Fluorocarbon Plasma-Polymerized Layer Increases the Release Time of Silver Ions and the Antibacterial Activity of Silver-Based Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Surface Characterization
2.3.1. X-ray Photoelectron Spectroscopy (XPS)
2.3.2. Contact Angle and Surface Energy
2.3.3. Atomic Force Microscopy (AFM)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
2.4. Release Profile
2.5. Antibacterial Essay
2.5.1. Bacterial Strain and Culture Preparation
2.5.2. Antibacterial Activity Test
2.5.3. Statistical Analysis
3. Results
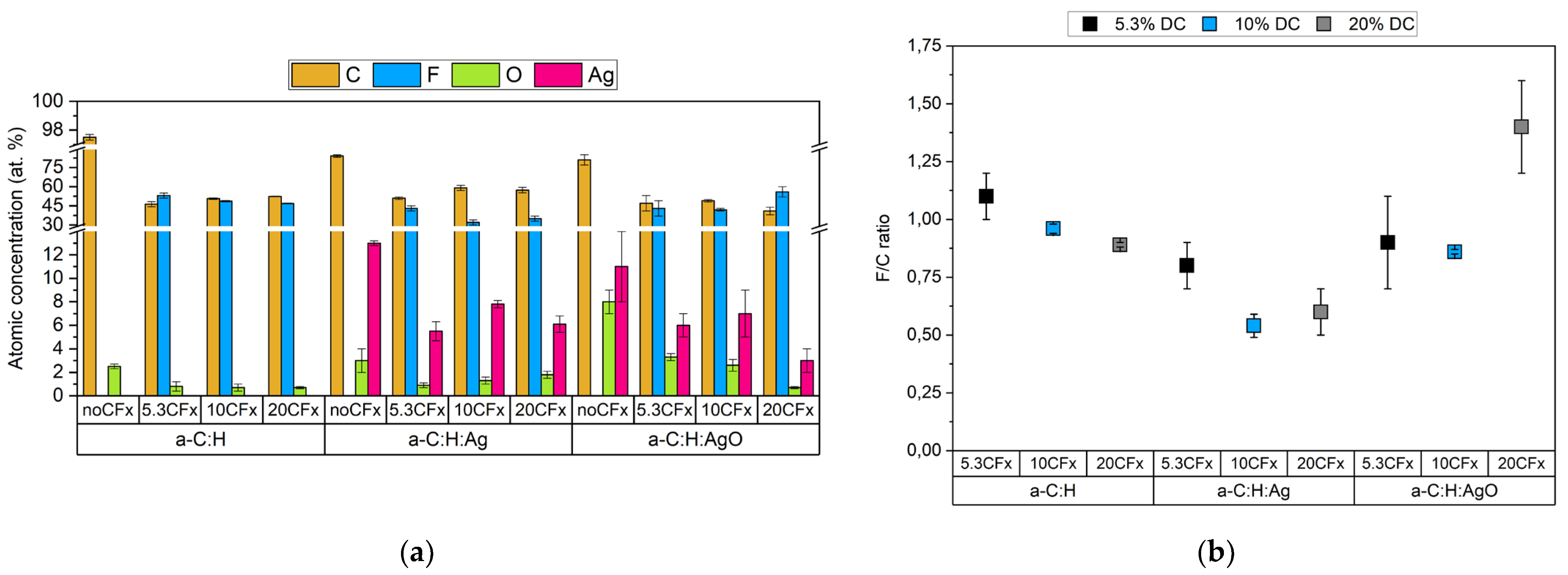
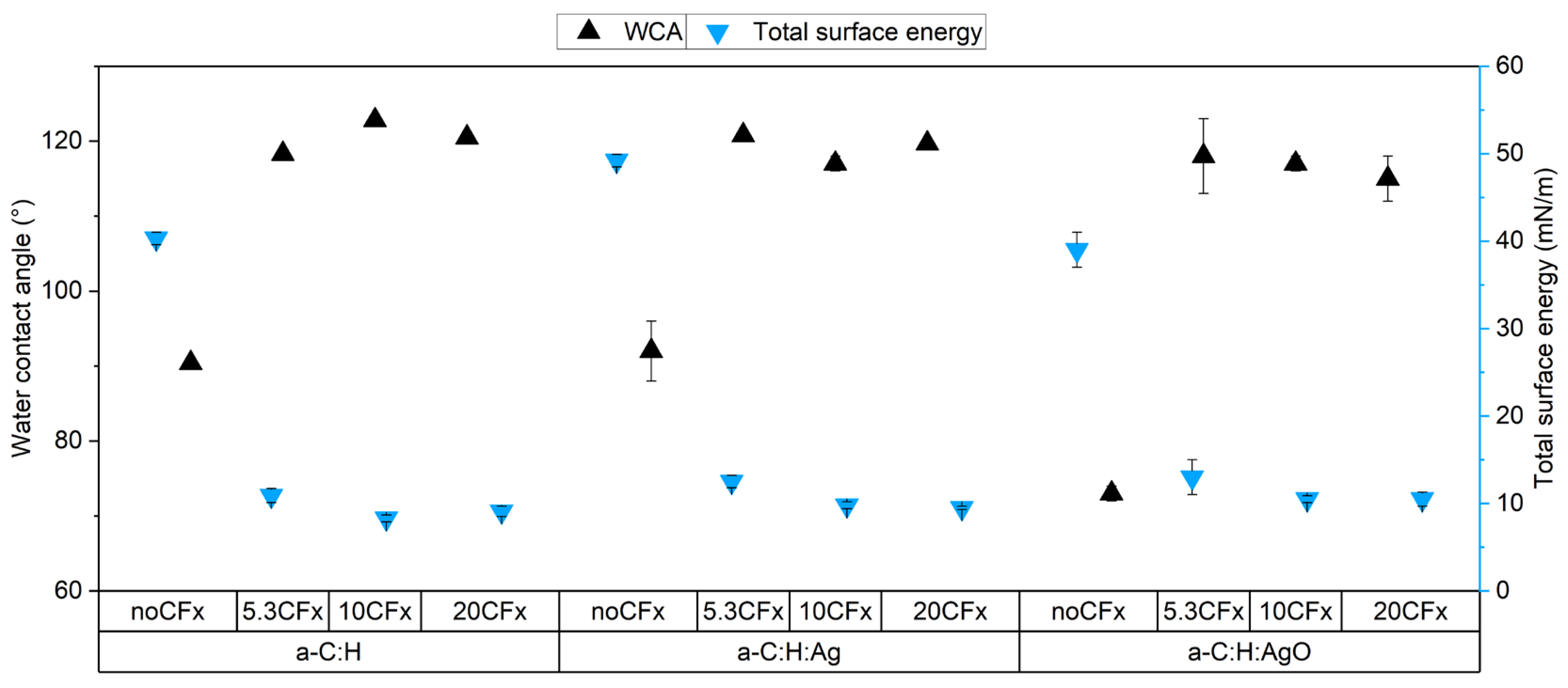
3.1. Chemical Characterization
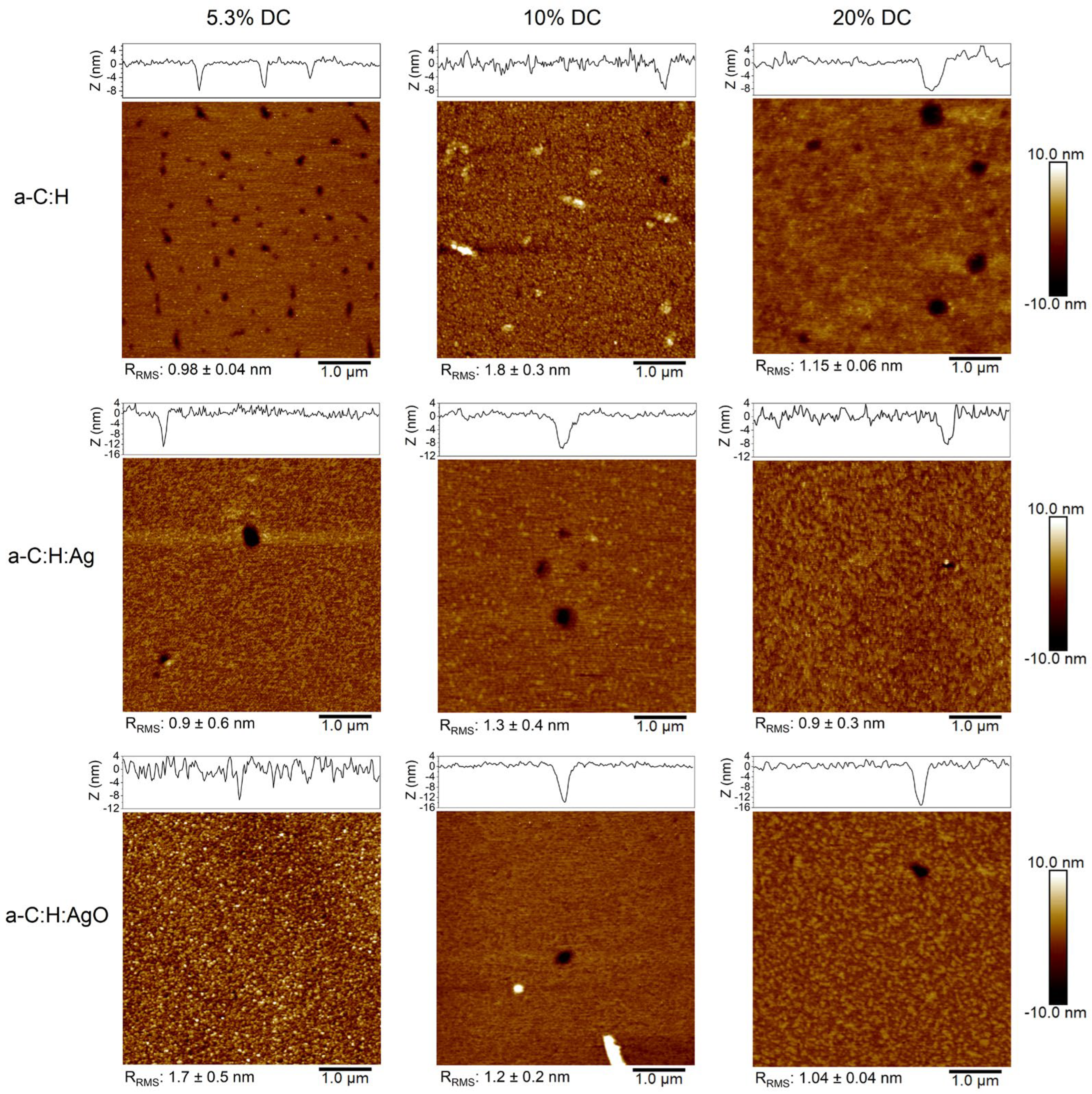
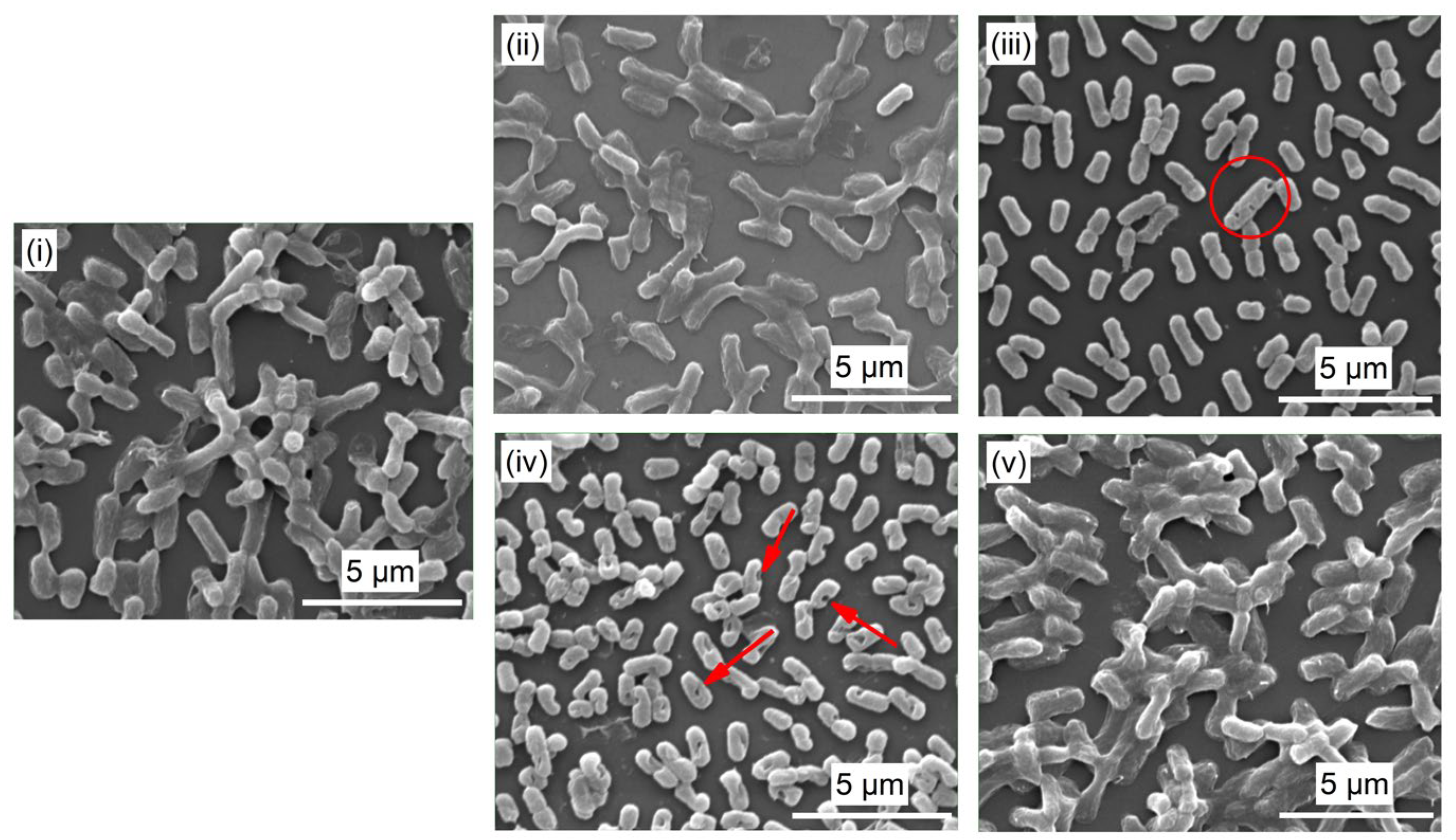
3.2. Surface Morphology
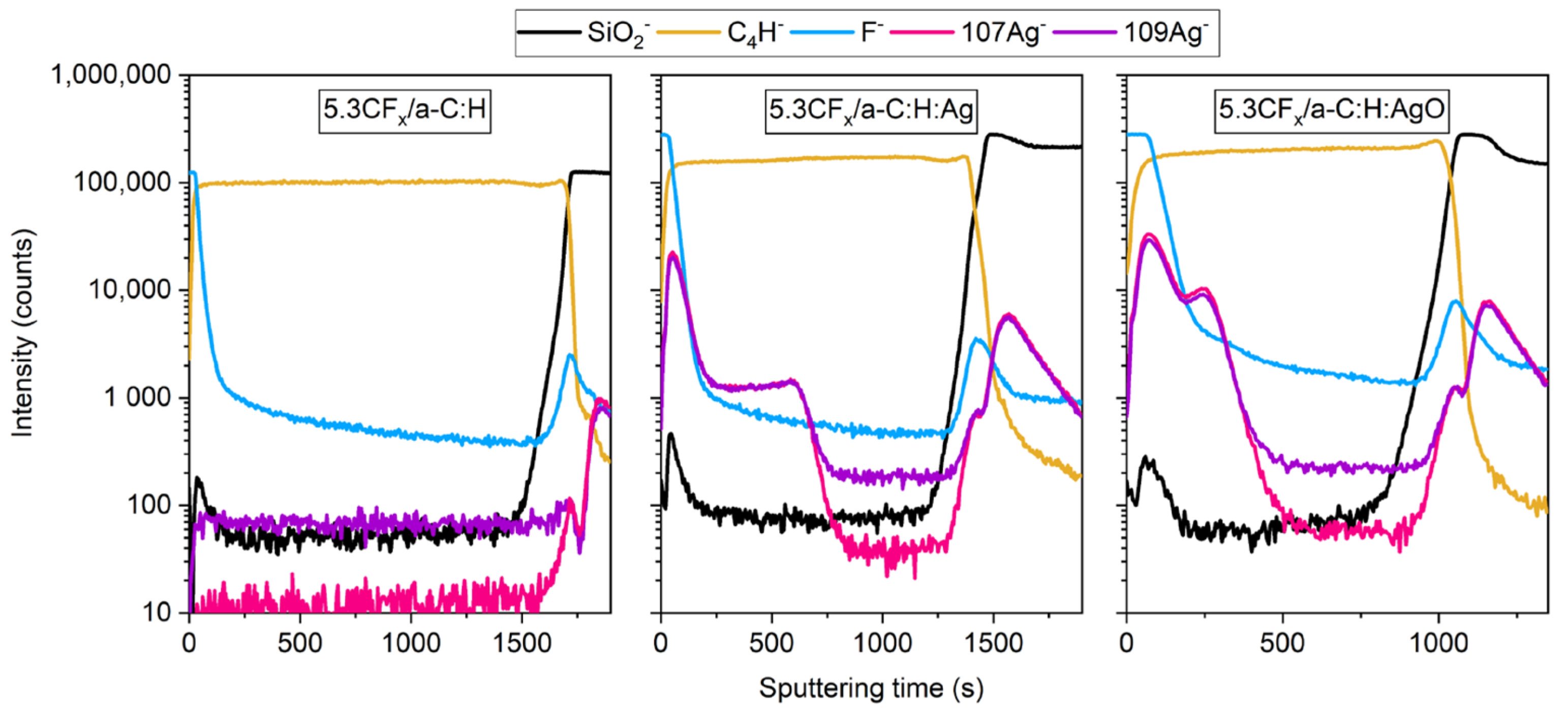
3.3. ToF-SIMS Analysis
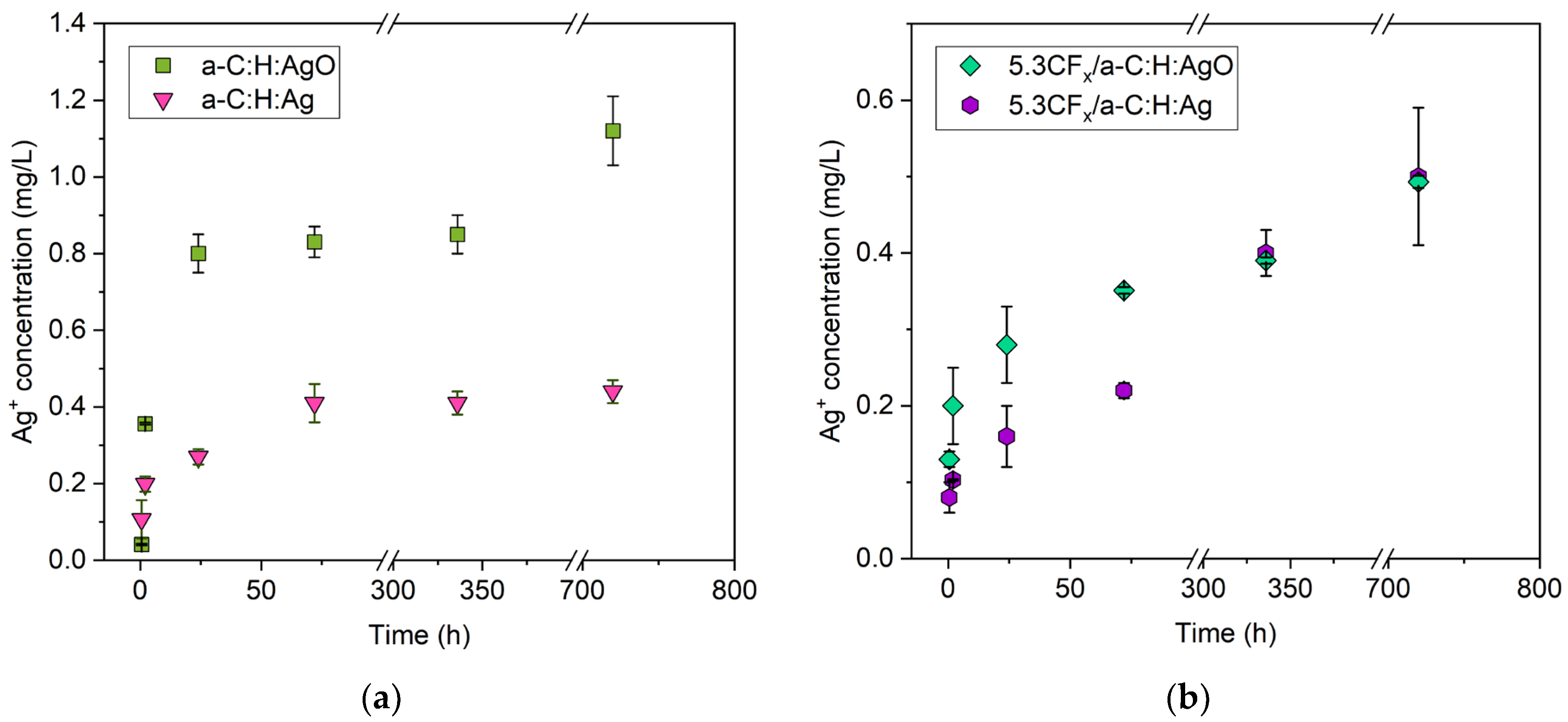
3.4. Release of Silver Ions
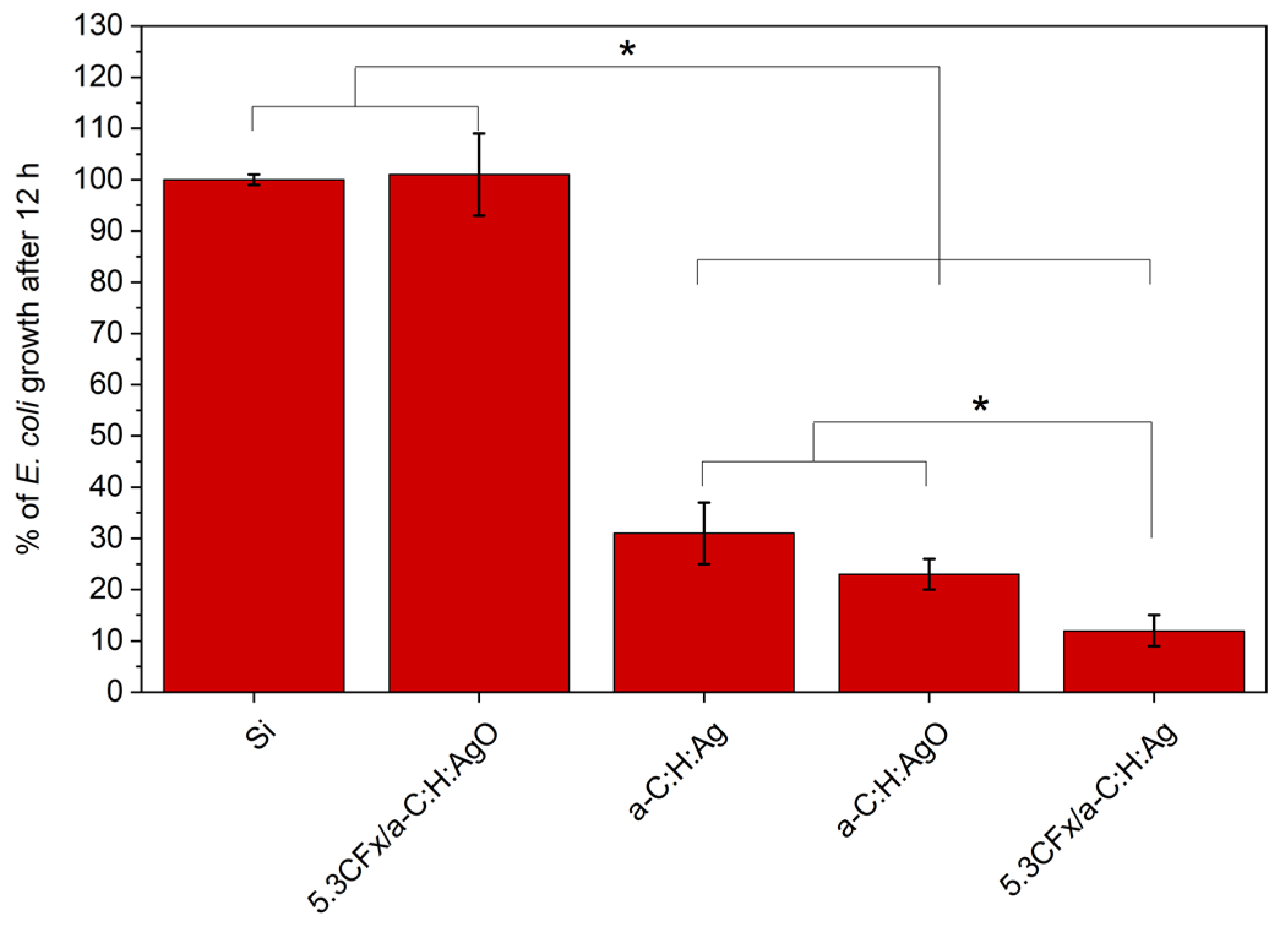
3.5. Antibacterial Activity
4. Discussion
4.1. Effect of the Duty Cycle on the Deposition of CFx Films
