In Situ Electrochemical Synthesis of Squamous-like Cu2S Induced by Sulfate-Reducing Bacteria as a Fenton-like Catalyst in Wastewater Treatment: Catalytic Performance and Mechanism
Abstract
1. Introduction
2. Experimental
2.1. Culture of SRB
2.2. Synthesis of Cu2S on Copper Mesh Induced by SRB
2.3. Characterization of CSCM
2.4. Fenton-like Degradation of Methylene Blue (MB) by CSCM-SRB
2.5. Disinfection Properties of CSCM-SRB against E. coli
2.6. Mechanism Study on CSCM-SRB
3. Results and Discussion
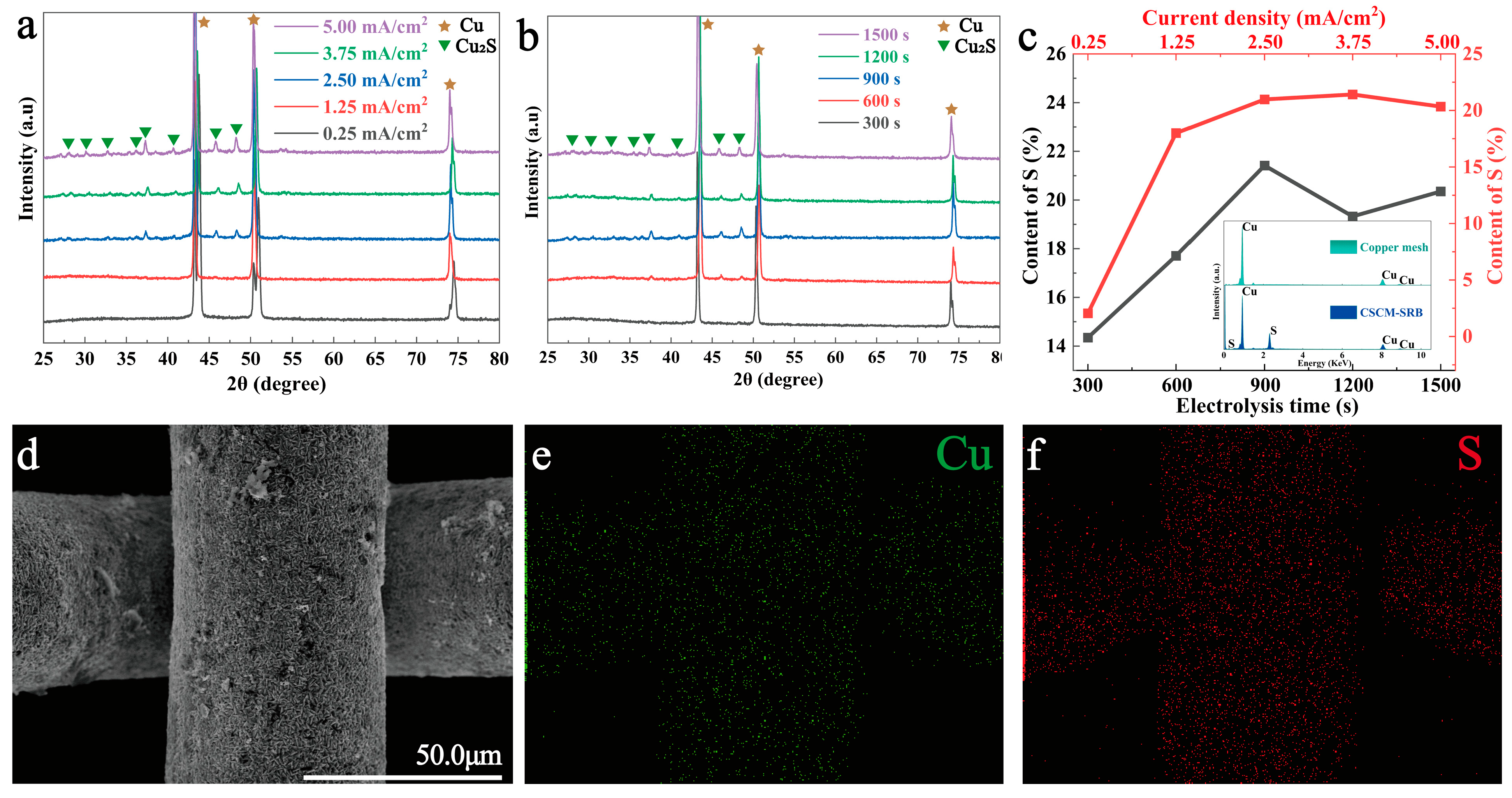
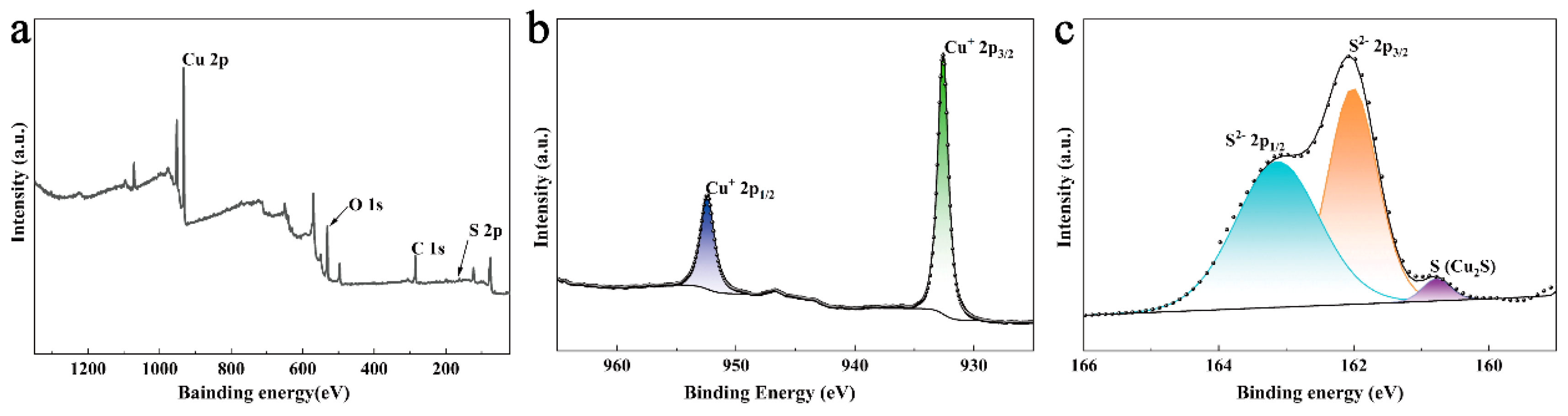
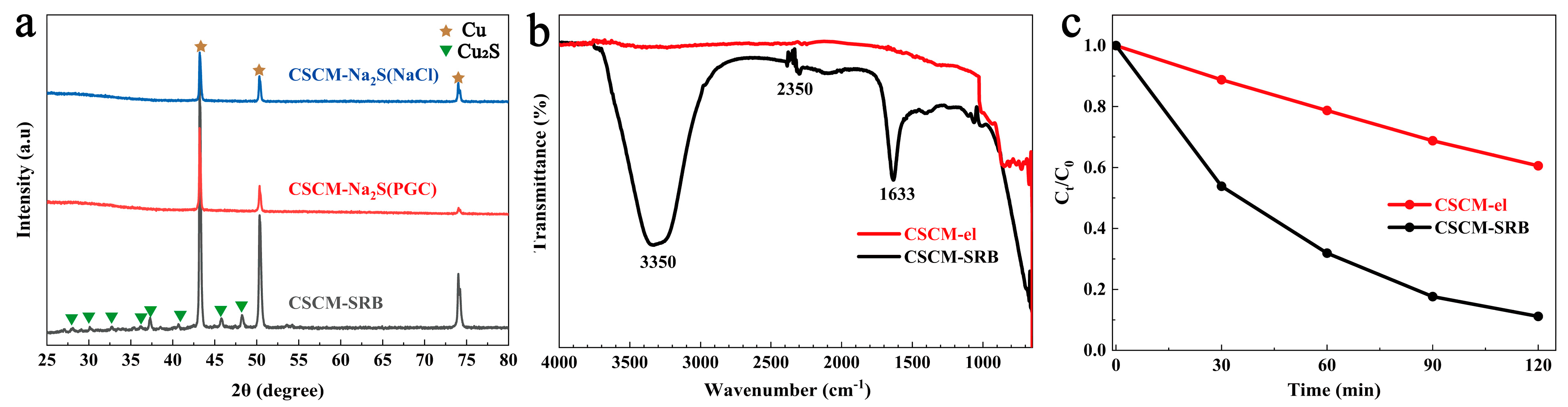
3.1. Characterization of CSCM-SRB
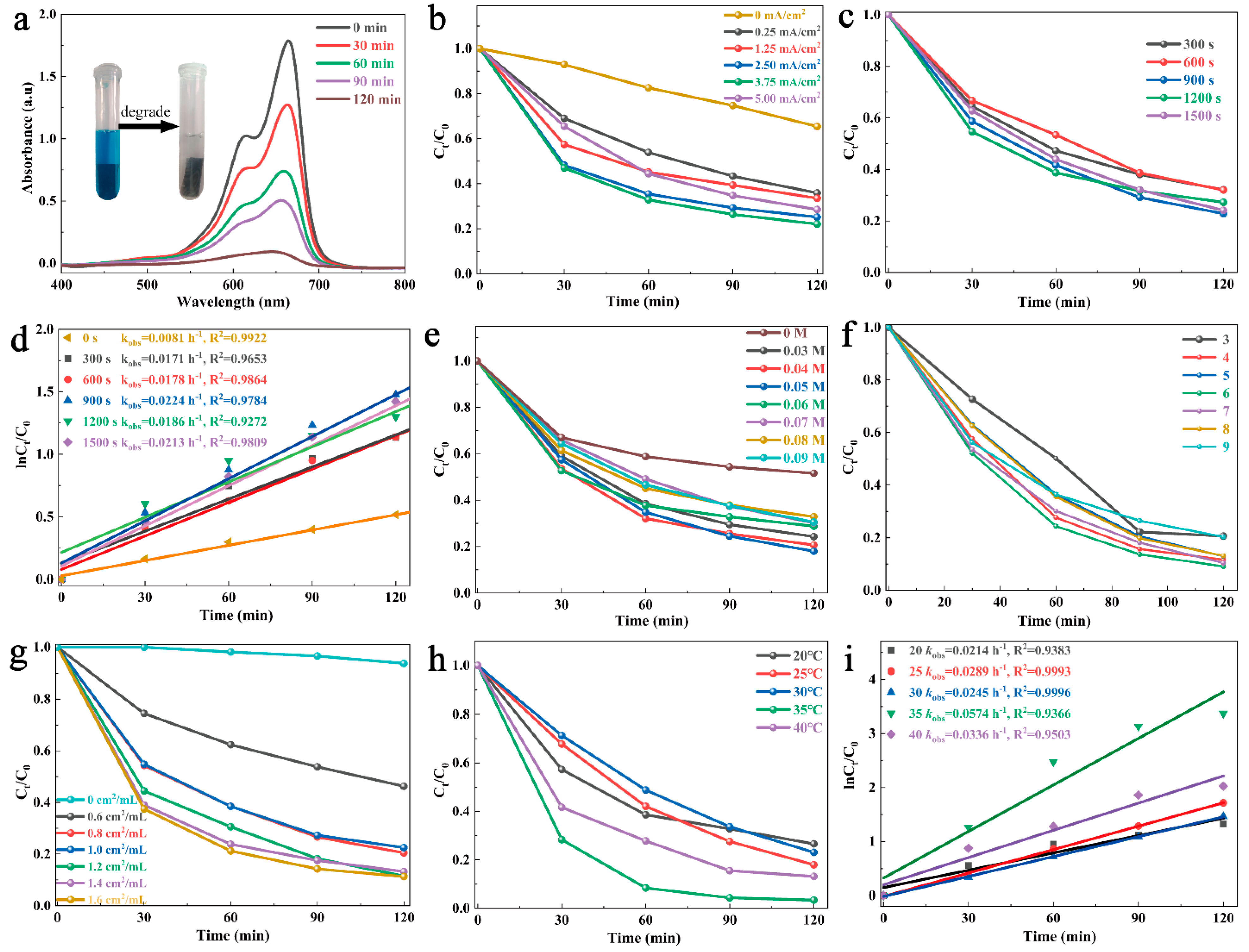
3.2. Fenton-like Degradation of MB by CSCM-SRB
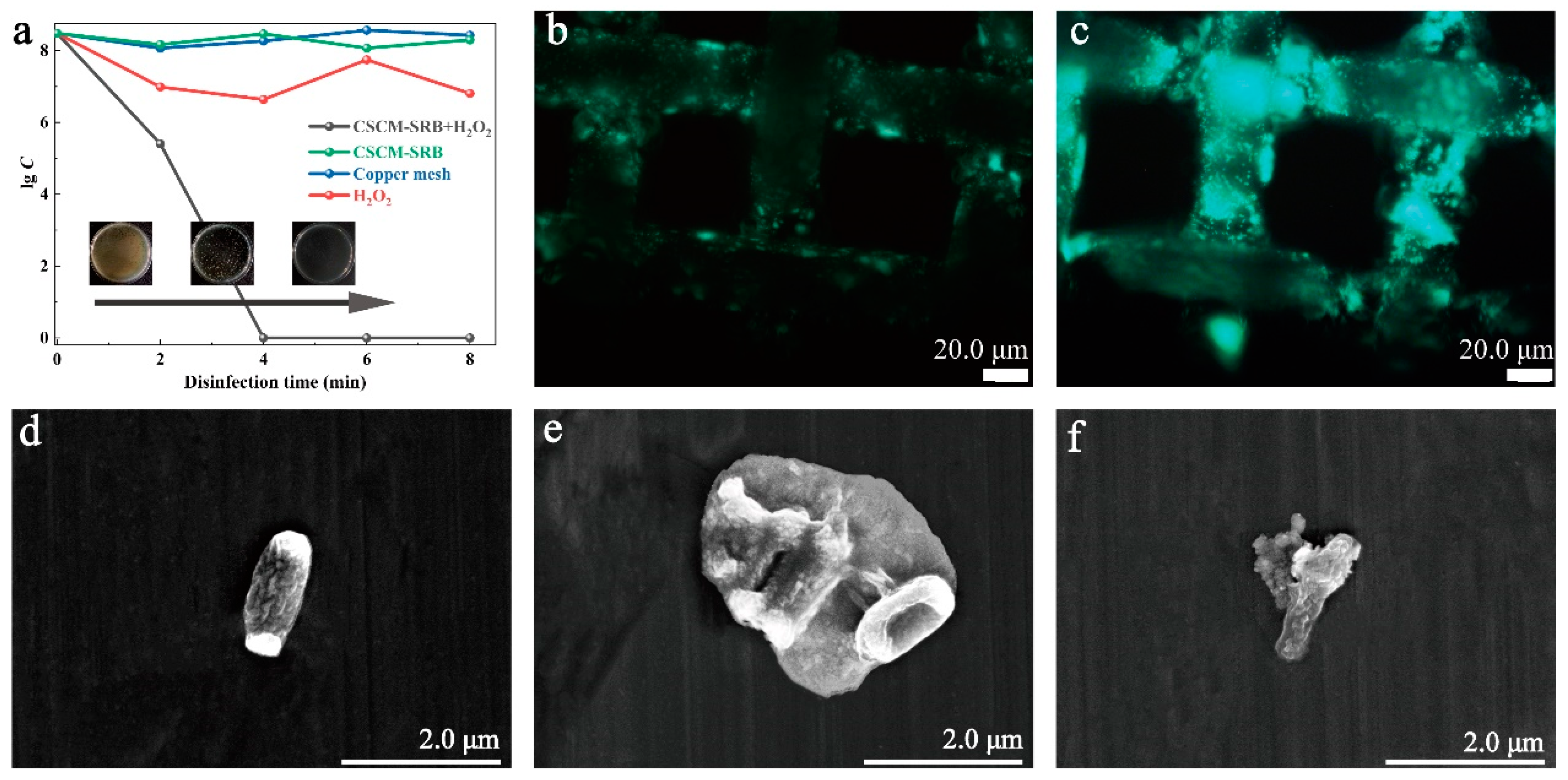
3.3. Fenton-like Disinfection Properties of CSCM-SRB
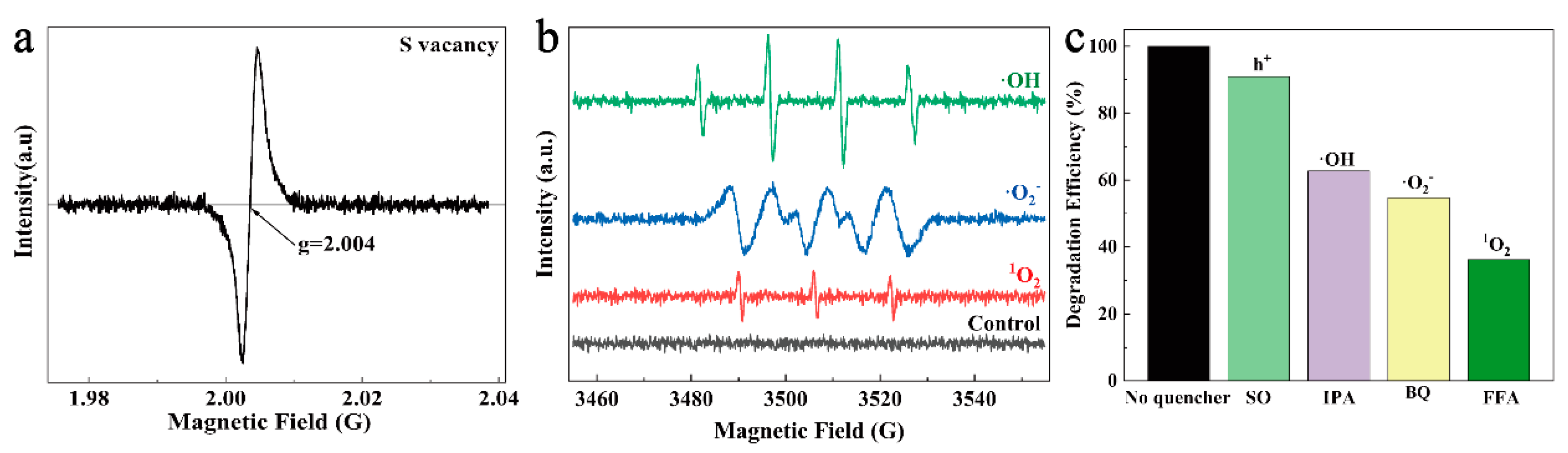
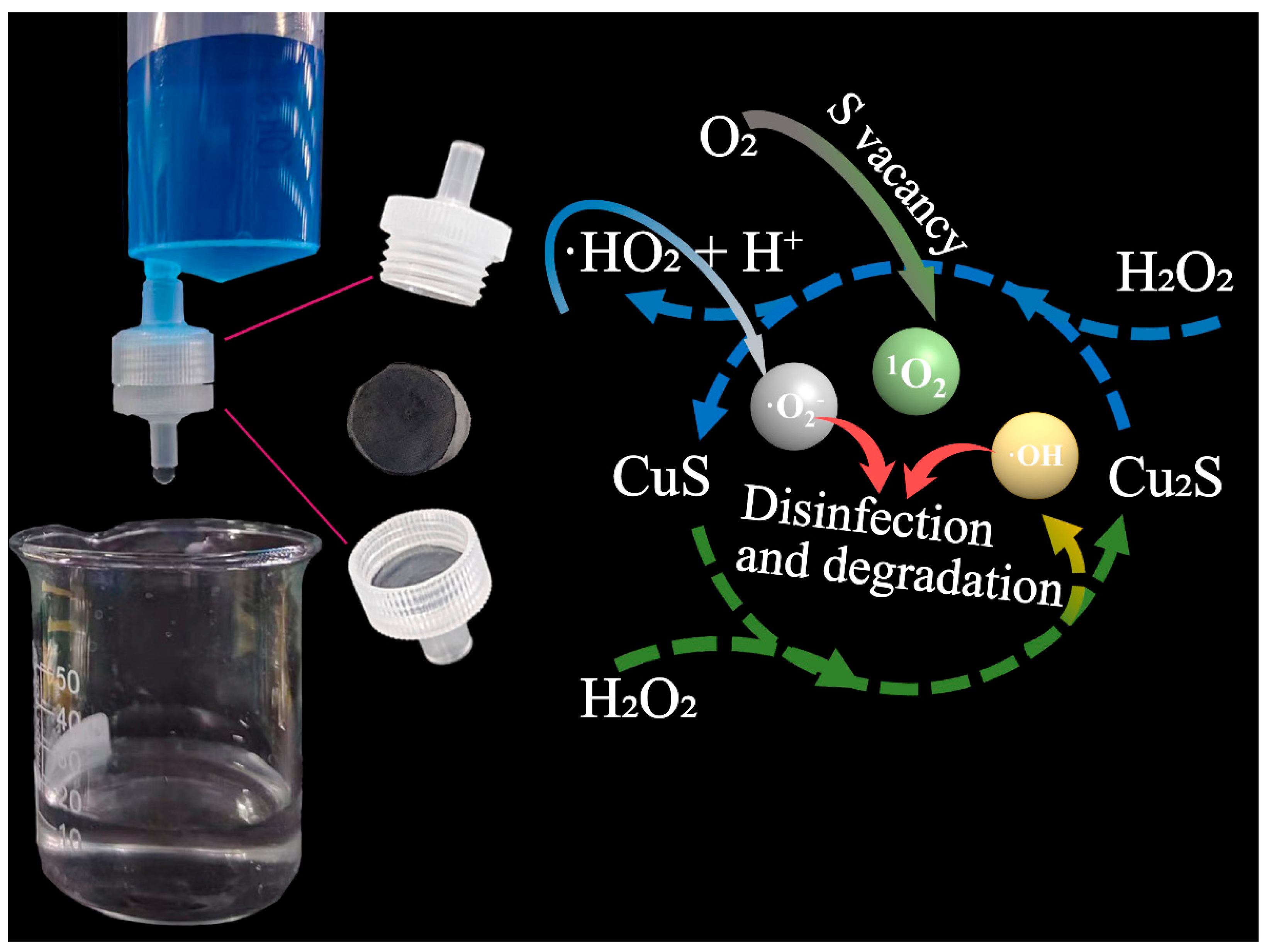
3.4. Study on the Fenton-like Mechanism of CSCM-SRB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.K.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Z.; Di, D.; Wang, H. Water resources allocation based on water resources supply-demand forecast and comprehensive values of water resources. J. Hydrol. Reg. Stud. 2023, 47, 101421. [Google Scholar] [CrossRef]
- George, J.S.; Ramos, A.; Shipley, H.J. Tanning facility wastewater treatment: Analysis of physical–chemical and reverse osmosis methods. J. Environ. Chem. Eng. 2015, 3, 969–976. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef]
