The Effects of Polystyrene Microplastics and Copper Ion Co-Contamination on the Growth of Rice Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preparation of Solutions
2.3. Measurement Methods
2.4. Data Analysis
3. Results and Discussion
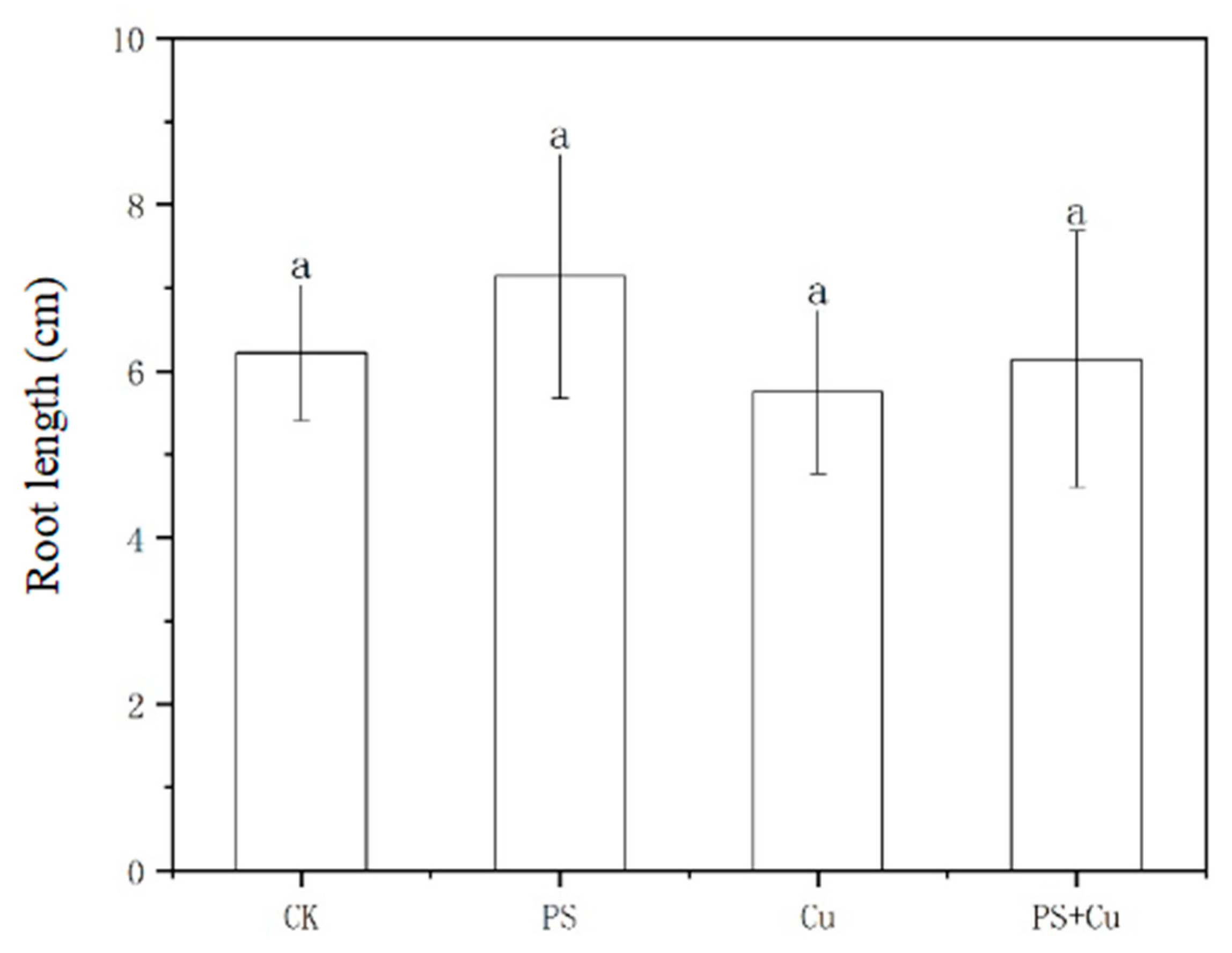
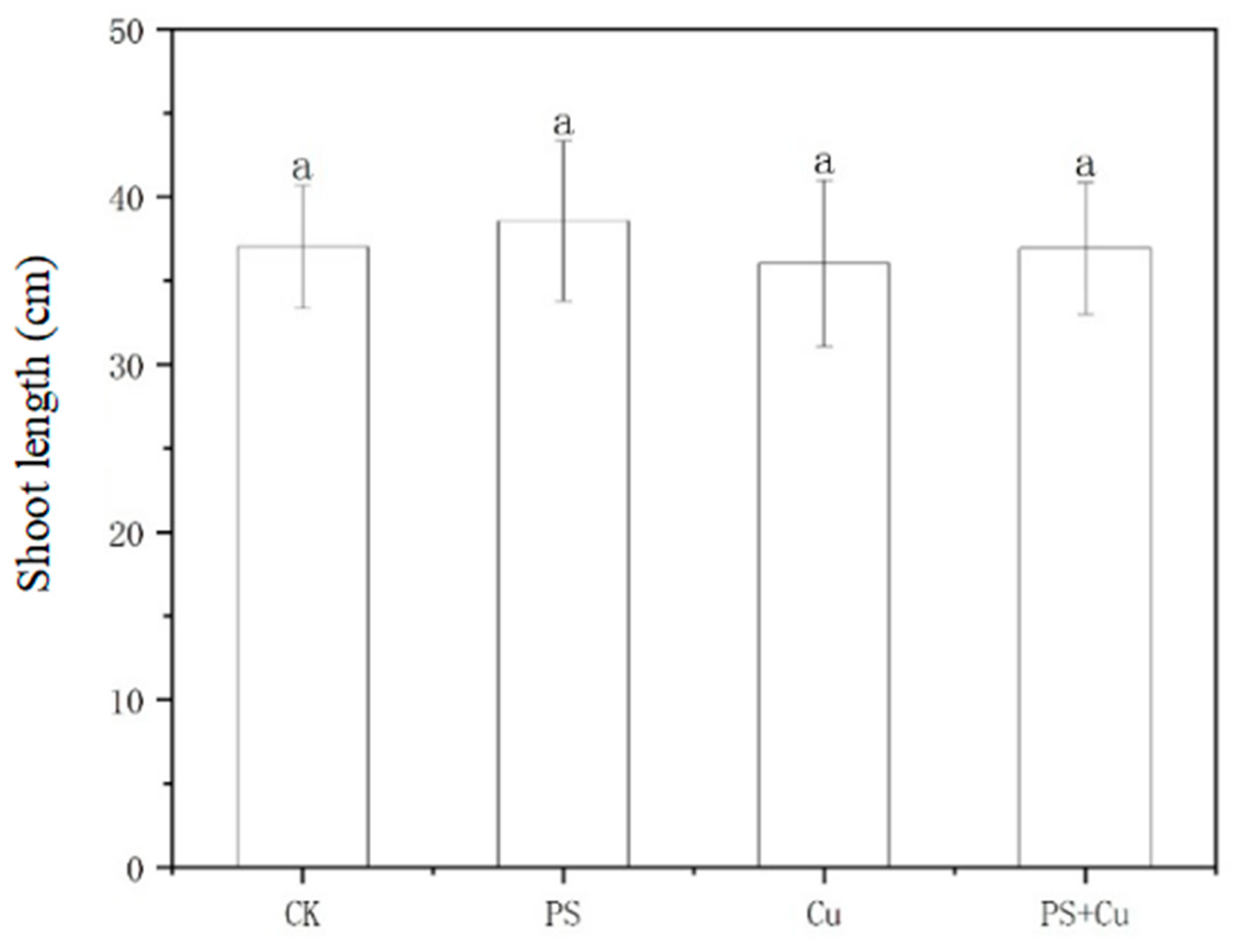
3.1. Effects of PS-MPs and Cu2+ on the Growth of Rice Seedlings
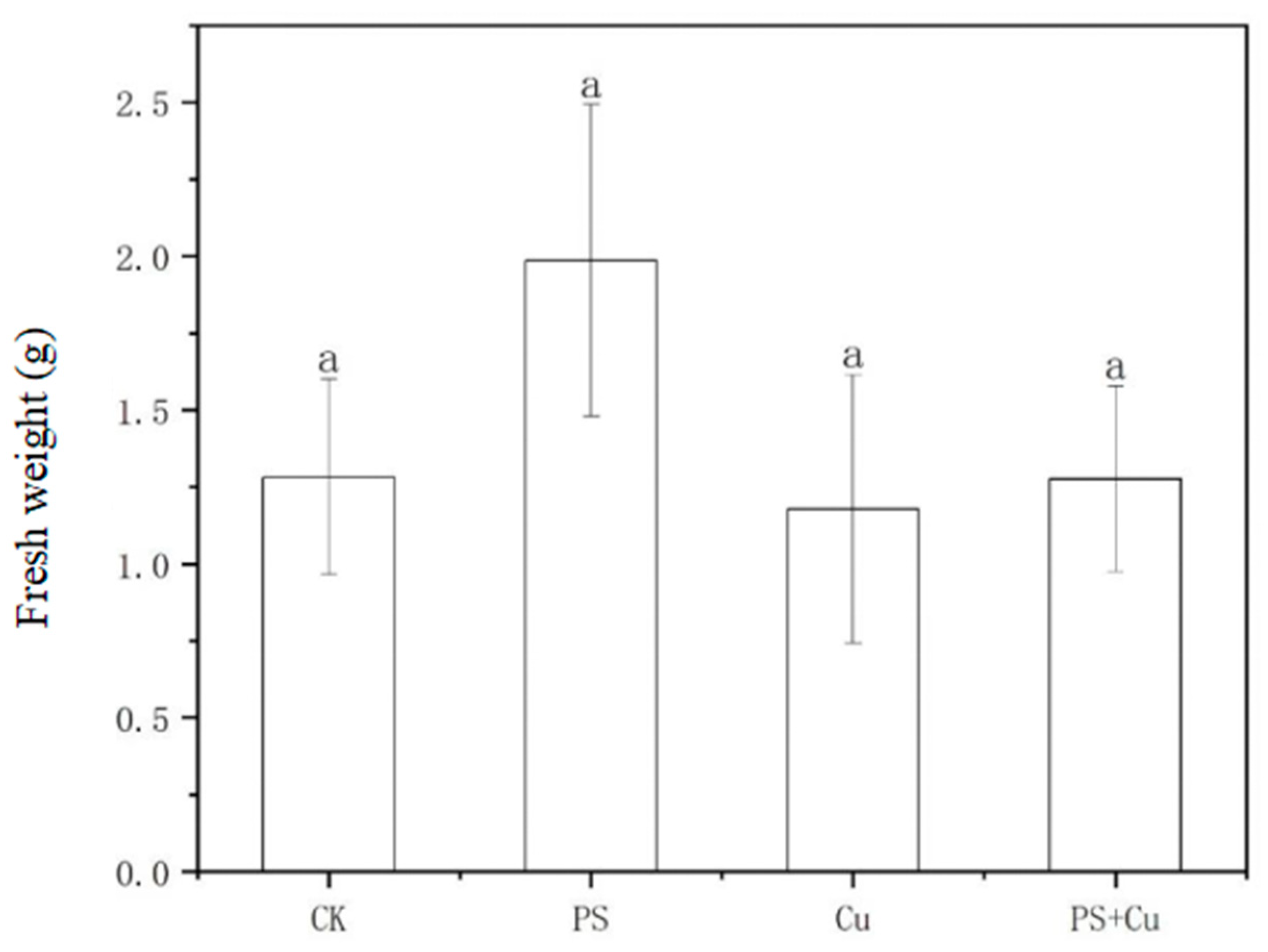
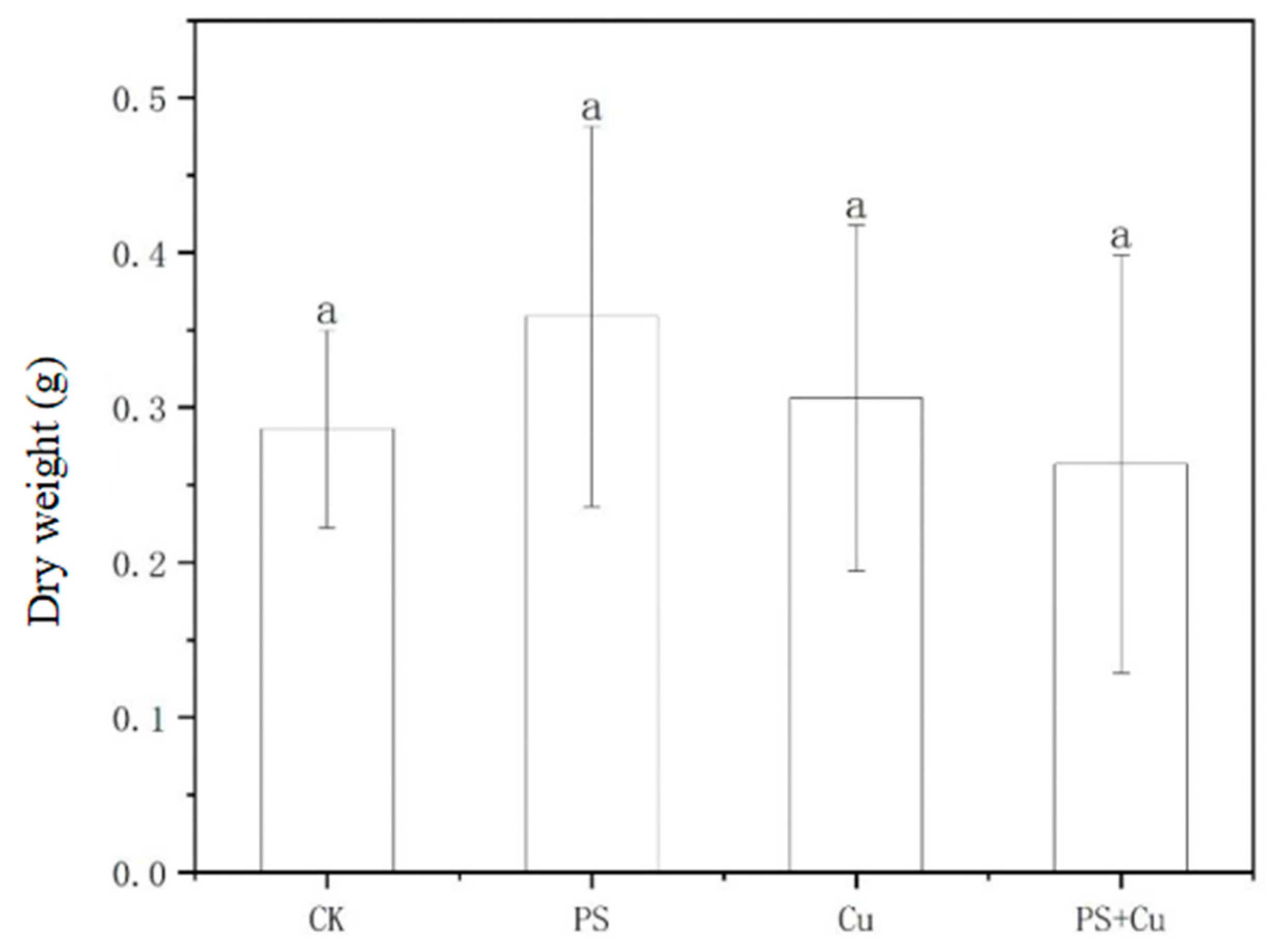
3.2. Effects of PS-MPs and Cu2+ on the Biomass of Rice Seedlings
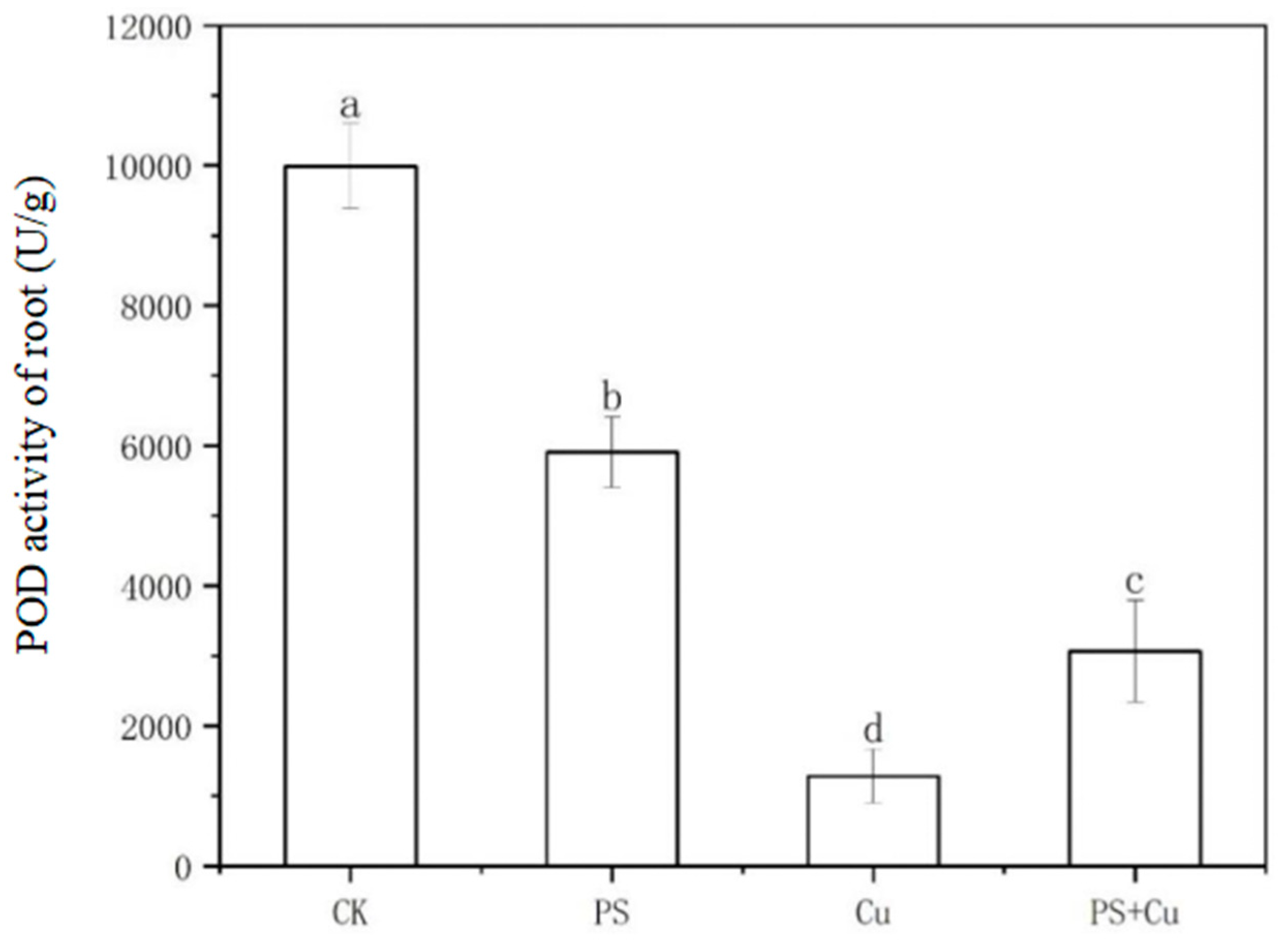
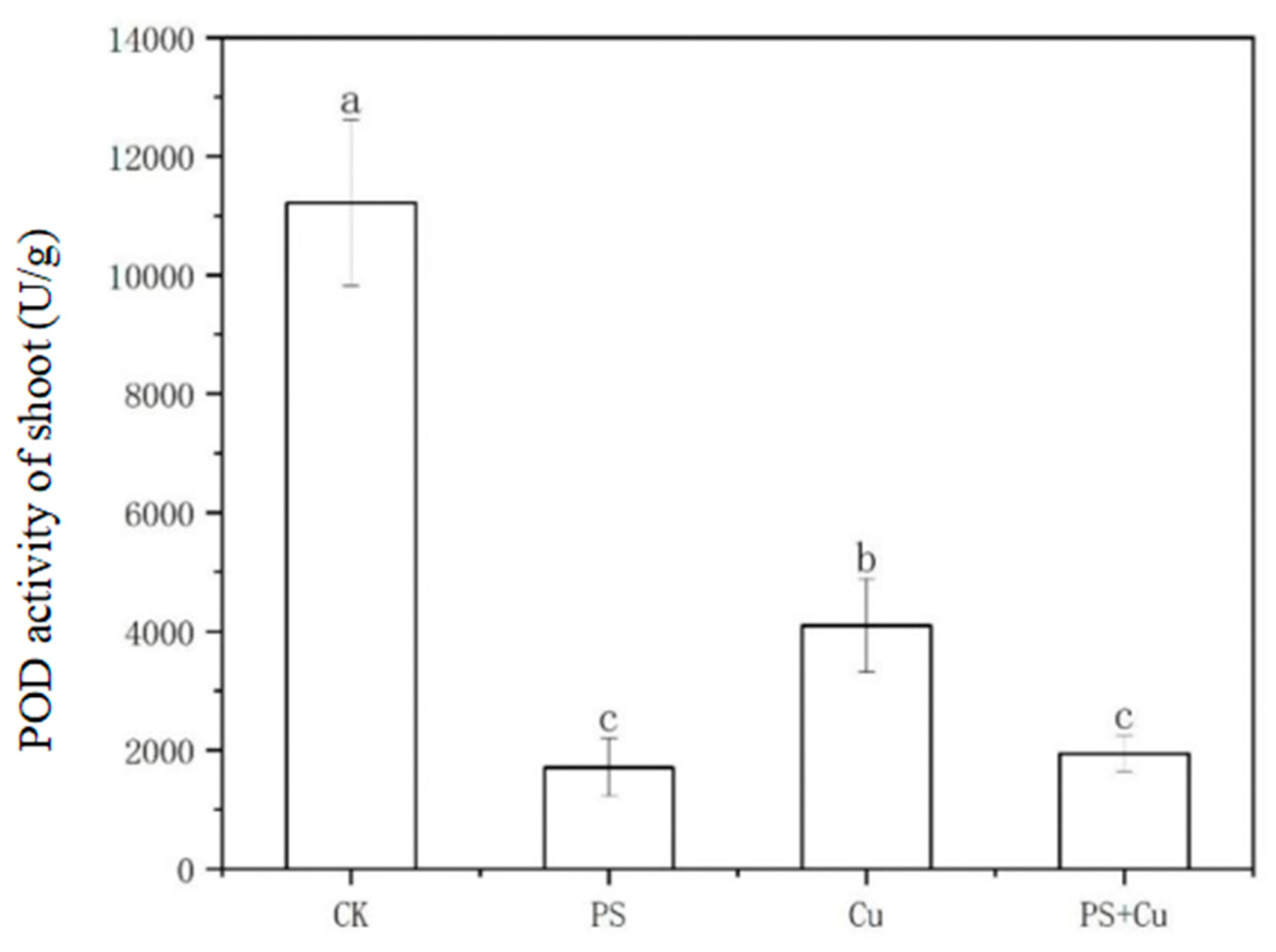
3.3. Effects of PS-MPs and Cu2+ on Peroxidase Activity in Rice Seedlings
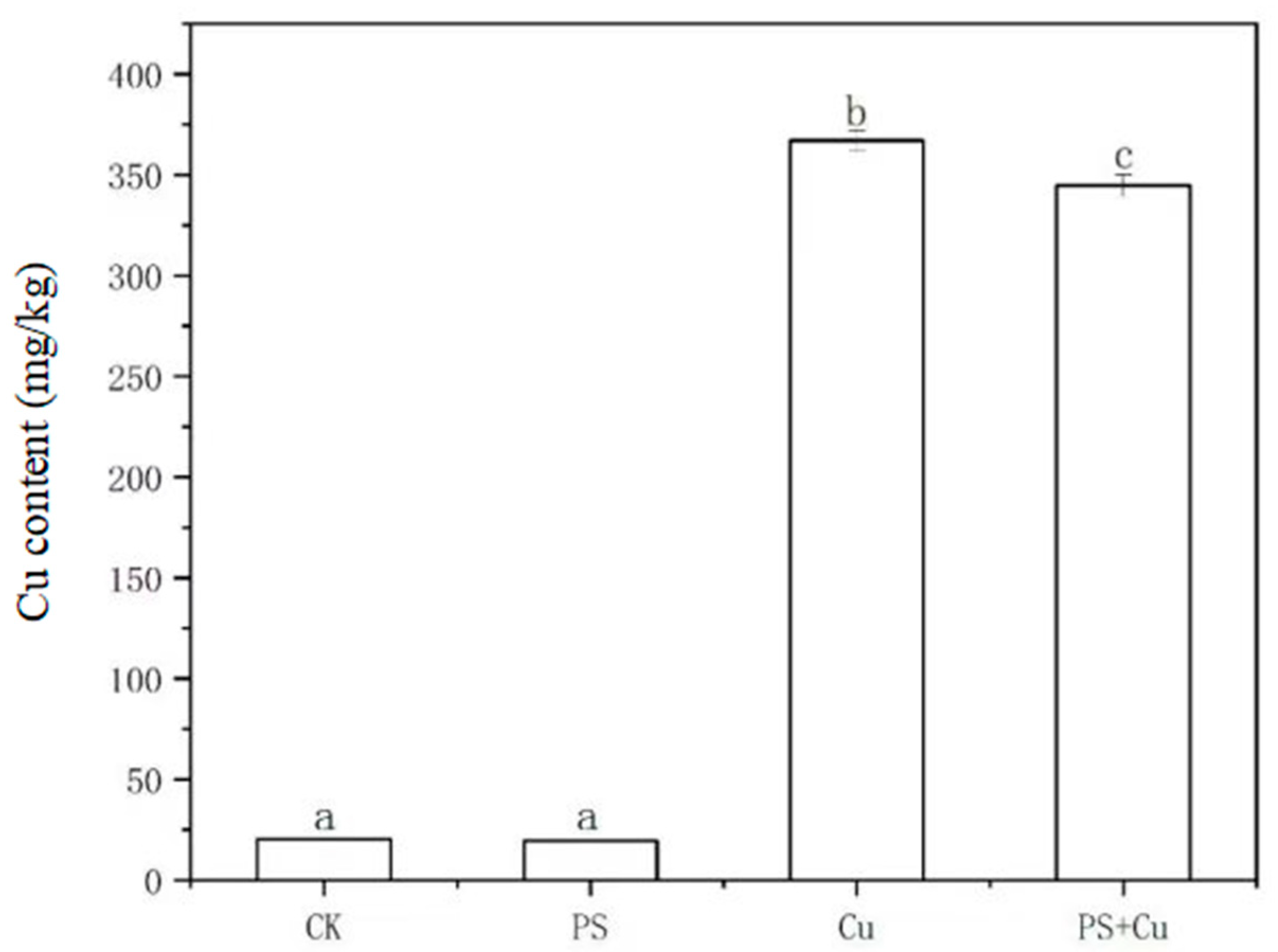
3.4. Effects of PS-MPs and Cu2+ on Cu Content in Rice Seedlings
4. Conclusions
- (1)
