Submicronic-Scale Mechanochemical Characterization of Oxygen-Enriched Materials
Abstract
1. Introduction
2. Theoretical Approach
3. Experiments and Results
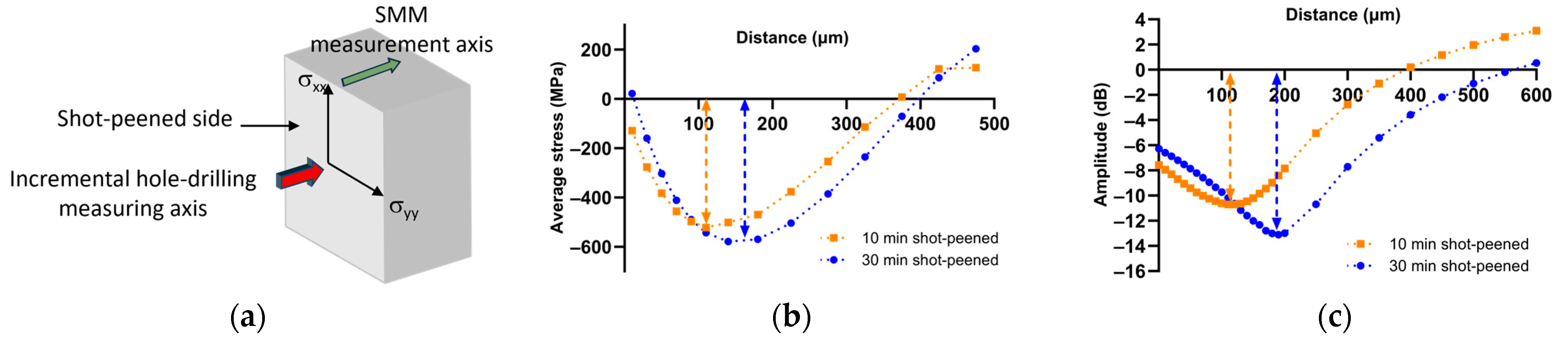
3.1. Preliminary Study on Pure Zirconium
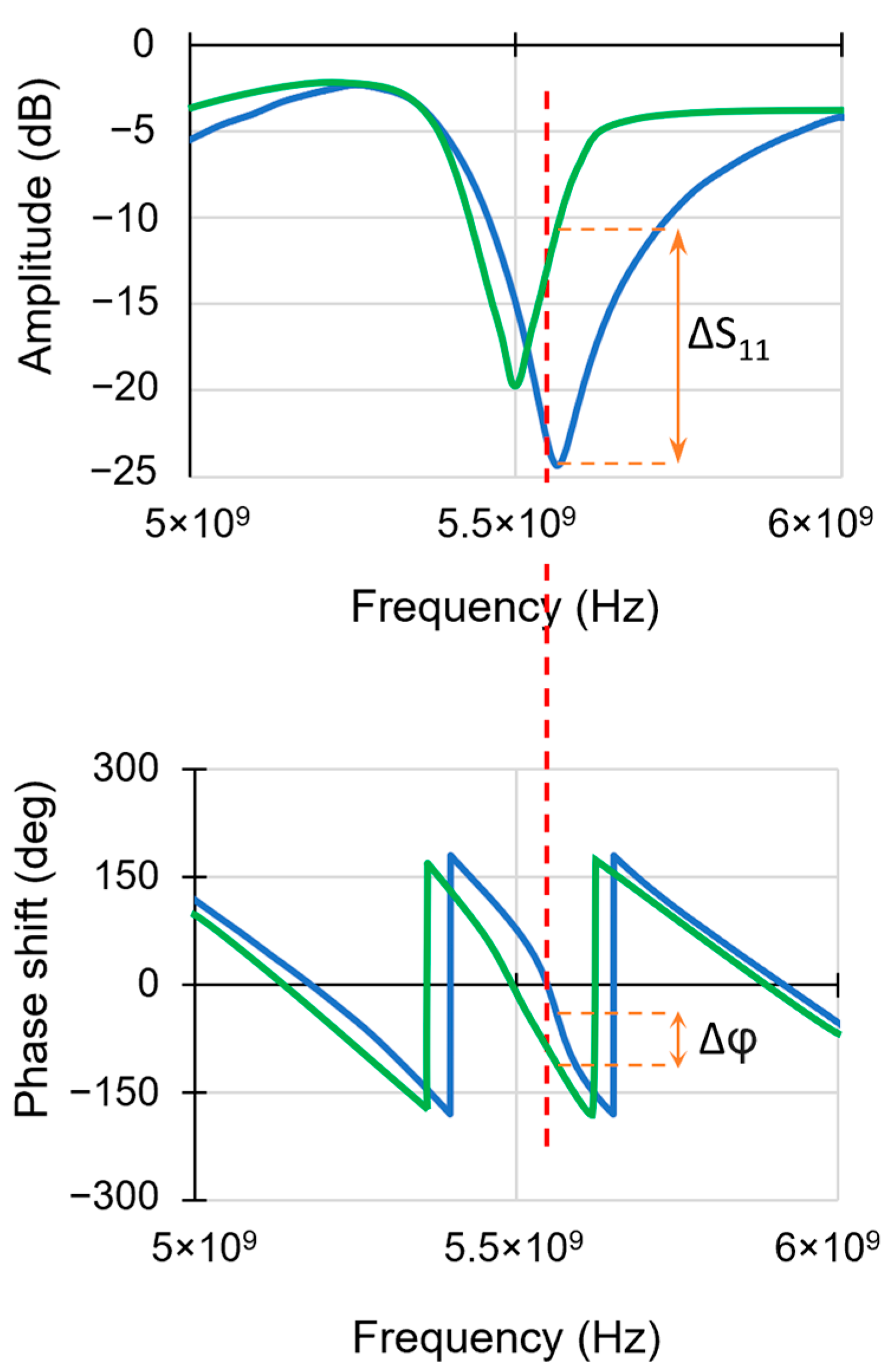
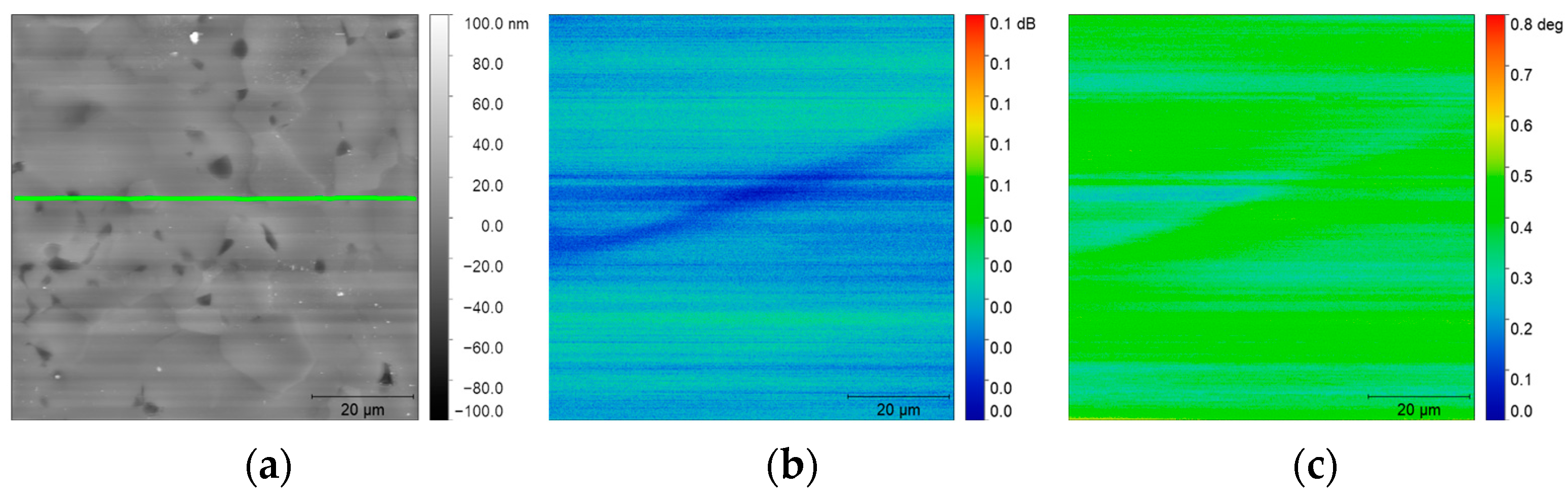
3.2. Simultaneous Detection of Amplitude and Phase Signal on Titanium Alloy
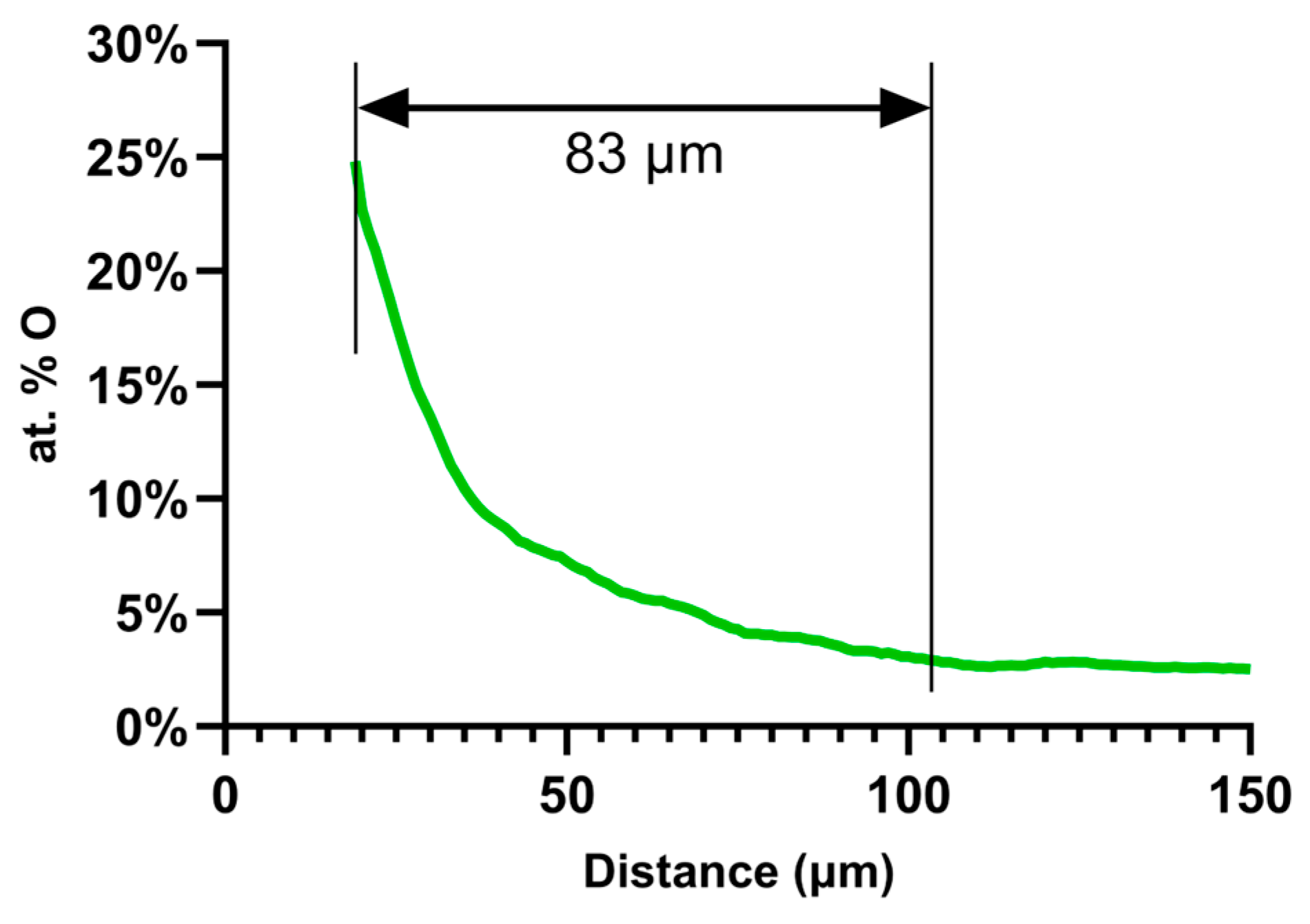
3.2.1. NRA Measurements
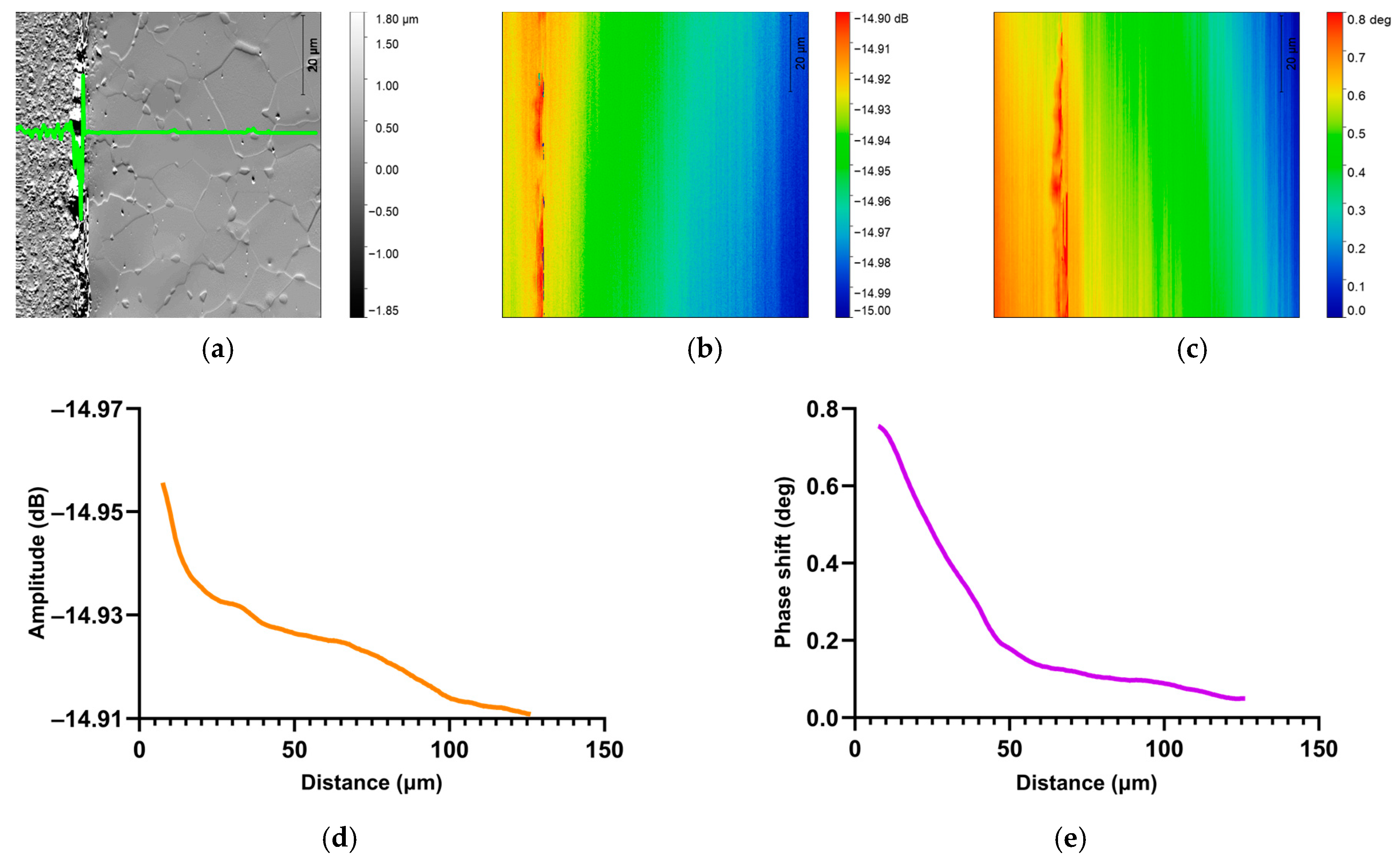
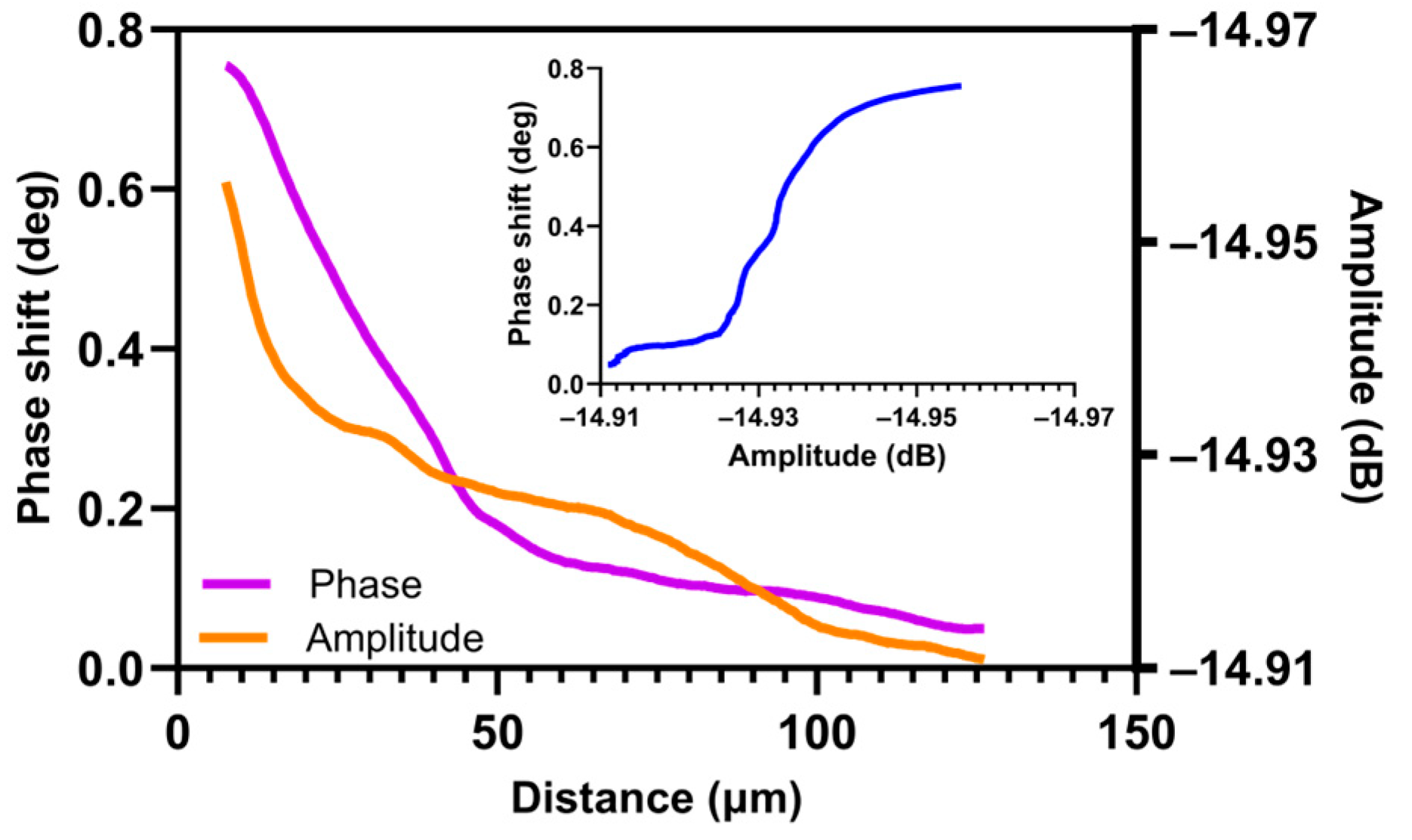
3.2.2. SMM Amplitude and Phase Measurements
3.2.3. Nanoindentation Measurements
4. Discussion
4.1. Chemical and Physical Characterization by NRA and Nanoindentation
4.2. SMM Characterization of Oxygen-Enriched Zone
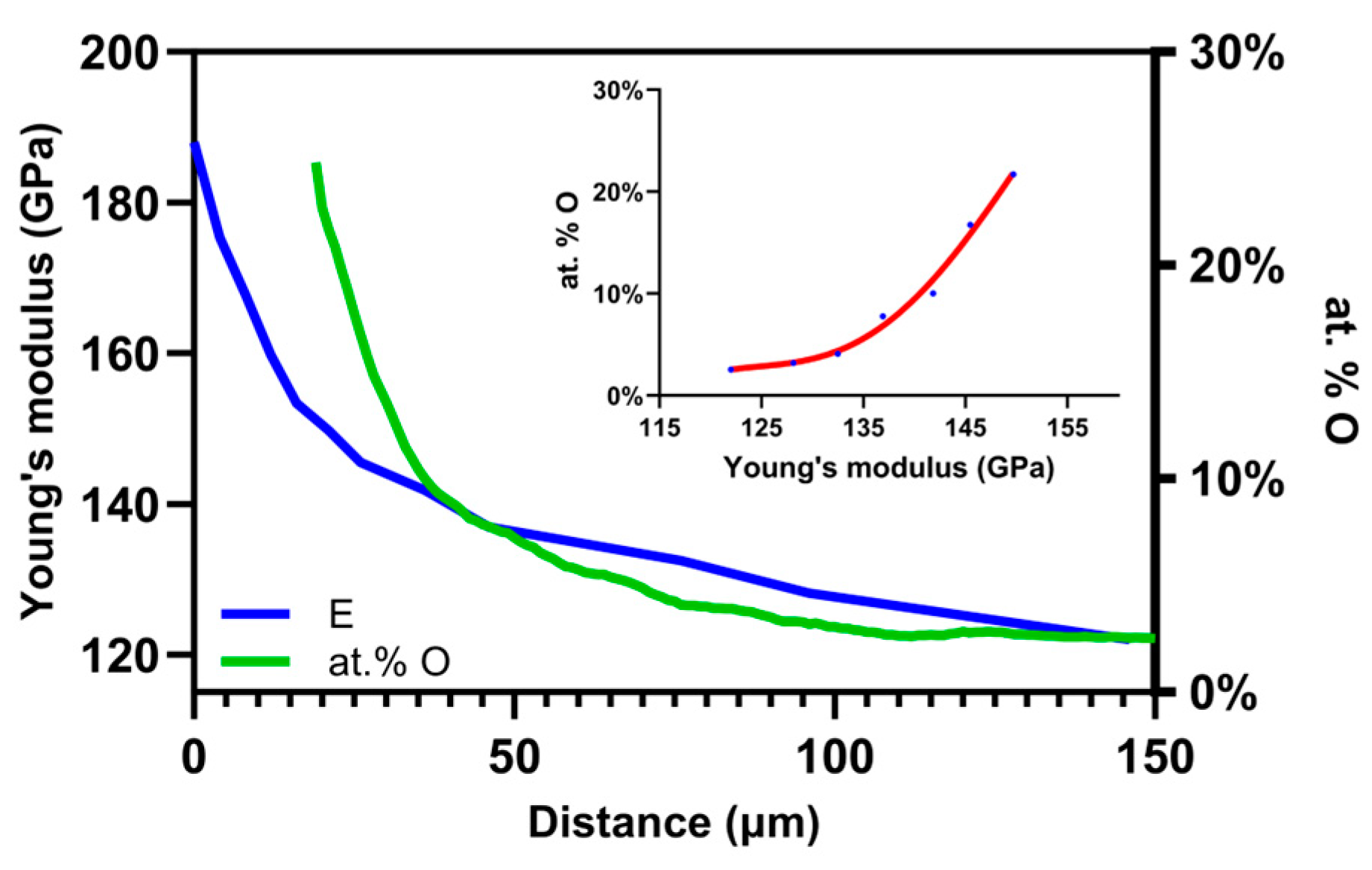
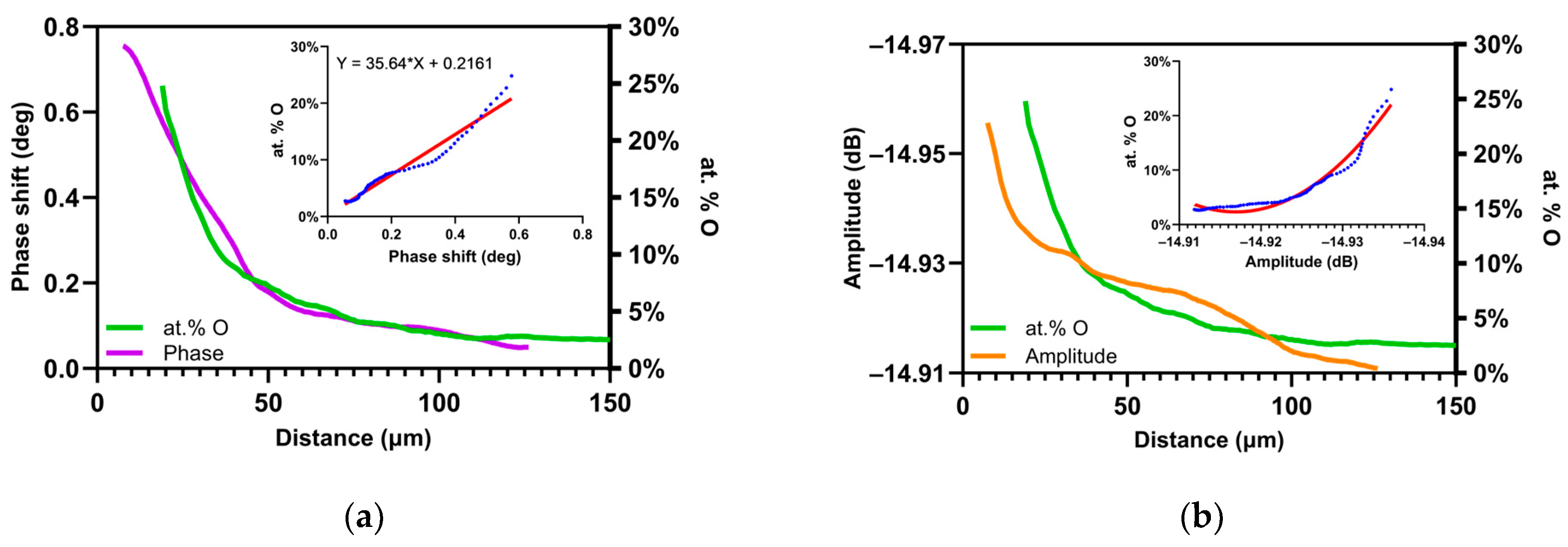
4.3. Comparison of the Oxygen Concentration and SMM Signals
4.4. Comparison of Young’s Modulus and SMM Signals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Welsch, G. Effects of Oxygen and Heat Treatment on the Mechanical Properties of Alpha and Beta Titanium Alloys. Metall. Trans. A 1988, 19, 527–542. [Google Scholar] [CrossRef]
- Evans, R.W.; Hull, R.J.; Wilshire, B. The Effects of Alpha-Case Formation on the Creep Fracture Properties of the High-Temperature Titanium Alloy IMI834. J. Mater. Process. Technol. 1996, 56, 492–501. [Google Scholar] [CrossRef]