- Cui, B.; Rong, H.; Tian, T.; Guo, D.; Duan, L.; Nkinahamira, F.; Ndagijimana, P.; Yan, W.; Naidu, R. Chemical methods to remove microplastics from wastewater: A review. Environ. Res. 2024, 249, 118416. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, Y.; Liu, X.; Zhang, A.; Yang, R.; Han, Y.; Liu, P.; He, H.B.; Li, Z. Regulation methods and enhanced mechanism on the efficient degradation of aromatics in biochemical treatment system of coal chemical wastewater. J. Environ. Manag. 2023, 348, 119358. [Google Scholar] [CrossRef]
- Deveci, E.Ü.; Dizge, N.; Yatmaz, H.C.; Aytepe, Y. Integrated process of fungal membrane bioreactor and photocatalytic membrane reactor for the treatment of industrial textile wastewater. Biochem. Eng. J. 2016, 105, 420–427. [Google Scholar] [CrossRef]
- Zhou, X.J.; Shi, P.H.; Qin, Y.F.; Fan, J.C.; Min, Y.L.; Yao, W.F. Synthesis of Co3O4/graphene composite catalysts through CTAB-assisted method for Orange II degradation by activation of peroxymonosulfate. J. Mater. Sci. Mater. Electron. 2015, 27, 1020–1030. [Google Scholar] [CrossRef]
- Kubendiran, H.; Hui, D.; Pulimi, M.; Chandrasekaran, N.; Murthy, P.S.; Mukherjee, A. Removal of methyl orange from aqueous solution using SRB supported Bio-Pd/Fe NPs. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100561. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Y.; Xiang, Y.; Shang, C. Influence of pre-ozonation of DOM on micropollutant abatement by UV-based advanced oxidation processes. J. Hazard. Mater. 2020, 391, 122201. [Google Scholar] [CrossRef] [PubMed]