- PS-MPs exhibited a slight stimulatory effect on root growth, while Cu2+ alone slightly inhibited root elongation. The combined treatment showed no significant synergistic or additive toxicity. Similarly, shoot lengths, as well as fresh and dry weights, were not significantly affected under any treatment, indicating limited impact of low-concentration PS-MPs and Cu on overall rice growth;
- (2)
- Both PS-MPs and Cu2+ significantly reduced POD activity in roots and shoots. While the combined treatment partially alleviated Cu toxicity, the suppressive effects of PS-MPs on the antioxidant system were predominant;
- (3)
- PS-MPs reduced Cu2+ bioavailability through adsorption, resulting in decreased Cu accumulation in rice seedlings under the combined treatment and mitigating Cu toxicity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope. Plastics—The Fast Facts 2023; PlasticsEurope: Brussels, Belgium, 2023; Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 1 March 2024).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.; Nguyen, M.K.; Hoang, T.D.; Ha, M.C.; Huyen, N.T.T.; Bui, V.K.H.; Pham, M.-T.; Nguyen, C.-M.; Chang, S.W.; Nguyen, D.D. Sources, environmental fate, and impacts of microplastic contamination in agricultural soils: A comprehensive review. Sci. Total Environ. 2024, 950, 175276. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A.; Machado, A.A.D.; Yang, G. Microplastic impacts on terrestrial ecosystems. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation, and allergen production in basil (Ocimum basilicum L.). Plants 2018, 9, 862. [Google Scholar] [CrossRef]
- Sarkar, A.; Rubin, A.; Zucker, I. Engineered Polystyrene-Based Microplastics of High Environmental Relevance. Environ. Sci. Technol. 2021, 55, 10491–10501. [Google Scholar] [CrossRef]