- Ren, D.; Jiang, Y.; Hu, X.; Zhang, X.; Xiang, X.; Huang, K.; Ling, H. Investigation of Tensile and High Cycle Fatigue Failure Behavior on a TIG Welded Titanium Alloy. Intermetallics 2021, 132, 107115. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. (Eds.) Titanium and Titanium Alloys: Fundamentals and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-3-527-30534-6. [Google Scholar]
- Welsch, G.; Boyer, R.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Detroit, MI, USA, 1993; ISBN 978-0-87170-481-8. [Google Scholar]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Detroit, MI, USA, 2000; ISBN 978-1-61503-062-0. [Google Scholar]
- Bundy, K.J. Corrosion and Other Electrochemical Aspects of Biomaterials. Crit. Rev. Biomed. Eng. 1994, 22, 139–251. [Google Scholar] [PubMed]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion Behavior of β Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Dhiflaoui, H.; Ben Salem, S.; Salah, M.; Dabaki, Y.; Chayoukhi, S.; Gassoumi, B.; Hajjaji, A.; Ben Cheikh Larbi, A.; Amlouk, M.; Benhayoune, H. Influence of TiO2 on the Microstructure, Mechanical Properties and Corrosion Resistance of Hydroxyapatite HaP + TiO2 Nanocomposites Deposited Using Spray Pyrolysis. Coatings 2023, 13, 1283. [Google Scholar] [CrossRef]