4.2. Use of 5.3% DC Fluoropolymeric Film as a Barrier Coating to Enhance Antibacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gazzarata, R.; Monteverde, M.E.; Ruggiero, C.; Maggi, N.; Palmieri, D.; Parruti, G.; Giacomini, M. Healthcare Associated Infections: An Interoperable Infrastructure for Multidrug Resistant Organism Surveillance. Int. J. Environ. Res. Public Health 2020, 17, 465. [Google Scholar] [CrossRef]
- Bonilla-Gameros, L.; Chevallier, P.; Sarkissian, A.; Mantovani, D. Silver-based antibacterial strategies for healthcare-associated infections: Processes, challenges, and regulations. An integrated review. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102142. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Bonilla-Gameros, L.; Chevallier, P.; Copes, F.; Sarkissian, A.; Mantovani, D. The oxidation state of Ag nanoparticles highly affects the release of Ag ions without compromising the mechanical performance and the safety of amorphous hydrogenated carbon coatings. Diam. Relat. Mater. 2022, 130, 109430. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Houdt, R.V. Silver as an Antimicrobial Agent: The Resistance Issue. In Silver Nanoparticles for Antibacterial Devices; Cao, H., Ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 165. [Google Scholar]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef]
- Terriza, A.; Álvarez, R.; Borrás, A.; Cotrino, J.; Yubero, F.; González-Elipe, A.R. Roughness assessment and wetting behavior of fluorocarbon surfaces. J. Colloid Interface Sci. 2012, 376, 274–282. [Google Scholar] [CrossRef]
- Dalvia, V.H.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef]
- Jung, S.-B.; Cho, Y.-J.; Lee, J.; Lee, K.-B.; Lee, Y. Investigation of Surface Properties of Fluorocarbon Films Produced Using Plasma Techniques. J. Nanosci. Nanotechnol. 2018, 18, 6288–6293. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jedrzejewski, R.; Moszynski, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Favia, P.; Cicala, G.; Milella, A.; Palumbo, F.; Rossini, P.; d’Agostino, R. Deposition of super-hydrophobic fluorocarbon coatings in modulated RF glow discharges. Surf. Coat. Technol. 2003, 169, 609–612. [Google Scholar] [CrossRef]