- Ateş, H.; Argun, M.E. Advanced oxidation of landfill leachate: Removal of micropollutants and identification of by-products. J. Hazard. Mater. 2021, 413, 125326. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Albornoz, M.; Mansilla, H.; Vidal, G.; Contreras, D. Effects of pH and substituted 1,2-dihydroxybenzenes on the reaction pathway of Fenton-like systems. Appl. Catal. B Environ. 2018, 226, 93–102. [Google Scholar] [CrossRef]
- Strlic, M.; Kolar, J.; Selih, V.; Kocar, D.; Pihlar, B. A comparative study of several transition metals in Fenton-like reaction systems at circum-neutral pH. Acta Chim. Slov. 2003, 50, 619–632. [Google Scholar]
- Trivedi, N.; Joshi, K.K.; Siraj, S.; Sahatiya, P.; Patel, V.; Sumesh, C.K.; Pataniya, P.M. Self-supported Cr–Cu2S nanoflakes for hydrogen production from seawater. Int. J. Hydrogen Energy 2024, 49, 1113–1122. [Google Scholar] [CrossRef]
- Zhao, B.; Li, S.; Zhang, Q.; Wang, Y.; Song, C.; Zhang, Z.; Yu, K. Controlled synthesis of Cu2S microrings and their photocatalytic and field emission properties. Chem. Eng. J. 2013, 230, 236–243. [Google Scholar] [CrossRef]
- Niu, L.; Kang, Z. A facile approach for the fabrication of 3D flower-like Cu2S nanostructures on brass mesh with temperature-induced wetting transition for efficient oil-water separation. Appl. Surf. Sci. 2017, 422, 456–468. [Google Scholar] [CrossRef]