- Vaché, N.; Monceau, D. Oxygen Diffusion Modeling in Titanium Alloys: New Elements on the Analysis of Microhardness Profiles. Oxid. Met. 2020, 93, 215–227. [Google Scholar] [CrossRef]
- Vaché, N.; Cadoret, Y.; Dod, B.; Monceau, D. Modeling the Oxidation Kinetics of Titanium Alloys: Review, Method and Application to Ti-64 and Ti-6242s Alloys. Corros. Sci. 2021, 178, 109041. [Google Scholar] [CrossRef]
- Chen, W.; Cao, S.; Kou, W.; Zhang, J.; Wang, Y.; Zha, Y.; Pan, Y.; Hu, Q.; Sun, Q.; Sun, J. Origin of the Ductile-to-Brittle Transition of Metastable β-Titanium Alloys: Self-Hardening of ω-Precipitates. Acta Mater. 2019, 170, 187–204. [Google Scholar] [CrossRef]
- Gardner, H.M.; Gopon, P.; Magazzeni, C.M.; Radecka, A.; Fox, K.; Rugg, D.; Wade, J.; Armstrong, D.E.J.; Moody, M.P.; Bagot, P.A.J. Quantifying the Effect of Oxygen on Micro-Mechanical Properties of a near-Alpha Titanium Alloy. J. Mater. Res. 2021, 36, 2529–2544. [Google Scholar] [CrossRef]
- Gramse, G.; Kasper, M.; Fumagalli, L.; Gomila, G.; Hinterdorfer, P.; Kienberger, F. Calibrated Complex Impedance and Permittivity Measurements with Scanning Microwave Microscopy. Nanotechnology 2014, 25, 145703. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Gramse, G.; Niegemann, J.; Zeier, M.; Kienberger, F. Measuring Low Loss Dielectric Substrates with Scanning Probe Microscopes. Appl. Phys. Lett. 2014, 105, 013102. [Google Scholar] [CrossRef]