- Guoa, R.; Yina, G.; Shaa, X.; Zhaob, Q.; Weia, L.; Wang, H. The significant adhesion enhancement of Ag–polytetrafluoroethylene antibacterial coatings by using of molecular bridge. Appl. Surf. Sci. 2015, 341, 13–18. [Google Scholar] [CrossRef]
- Marciano, F.; Lima-Oliveira, D.; Da-Silva, N.; Corat, E.; Trava-Airoldi, V. Antibacterial activity of fluorinated diamond-like carbon films produced by PECVD. Surf. Coat. Technol. 2010, 204, 2986–2990. [Google Scholar] [CrossRef]
- Onodera, S.; Fujii, S.; Moriguchi, H.; Tsujioka, M.; Hirakuri, K. Antibacterial property of F doped DLC film with plasma treatment. Diam. Relat. Mater. 2020, 107, 107835. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Ellinas, K.; Kaprou, G.D.; Mastellos, D.C.; Tserepi, A.; Gogolides, E. Bactericidal Action of Smooth and Plasma Micro-Nanotextured Polymeric Surfaces with Varying Wettability, Enhanced by Incorporation of a Biocidal Agent. Macromol. Mater. Eng. 2021, 306, 2000694. [Google Scholar] [CrossRef]
- Prado, G.D.; Terriza, A.; Ortiz-Perez, A.; Molina-Manso, D.; Mahillo, I.; Yubero, F.; Puertolas, J.A.; Manrubia-Cobo, M.; Barrena, E.G.m.; Esteban, J. DLC coatings for UHMWPE: Relationship between bacterial adherence and surface properties. J. Biomed. Mater. Res. Part A 2012, 100A, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, G.; Sportelli, M.C.; Heinzmann, A.; Picca, R.A.; Valentini, A.; Barth, H.; Mizaikoff, B.; Cioffi, N.; Kranza, C. Silver-fluoropolymer (Ag-CFX) films: Kinetic study of silver release, and spectroscopic-microscopic insight into the inhibition of P. fluorescens biofilm formation. Anal. Chem. Acta 2022, 1212, 339892. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Tütüncü, E.; Picca, R.A.; Valentini, M.; Valentini, A.; Kranz, C.; Mizaikoff, B.; Barth, H.; Cioffi, N.; Inhibiting, P. fluorescens biofilms with fluoropolymer-embedded silver nanoparticles: An in-situ spectroscopic study. Sci. Rep. 2017, 7, 11870. [Google Scholar] [CrossRef]