- Yue, X.; Hu, R.; Zhu, D.; Qi, J.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Sui, Y. Controlled synthesis and formation mechanism of flower-like CuS/NiS microspheres for supercapacitors. Surf. Interfaces 2021, 22, 100871. [Google Scholar] [CrossRef]
- Yang, M.; Yang, X.; Huai, L.; Liu, W. Synthesis and characterization of spherical hollow composed of Cu2S nanoparticles. Appl. Surf. Sci. 2008, 255, 1750–1753. [Google Scholar] [CrossRef]
- Su, Z.; Su, Y.; Huynen, I.; Kannan, S.; Mehrez, S.; Aly, W.H.F.; Mohanavel, V.; Mahariq, I. Decoration of worm-like Cu2S particles on CuCo2S4 micro-spheres: An effective strategy to improve the electromagnetic dissipation feature. Ceram. Int. 2023, 49, 10702–10713. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, S.; Yuan, H.; Wu, Z.; Li, M. 3D hierarchically branched Cu2S/ZnO heterojunction nanowire arrays for enhanced solar water splitting. Mater. Today Commun. 2023, 34, 105417. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, D.; Liang, J.; Shen, J.; Zhang, S.; Qian, Y. Growth of Cu2S Ultrathin Nanowires in a Binary Surfactant Solvent. J. Phys. Chem. B 2005, 109, 10699–10704. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Ma, L.-L.; Zhang, Y.-G.; Tan, M.; Wang, J.-B.; Yu, Y. Controllable synthesis of self-assembled Cu2S nanostructures through a template-free polyol process for the degradation of organic pollutant under visible light. Mater. Res. Bull. 2009, 44, 1834–1841. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kalita, M.P.C. SnO2-Cu2S nanocomposites: Structural and optical properties with enhanced photocatalytic activities for ciprofloxacin antibiotic and methylene blue dye degradation. Opt. Mater. 2023, 140, 113845. [Google Scholar] [CrossRef]
- Xu, F.; Costa, M.F.; Sun, S.; Teixeira, V.; Wang, Y.; Bian, F.; Wang, H.; Cui, H. Structure optimization on the photoelectric and photocatalytic properties of Cu2S and ZnO complex films. Mater. Today Proc. 2015, 2, 253–260. [Google Scholar] [CrossRef]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Kniemeyer, O.; Musat, F.; Sievert, S.M.; Knittel, K.; Wilkes, H.; Blumenberg, M.; Michaelis, W.; Classen, A.; Bolm, C.; Joye, S.B.; et al. Anaerobic oxidation of short-chain hydrocarbons by marine sulphate-reducing bacteria. Nature 2007, 449, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Deng, L.; Ren, W.; Li, M.; Wu, C.; Qu, A.; Wan, C. Photoelectrochemical and energy storage properties for metal sulfides regulated by biomineralization of sulfate reducing bacteria. J. Clean. Prod. 2022, 340, 130741. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Toxicity of hydrogen sulfide toward sulfate-reducing bacteria Desulfovibrio piger Vib-7. Arch. Microbiol. 2019, 201, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gong, L.; Wang, H.; Dong, C.; Wang, J.; Qi, K.; Liu, H.; Guo, X.; Xia, B.Y. Preparation of nickel-iron hydroxides by microorganism corrosion for efficient oxygen evolution. Nat. Commun. 