- Lai, K.; Kundhikanjana, W.; Kelly, M.A.; Shen, Z.X. Calibration of Shielded Microwave Probes Using Bulk Dielectrics. Appl. Phys. Lett. 2008, 93, 123105. [Google Scholar] [CrossRef]
- Berweger, S.; Weber, J.C.; John, J.; Velazquez, J.M.; Pieterick, A.; Sanford, N.A.; Davydov, A.V.; Brunschwig, B.; Lewis, N.S.; Wallis, T.M.; et al. Microwave Near-Field Imaging of Two-Dimensional Semiconductors. Nano Lett. 2015, 15, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, A.; Wallis, T.M.; Kabos, P. Near-Field Scanning Microwave Microscopy: An Emerging Research Tool for Nanoscale Metrology. IEEE Microw. 2014, 15, 52–64. [Google Scholar] [CrossRef]
- Bourillot, E.; Vitry, P.; Optasanu, V.; Plassard, C.; Lacroute, Y.; Montessin, T.; Lesniewska, E. Solid Solution Characterization in Metal by Original Tomographic Scanning Microwave Microscopy Technique. In Characterization of Minerals, Metals, and Materials 2015; Carpenter, J.S., Bai, C., Escobedo, J.P., Hwang, J., Ikhmayies, S., Li, B., Li, J., Monteiro, S.N., Peng, Z., Zhang, M., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 65–72. ISBN 978-1-119-08246-0. [Google Scholar]
- Optasanu, V.; Bourillot, E.; Selon, R.; Lavisse, L.; Sanchot, A.; Vitry, P.; Kanjer, A.; Berger, P.; Lesniewska, E.; Montesin, T. Characterization of Oxygen-Enriched Layers of TA6V, Titanium, and Zirconium by Scanning Microwave Microscopy. Oxid. Met. 2017, 88, 531–542. [Google Scholar] [CrossRef]
