Heteropolyacids@Silica Heterogeneous Catalysts to Produce Solketal from Glycerol Acetalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Catalyst Characterization
2.4. Catalytic Experiments
3. Results and Discussion
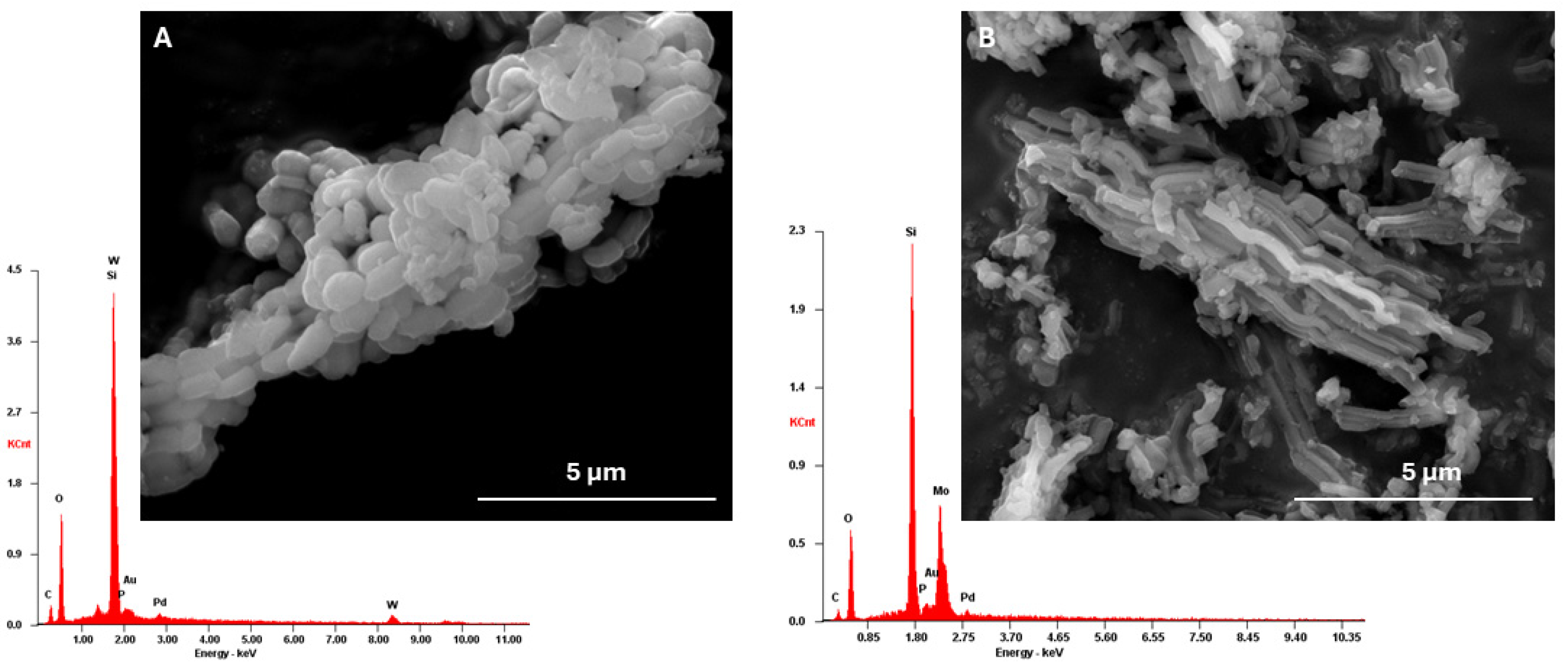
3.1. Catalysts Characterization
3.2. Acidity Characterization
3.3. Catalytic Activity of HPAs
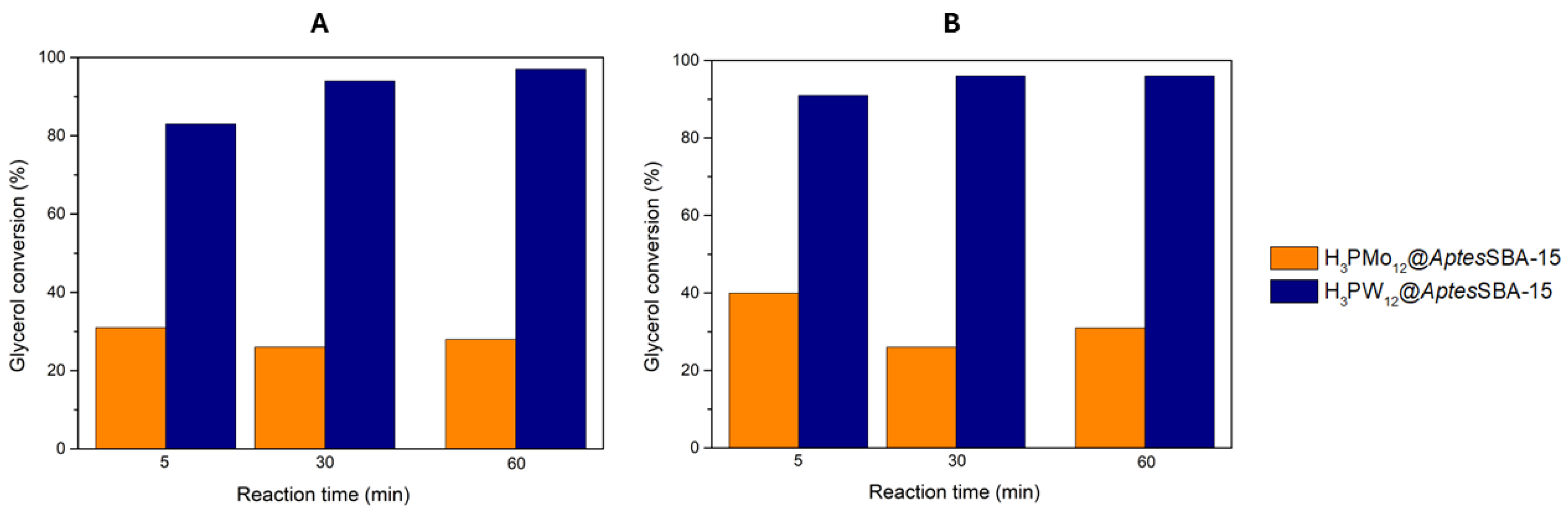
3.4. HPAs@AptesSBA-15 Catalytic Performance
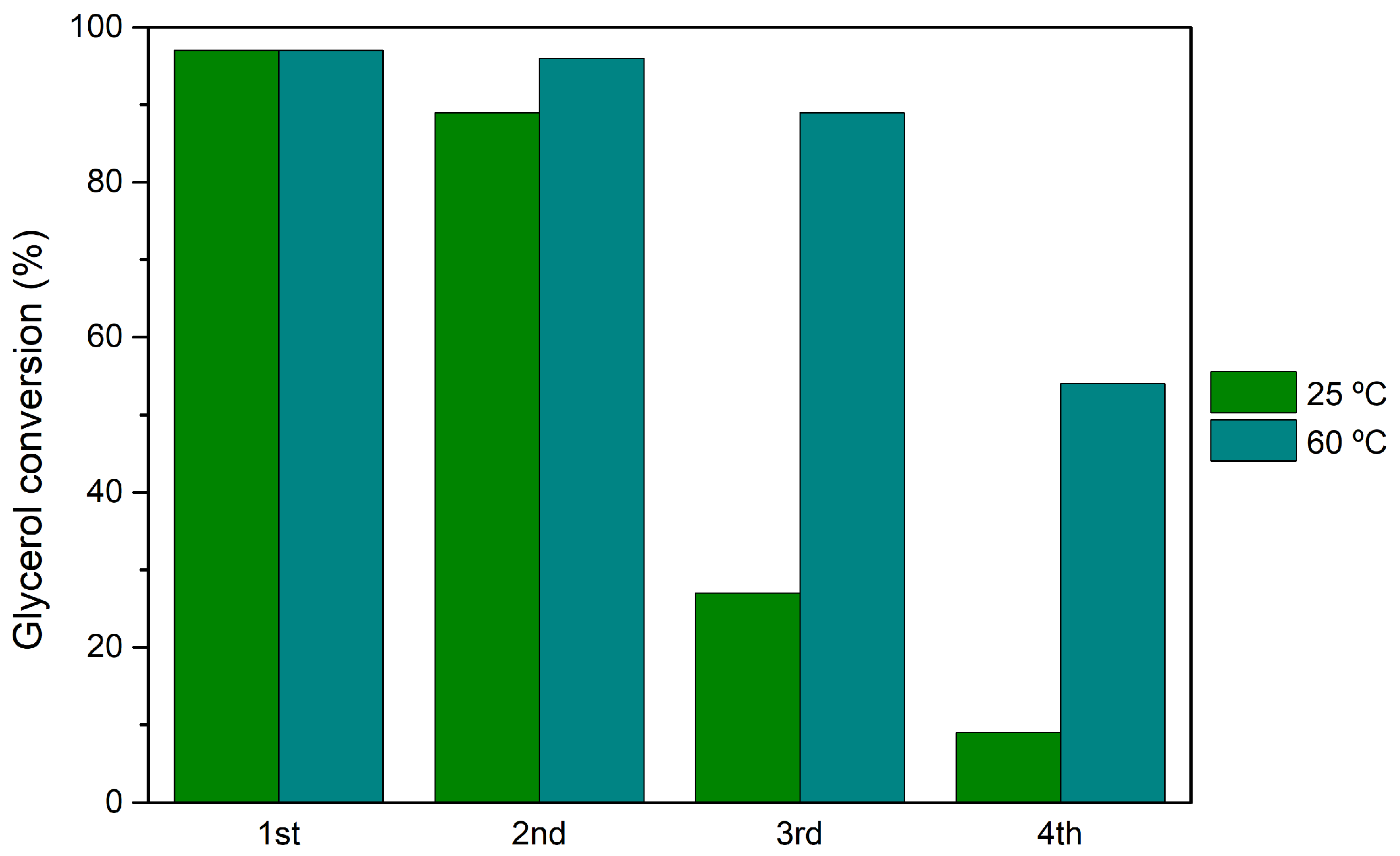
3.5. Catalyst Reutilization and Recycling
3.6. Comparison with Other Catalysts
3.7. Catalyst Characterization after Use
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustain. Chem. 2021, 2, 286–324. [Google Scholar] [CrossRef]
- Akram, F.; Haq, I.u.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current trends in biodiesel production technologies and future progressions: A possible displacement of the petro-diesel. J. Clean. Prod. 2022, 370, 133479. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 573. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G.; Chaves, D.M.; Siqueira, L. An efficient process to synthesize solketal from glycerol over tin (II) silicotungstate catalyst. Fuel 2020, 281, 118724. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Smirnov, A.; Selishcheva, S.; Yakovlev, V. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Glycerol to Solketal for Fuel Additive: Recent Progress in Heterogeneous Catalysts. Energies 2019, 12, 2872. [Google Scholar] [CrossRef]