- Ranjani, M.; Veerasingam, S.; Venkatachalapathy, R.; Mugilarasan, M.; Bagaev, A.; Mukhanov, V.; Vethamony, P. Assessment of Potential Ecological Risk of Microplastics in the Coastal Sediments of India: A Meta-Analysis. Mar. Pollut. Bull. 2021, 163, 111969. [Google Scholar] [CrossRef]
- Li, W.; Zu, B.; Yang, Q.; Guo, J.; Li, J. Sources, Distribution, and Environmental Effects of Microplastics: A Systematic Review. RSC Adv. 2023, 13, 15566–15574. [Google Scholar] [CrossRef]
- Liang, S.; Wang, K.; Wang, K.; Kou, Y.; Wang, T.; Guo, C.; Wang, W.; Wang, J. Adsorption of Diclofenac Sodium by Aged Degradable and Non-Degradable Microplastics: Environmental Effects, Adsorption Mechanisms. Toxics 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, J.; Gong, Y.; Li, Z.; Zeng, E. Strong Influence of Surfactants on Virgin Hydrophobic Microplastics Adsorbing Ionic Organic Pollutants. Environ. Pollut. 2020, 265 Pt B, 115061. [Google Scholar] [CrossRef] [PubMed]
- An, Q.Y.; Wen, C.; Yan, C.Z. Meta-analysis reveals the combined effects of microplastics and heavy metal on plants. J. Hazard. Mater. 2024, 476, 135028. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Cui, Q.L.; Chen, L.; Zhu, X.; Zhao, S.; Duan, C.; Zhang, X.; Song, D.; Fang, L. A critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity, and prevention. J. Hazard. Mater. 2024, 424, 127750. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity, and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Cui, X.; Ding, J.; Ji, C.; Yu, L.; Cai, H. High level of copper affects both nitrogen transport in rice plant and nitrogen transformation in rhizosphere soil. Plant Soil 2024, 502, 351–372. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Han, Z.; Osman, R.; Liu, Y.; Wei, Z.; Wang, L.; Xu, M. Analyzing the impacts of cadmium alone and in co-existence with polypropylene microplastics on wheat growth. Front. Plant Sci. 2023, 14, 1240472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Liu, X.N.; Jiang, H.S.; Li, X.; Li, W.; Cao, Y. Antidote or Trojan horse for submerged macrophytes: Role of microplastics in copper toxicity in aquatic environments. Water Res. 2022, 216, 118354. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Su, H.L.; Wang, F.F.; Ma, B.; Tao, Y.; Cao, K.; Shen, Y.; Zhao, W.; Wei, Y.; Wu, F. Understanding the role of low-dose polystyrene microplastic in copper toxicity to rice seed (Oryza sativa L.). Environ. Toxicol. Chem. 2024, 43, 1870–1879. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Chen, C.; Zhuang, K.; Song, Y.; Shen, Z. Analysis of copper-binding proteins in rice radicles exposed to excess copper and hydrogen peroxide stress. Front. Plant Sci. 2016, 7, 1216. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhu, J.; Zhang, L.; Jiang, L.; Yin, Y.; Guo, H. Effects of polystyrene microplastic on uptake and toxicity of copper and cadmium in hydroponic wheat seedlings (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2021, 217, 112217. [Google Scholar] [CrossRef]
- Chen, L.; Chang, N.; Qiu, T.Y.; Wang, N.; Cui, Q.; Zhao, S.; Huang, F.; Chen, H.; Zeng, Y.; Dong, F.; et al. Meta-analysis of impacts of microplastics on plant heavy metal (loid) accumulation. Environ. Pollut. 2024, 348, 123787. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Lin, G.; Ma, M.; Wang, L.; Liu, L. The Effects of Polystyrene Microplastics and Copper Ion Co-Contamination on the Growth of Rice Seedlings. Nanomaterials 2025, 15, 17. https://doi.org/10.3390/nano15010017
Jin H, Lin G, Ma M, Wang L, Liu L. The Effects of Polystyrene Microplastics and Copper Ion Co-Contamination on the Growth of Rice Seedlings. Nanomaterials. 2025; 15(1):17. https://doi.org/10.3390/nano15010017
Chicago/Turabian StyleJin, Huiyu, Guohe Lin, Mingzi Ma, Lin Wang, and Lixiang Liu. 2025. "The Effects of Polystyrene Microplastics and Copper Ion Co-Contamination on the Growth of Rice Seedlings" Nanomaterials 15, no. 1: 17. https://doi.org/10.3390/nano15010017
APA StyleJin, H., Lin, G., Ma, M., Wang, L., & Liu, L. (2025). The Effects of Polystyrene Microplastics and Copper Ion Co-Contamination on the Growth of Rice Seedlings. Nanomaterials, 15(1), 17. https://doi.org/10.3390/nano15010017