- Optasanu, V.; Bourillot, E.; Vitry, P.; Plassard, C.; Beaurenaut, L.; Jacquinot, P.; Herbst, F.; Berger, P.; Lesniewska, E.; Montessin, T. High-Resolution Characterization of the Diffusion of Light Chemical Elements in Metallic Components by Scanning Microwave Microscopy. Nanoscale 2014, 6, 14932–14938. [Google Scholar] [CrossRef]
- Convert, L.; Bourillot, E.; François, M.; Pocholle, N.; Baras, F.; Politano, O.; Costil, S. Laser Textured Titanium Surface Characterization. Appl. Surf. Sci. 2022, 586, 152807. [Google Scholar] [CrossRef]
- Anlage, S.M.; Talanov, V.V.; Schwartz, A.R. Principles of Near-Field Microwave Microscopy. In Scanning Probe Microscopy; Kalinin, S., Gruverman, A., Eds.; Springer: New York, NY, USA, 2007; pp. 215–253. ISBN 978-0-387-28667-9. [Google Scholar]
- Michalas, L.; Brinciotti, E.; Lucibello, A.; Gramse, G.; Joseph, C.H.; Kienberger, F.; Proietti, E.; Marcelli, R. De-Embedding Techniques for Nanoscale Characterization of Semiconductors by Scanning Microwave Microscopy. Microelectron. Eng. 2016, 159, 64–69. [Google Scholar] [CrossRef]
- Hommel, S.; Killat, N.; Altes, A.; Schweinboeck, T.; Kreupl, F. Determination of Doping Type by Calibrated Capacitance Scanning Microwave Microscopy. Microelectron. Reliab. 2017, 76–77, 218–221. [Google Scholar] [CrossRef]