- Lopez, X.; Carbo, J.J.; Bo, C.; Poblet, J.M. Structure, properties and reactivity of polyoxometalates: A theoretical perspective. Chem. Soc. Rev. 2012, 41, 7537–7571. [Google Scholar] [CrossRef]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A Gen. 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Borrás-Almenar, J.J.; Coronado, E.; Müller, A.; Pope, M. Polyoxometalate Molecular Science; Springer: Berlin/Heidelberg, Germany, 2003; Volume 98, p. 472. [Google Scholar] [CrossRef]
- Katsoulis, D.E. A Survey of Applications of Polyoxometalates. Chem. Rev. 1998, 98, 359–387. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.J.; Julio, A.A.; Dorigetto, F.C.S. Solvent-free heteropolyacid-catalyzed glycerol ketalization at room temperature. RSC Adv. 2015, 5, 44499–44506. [Google Scholar] [CrossRef]
- Juliao, D.; Mirante, F.; Balula, S.S. Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. Molecules 2022, 27, 6573. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.O.; Granadeiro, C.M.; Almeida, P.L.; Pires, J.; Capel-Sanchez, M.C.; Campos-Martin, J.M.; Gago, S.; de Castro, B.; Balula, S.S. Oxidative desulfurization strategies using Keggin-type polyoxometalate catalysts: Biphasic versus solvent-free systems. Catal. Today 2019, 333, 226–236. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Hu, G.; Zhao, J. Heteropolyacid supported MOF fibers for oxidative desulfurization of fuel. Chem. Eng. J. 2020, 388, 124325. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Vital, J.; Fonseca, I.M.; Ramos, A.M. Glycerol conversion into biofuel additives by acetalization with pentanal over heteropolyacids immobilized on zeolites. Catal. Today 2020, 346, 76–80. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Highly Efficient Glycerol Acetalization over Supported Heteropoly Acid Catalysts. ChemCatChem 2018, 10, 1918–1925. [Google Scholar] [CrossRef]
- Castanheiro, J. Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6. Catalysts 2022, 12, 81. [Google Scholar] [CrossRef]
- Mirante, F.; Ribeiro, S.O.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Sustainable Desulfurization Processes Catalyzed by Titanium-Polyoxometalate@TM-SBA-15. Top. Catal. 2017, 60, 1140–1150. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I. APTES-functionalized mesoporous silica as a vehicle for antipyrine—Adsorption and release studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 187–196. [Google Scholar] [CrossRef]
- Mirante, F.; Gomes, N.; Branco, L.C.; Cunha-Silva, L.; Almeida, P.L.; Pillinger, M.; Gago, S.; Granadeiro, C.M.; Balula, S.S. Mesoporous nanosilica-supported polyoxomolybdate as catalysts for sustainable desulfurization. Microporous Mesoporous Mater. 2019, 275, 163–171. [Google Scholar] [CrossRef]