2020, 11, 5075. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhai, X.; Yang, J.; Guan, F.; Zhang, Y.; Duan, J.; Hou, B. Two metabolic stages of SRB strain Desulfovibrio bizertensis affecting corrosion mechanism of carbon steel Q235. Corros. Commun. 2023, 10, 56–68. [Google Scholar] [CrossRef]
- He, R.-M.; Yang, Y.-L.; Chen, H.-J.; Liu, J.-J.; Sun, Y.-M.; Guo, W.-N.; Li, D.-H.; Hou, X.-J.; Suo, G.-Q.; Ye, X.-H.; et al. In situ controllable growth of Cu7S4 nanosheets on copper mesh for catalysis: The synergistic effect of photocatalytic Fenton-like process. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128651. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, Y.; Hao, L.; Cao, J.; Zhai, X.; Dou, K.; Jiang, F.; Sun, C. 1D Bi2S3 nanorods modified 2D BiOI nanoplates for highly efficient photocatalytic activity: Pivotal roles of oxygen vacancies and Z-scheme heterojunction. J. Mater. Sci. Technol. 2023, 142, 45–59. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.-W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ju, P.; Zhang, Y.; Zhai, X.; Sun, C.; Duan, J.; Su, Y.; Lu, Z.; Liao, D. Fabrication of hierarchical flower-like BiOI/MoS2 heterostructures with highly enhanced visible-light photocatalytic activities. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125714. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumaraguru, S.P.; Popov, B.N. Nanostructured Zn-Ni Alloys by Pulse Electrodeposition. In Proceedings of the Proceedings-AESF SUR/FIN Annual International Technical Conference, Columbia, SC, USA, 28–30 June 2004; pp. 1064–1075. [Google Scholar]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O’Shea, K.E. UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin. Catal. Today 2014, 224, 70–76. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Effect of solution pH on the performance of three electrolytic advanced oxidation processes for the treatment of textile wastewater and sludge characteristics. RSC Adv. 2014, 4, 27946–27954. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Kollu, P.; Jeong, S.K.; Grace, A.N. A template-free facile approach for the synthesis of CuS–rGO nanocomposites towards enhanced photocatalytic reduction of organic contaminants and textile effluents. RSC Adv. 2015, 5, 15831–15840. [Google Scholar] [CrossRef]
- Lim, C.L.; Morad, N.; Teng, T.T.; Ismail, N. Treatment of Terasil red R dye wastewater using H2O2/pyridine/Cu(II) system. J. Hazard. Mater. 2009, 168, 383–389. [Google Scholar] [CrossRef]
- Xiang, W.; Zhou, T.; Wang, Y.; Huang, M.; Wu, X.; Mao, J.; Lu, X.; Zhang, B. Catalytic oxidation of diclofenac by hydroxylamine-enhanced Cu nanoparticles and the efficient neutral heterogeneous-homogeneous reactive copper cycle. Water Res. 2019, 153, 274–283. [Google Scholar] [CrossRef]