- Hommel, S.; Killat, N.; Schweinboeck, T.; Altes, A.; Kreupl, F. Resolving Trapping Effects by Scanning Microwave Microscopy. Microelectron. Reliab. 2019, 92, 179–181. [Google Scholar] [CrossRef]
- Joseph, C.H.; Luzi, F.; Azman, S.N.A.; Forcellese, P.; Pavoni, E.; Fabi, G.; Mencarelli, D.; Gentili, S.; Pierantoni, L.; Morini, A.; et al. Nanoscale Characterization of Graphene Oxide-Based Epoxy Nanocomposite Using Inverted Scanning Microwave Microscopy. Sensors 2022, 22, 9608. [Google Scholar] [CrossRef] [PubMed]
- Plassard, C.; Bourillot, E.; Rossignol, J.; Lacroute, Y.; Lepleux, E.; Pacheco, L.; Lesniewska, E. Detection of Defects Buried in Metallic Samples by Scanning Microwave Microscopy. Phys. Rev. B 2011, 83, 121409. [Google Scholar] [CrossRef]
- Vitry, P. Applications and Development of Acoustic and Microwave Atomic Force Microscopy for High Resolution Tomography Analysis. Doctoral Dissertation, Université de Bourgogne, Dijon, France, 2016. [Google Scholar]
- ASTM E837-20; Standard Test Method for Determining Residual Stresses by the Hole-Drillong Strain-Gage Method. ASTM International: West Conshohocken, PA, USA, 2021.
- Garbacz, H.; Kurzydlowski, K.J. Properties of Nanotitanium for Potential Medical Applications. Macromol. Symp. 2007, 253, 128–133. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Biomaterial Surface Modification of Titanium and Titanium Alloys for Medical Applications. Nanomedicine 2014, 111, 111. [Google Scholar]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science Publishers: Barking, UK, 1988. [Google Scholar]