- Maria Chong, A.S.; Zhao, X.S. Functionalization of SBA-15 with APTES and Characterization of Functionalized Materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Aouissi, A.; Al-Othman, Z.A.; Al-Anezi, H. Reactivity of heteropolymolybdates and heteropolytungstates in the cationic polymerization of styrene. Molecules 2010, 15, 3319–3328. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Borges, S.; Carvalho, A.; Pereira, C.; Pereira, A.M.; Fernandes, C.; Araújo, J.P.; Freire, C. Magnetically recyclable mesoporous iron oxide–silica materials for the degradation of acetaminophen in water under mild conditions. Polyhedron 2016, 106, 125–131. [Google Scholar] [CrossRef]
- Sujandi; Park, S.E.; Han, D.S.; Han, S.C.; Jin, M.J.; Ohsuna, T. Amino-functionalized SBA-15 type mesoporous silica having nanostructured hexagonal platelet morphology. Chem. Commun. 2006, 39, 4131–4133. [Google Scholar] [CrossRef] [PubMed]
- Gadamsetti, S.; Rajan, N.P.; Rao, G.S.; Chary, K.V.R. Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts. J. Mol. Catal. A Chem. 2015, 410, 49–57. [Google Scholar] [CrossRef]
- Ammaji, S.; Rao, G.S.; Chary, K.V.R. Acetalization of glycerol with acetone over various metal-modified SBA-15 catalysts. Appl. Petrochem. Res. 2018, 8, 107–118. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Matkala, B.; Boggala, S.; Basavaraju, S.; Sarma Akella, V.S.; Aytam, H.P. Influence of sulphonation on Al-MCM-41 catalyst for effective bio-glycerol conversion to Solketal. Microporous Mesoporous Mater. 2024, 363, 112830. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, Y.; Zhao, H.; Ye, B.; Wang, L.; Hou, Z. Synthesis of solketal from glycerol over modified SiO2 supported p-phenolsulfonic acid catalyst. Fuel 2021, 291, 120207. [Google Scholar] [CrossRef]


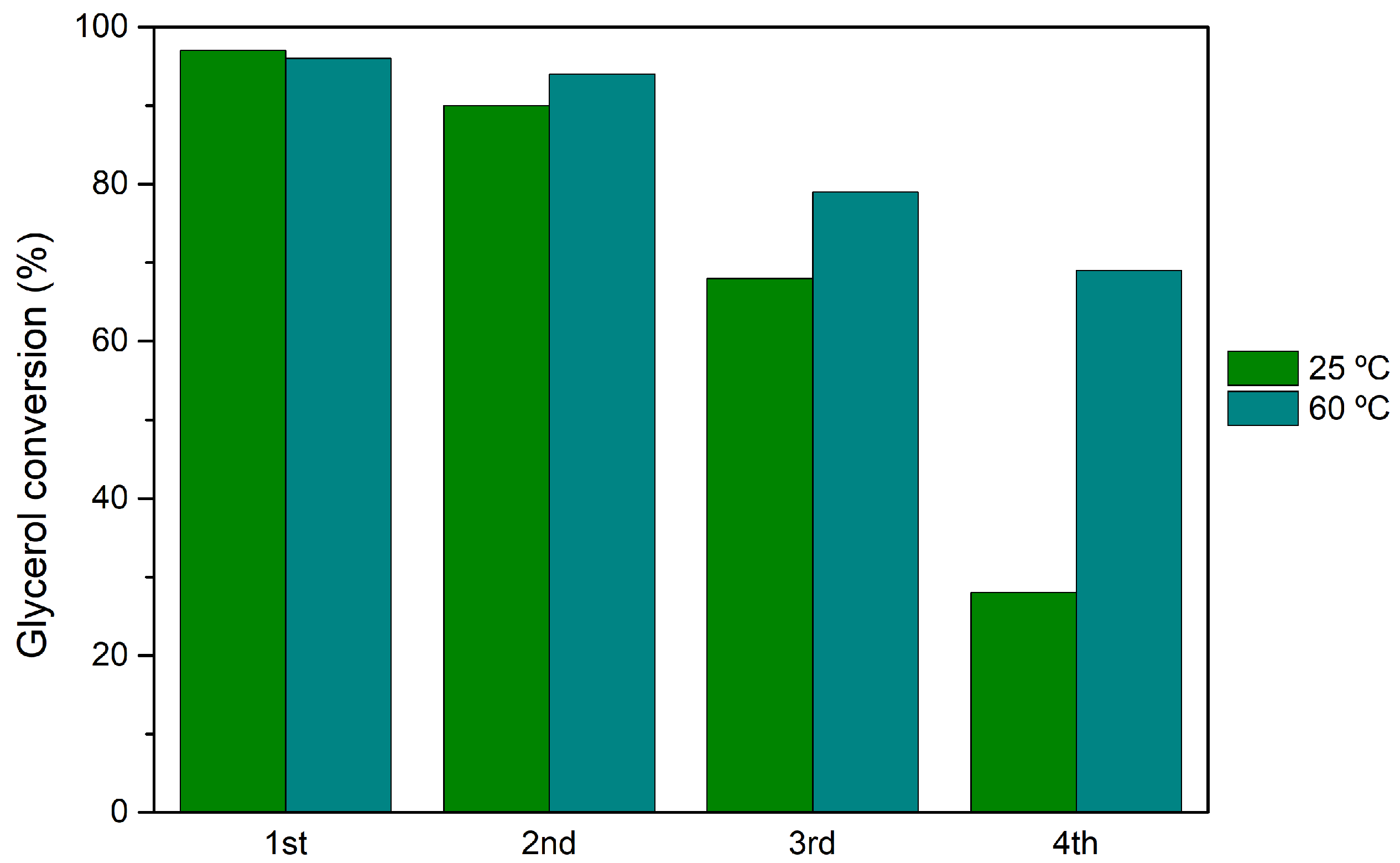
| Material | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|
| H3PW12@AptesSBA-15 | 129 | 0.245 |
| H3PMo12@AptesSBA-15 | 188 | 0.293 |
| AptesSBA-15 | 337 | 0.589 |
| SBA-15 | 725 | 0.971 |
| Material | pH | Acidity (mmol H+/g) |
|---|---|---|
| H3PMo12 | 2.2 | 9.74 |
| H3PW12 | 2.2 | 1.93 |
| H4SiW12 | 2.6 | 0.995 |
| H3PMo12@AptesSBA-15 | 3.41 | 3.36 |
| H3PW12@AptesSBA-15 | 3.39 | 0.470 |
| Material | Acidity (mmol H+/g) | Catalyst Amount (µmol) |
|---|---|---|
| H3PMo12 | 0.373 | 0.525 |
| H3PW12 | 0.373 | 1.70 |
| H4SiW12 | 0.373 | 3.26 |
| Catalyst | Ratio of Glycerol/Acetone | T (°C) | Time (h) | Conversion (%) | Selectivity to Solketal (%) | Ref. |
|---|---|---|---|---|---|---|
| SO4-Al-MCM-41 | 1:10 | RT | 2 | 94.8 | 99 | [33] |
| PSF@SiO2 | 1:10 | RT | 1.5 | 86.6 | 98 | [34] |
| Ar-SBA-15 | 1:6 | 70 | 0.5 | 82.5 | wi | [32] |
| Pr-SBA-15 | 1:6 | 70 | 0.5 | 79.0 | wi | [32] |
| Nb-SBA-15 | 1:3 | RT | 1 | 95 | 100 | [30] |
| Zr-SBA-15 | 1:3 | RT | 1 | 92 | 98 | [30] |
| Ti-SBA-15 | 1:3 | RT | 1 | 65 | 98 | [30] |
| Al-SBA-15 | 1:3 | RT | 1 | 60 | 98 | [30] |
| MoPo/SBA-15 | 1:3 | RT | 1 | 100 | 98 | [29] |