- Rebrov, A.K.; Safonov, A.I.; Timoshenko, N.I.; Varnek, V.A.; Repin, V.E. Silver Nanoparticles in a Fluoropolymer Matrix: Production and Properties. Dokl. Chem. 2009, 428, 207–209. [Google Scholar] [CrossRef]
- Yin, B.; Liu, T.; Yin, Y. Prolonging the Duration of Preventing Bacterial Adhesion of Nanosilver-Containing Polymer Films through Hydrophobicity. Langmuir 2012, 28, 17019–17025. [Google Scholar] [CrossRef]
- Cho, E.; Kima, S.H.; Kim, M.; Park, J.-S.; Lee, S.-J. Super-hydrophobic and antimicrobial properties of Ag-PPFC nanocomposite thin films fabricated using a ternary carbon nanotube-Ag-PTFE composite sputtering target. Surf. Coat. Technol. 2019, 370, 18–23. [Google Scholar] [CrossRef]
- Lewis, F.; Cloutier, M.; Chevallier, P.; Turgeon, S.e.; Pireaux, J.-J.; Tatoulian, M.; Mantovani, D. Influence of the 316 L Stainless Steel Interface on the Stability and Barrier Properties of Plasma Fluorocarbon Films. ACS Appl. Mater. Interfaces 2011, 3, 2323–2331. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Kuzminova, A.; Kylián, O. State-of-the-Art, and Perspectives of, Silver/Plasma Polymer Antibacterial Nanocomposites. Antibiotics 2018, 7, 78. [Google Scholar] [CrossRef]