- Amsel, G.; Béranger, G.; de Gélas, B.; Lacombe, P. Use of the Nuclear Reaction O16(d, p)O17 to Study Oxygen Diffusion in Solids and Its Application to Zirconium. J. Appl. Phys. 1968, 39, 2246–2255. [Google Scholar] [CrossRef]
- Lavisse, L.; Berger, P.; Kanjer, A.; Optasanu, V.; Gorny, C.; Peyre, P.; François, M.; Montesin, T.; Marco de Lucas, M.C. Tracking the Role of Nitrogen in the Improvement of the High Temperature Oxidation Resistance of Titanium by Mechanical Treatments. Corros. Sci. 2022, 197, 110080. [Google Scholar] [CrossRef]
- Hay, J. Introduction to Instrumented Indentation Testing. Exp. Tech. 2009, 33, 66–72. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. Nanoindentation; Mechanical Engineering Series; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9871-2. [Google Scholar]
- Pethica, J.B.; Oliver, W.C. Tip Surface Interactions in STM and AFM. Phys. Scr. 1987, T19A, 61–66. [Google Scholar] [CrossRef]
- Oliver, W.; Pethica, J. Method for Continuous Determination of Elastic Stiffness of Contact between Two Bodies. U.S. Patent No 4,848,141, 1989. [Google Scholar]
