |
| H2O + e− → eaq− | −2.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wu, D.; Zheng, J.; Han, B.; Qi, J.; Meng, F.; Li, J.; Liu, D. New Strategy for Microbial Corrosion Protection: Photocatalytic Antimicrobial Quantum Dots. Nanomaterials 2025, 15, 2. https://doi.org/10.3390/nano15010002
Liu S, Wu D, Zheng J, Han B, Qi J, Meng F, Li J, Liu D. New Strategy for Microbial Corrosion Protection: Photocatalytic Antimicrobial Quantum Dots. Nanomaterials. 2025; 15(1):2. https://doi.org/10.3390/nano15010002
Chicago/Turabian StyleLiu, Shijia, Dapeng Wu, Jie Zheng, Baochen Han, Jian Qi, Fanchun Meng, Jianhui Li, and Dan Liu. 2025. "New Strategy for Microbial Corrosion Protection: Photocatalytic Antimicrobial Quantum Dots" Nanomaterials 15, no. 1: 2. https://doi.org/10.3390/nano15010002
APA StyleLiu, S., Wu, D., Zheng, J., Han, B., Qi, J., Meng, F., Li, J., & Liu, D. (2025). New Strategy for Microbial Corrosion Protection: Photocatalytic Antimicrobial Quantum Dots. Nanomaterials, 15(1), 2. https://doi.org/10.3390/nano15010002