- Wang, J.; Yao, J.; Li, Y.; Wei, Z.; Gao, C.; Jiang, L.; Wu, X. S vacancies-introduced chalcopyrite switch radical to non-radical pathways via peroxymonosulfate activation: Vital roles of S vacancies. J. Hazard. Mater. 2024, 467, 133751. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, Z.; Yang, J.; Sun, J.; Zhai, X.; Ma, F.; Duan, J.; Ju, P.; Hou, B. In Situ Electrochemical Synthesis of Squamous-like Cu2S Induced by Sulfate-Reducing Bacteria as a Fenton-like Catalyst in Wastewater Treatment: Catalytic Performance and Mechanism. Nanomaterials 2024, 14, 621. https://doi.org/10.3390/nano14070621
Zhao L, Li Z, Yang J, Sun J, Zhai X, Ma F, Duan J, Ju P, Hou B. In Situ Electrochemical Synthesis of Squamous-like Cu2S Induced by Sulfate-Reducing Bacteria as a Fenton-like Catalyst in Wastewater Treatment: Catalytic Performance and Mechanism. Nanomaterials. 2024; 14(7):621. https://doi.org/10.3390/nano14070621
Chicago/Turabian StyleZhao, Liuhui, Zihao Li, Jing Yang, Jiawen Sun, Xiaofan Zhai, Fubin Ma, Jizhou Duan, Peng Ju, and Baorong Hou. 2024. "In Situ Electrochemical Synthesis of Squamous-like Cu2S Induced by Sulfate-Reducing Bacteria as a Fenton-like Catalyst in Wastewater Treatment: Catalytic Performance and Mechanism" Nanomaterials 14, no. 7: 621. https://doi.org/10.3390/nano14070621
APA StyleZhao, L., Li, Z., Yang, J., Sun, J., Zhai, X., Ma, F., Duan, J., Ju, P., & Hou, B. (2024). In Situ Electrochemical Synthesis of Squamous-like Cu2S Induced by Sulfate-Reducing Bacteria as a Fenton-like Catalyst in Wastewater Treatment: Catalytic Performance and Mechanism. Nanomaterials, 14(7), 621. https://doi.org/10.3390/nano14070621