- Schreiber, E. The Effect of Solid Solutions upon the Bulk Modulus and Its Pressure Derivative: Implications for Equations of State. Earth Planet. Sci. Lett. 1969, 7, 137–140. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnier, M.; Lesniewska, E.; Optasanu, V.; Guelorget, B.; Berger, P.; Lavisse, L.; François, M.; Custovic, I.; Pocholle, N.; Bourillot, E. Submicronic-Scale Mechanochemical Characterization of Oxygen-Enriched Materials. Nanomaterials 2024, 14, 628. https://doi.org/10.3390/nano14070628
Garnier M, Lesniewska E, Optasanu V, Guelorget B, Berger P, Lavisse L, François M, Custovic I, Pocholle N, Bourillot E. Submicronic-Scale Mechanochemical Characterization of Oxygen-Enriched Materials. Nanomaterials. 2024; 14(7):628. https://doi.org/10.3390/nano14070628
Chicago/Turabian StyleGarnier, Marie, Eric Lesniewska, Virgil Optasanu, Bruno Guelorget, Pascal Berger, Luc Lavisse, Manuel François, Irma Custovic, Nicolas Pocholle, and Eric Bourillot. 2024. "Submicronic-Scale Mechanochemical Characterization of Oxygen-Enriched Materials" Nanomaterials 14, no. 7: 628. https://doi.org/10.3390/nano14070628
APA StyleGarnier, M., Lesniewska, E., Optasanu, V., Guelorget, B., Berger, P., Lavisse, L., François, M., Custovic, I., Pocholle, N., & Bourillot, E. (2024). Submicronic-Scale Mechanochemical Characterization of Oxygen-Enriched Materials. Nanomaterials, 14(7), 628. https://doi.org/10.3390/nano14070628