| Cs2.5H0.5PW12O40@KIT-6 | 1:6 | RT | 0.25 | 95 | 98 | [20] |
| H3PW12@AptesSBA-15 | 1:15 | RT | 0.08 | 83 | 97 | This work |
| 60 | 91 | 97 | ||||
| H3PMo12@AptesSBA-15 | 1:15 | RT | 0.08 | 31 | 69 | This work |
| 60 | 40 | 76 |
| H3PW12@AptesSBA-15 | Acidity (mmol H+/g) | HPA Loading (µmol/g) |
|---|---|---|
| Before usage | 0.469 | 159 |
| after 4 catalytic cycles | 0.277 | 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.N.; Santos-Vieira, I.C.M.S.; Gomes, C.R.; Mirante, F.; Balula, S.S. Heteropolyacids@Silica Heterogeneous Catalysts to Produce Solketal from Glycerol Acetalization. Nanomaterials 2024, 14, 733. https://doi.org/10.3390/nano14090733
Dias CN, Santos-Vieira ICMS, Gomes CR, Mirante F, Balula SS. Heteropolyacids@Silica Heterogeneous Catalysts to Produce Solketal from Glycerol Acetalization. Nanomaterials. 2024; 14(9):733. https://doi.org/10.3390/nano14090733
Chicago/Turabian StyleDias, Catarina N., Isabel C. M. S. Santos-Vieira, Carlos R. Gomes, Fátima Mirante, and Salete S. Balula. 2024. "Heteropolyacids@Silica Heterogeneous Catalysts to Produce Solketal from Glycerol Acetalization" Nanomaterials 14, no. 9: 733. https://doi.org/10.3390/nano14090733
APA StyleDias, C. N., Santos-Vieira, I. C. M. S., Gomes, C. R., Mirante, F., & Balula, S. S. (2024). Heteropolyacids@Silica Heterogeneous Catalysts to Produce Solketal from Glycerol Acetalization. Nanomaterials, 14(9), 733. https://doi.org/10.3390/nano14090733