- Kylian, O.; Kratochvil, J.; Petr, M.; Kuzminova, A.; Slavinska, D. Ag/C:F Antibacterial and hydrophobic nanocomposite coatings. Funct. Mater. Lett. 2017, 10, 1750029. [Google Scholar] [CrossRef]
- Hale, P.; Turgeon, S.; Horny, P.; Lewis, F.; Brack, N.; Riessen, G.V.; Pigram, P.; Mantovani, D. X-ray Photoelectron Emission Microscopy and Time-of-Flight Secondary Ion Mass Spectrometry Analysis of Ultrathin Fluoropolymer Coatings for Stent Applications. Langmuir 2008, 24, 7897–7905. [Google Scholar] [CrossRef]
- Haïdopoulos, M.; Turgeon, S.; Laroche, G.; Mantovani, D. Chemical and Morphological Characterization of Ultra-Thin Fluorocarbon Plasma-Polymer Deposition on 316 Stainless Steel Substrates: A First Step Toward the Improvement of the Long-Term Safety of Coated-Stents. Plasma Process. Polym. 2005, 2, 424–440. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive Forces at Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Montaño-Machado, V.; Noël, C.; Chevallier, P.; Turgeon, S.; Houssiau, L.; Pauthe, E.; Pireaux, J.-J.; Mantovani, D. Interaction of phosphorylcholine with fibronectin coatings: Surface characterization and biological performances. Appl. Surf. Sci. 2017, 396, 1613–1622. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.c.; Rego, A.M.B.d. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Cloutier, M.; Turgeon, S.; Busby, Y.; Tatoulian, M.; Pireaux, J.J.; Mantovani, D. Controlled Distribution and Clustering of Silver in Ag-DLC Nanocomposite Coatings Using a Hybrid Plasma Approach. ACS Appl. Mater. Interfaces 2016, 8, 21020–21027. [Google Scholar] [CrossRef]
- Lewis, F.; Horny, P.; Hale, P.; Turgeon, S.; Tatoulian, M.; Mantovani, D. Study of the adhesion of thin plasma fluorocarbon coatings resisting plastic deformation for stent applications. J. Phys. D Appl. Phys. 2008, 41, 045310. [Google Scholar] [CrossRef]
- Cooks, R.G.; Busch, K.L. Matrix effects, internal energies and MS/MS spectra of molecular ions sputtered from surfaces. Int. J. Mass Spectrom. Ion Phys. 1983, 53, 111–124. [Google Scholar] [CrossRef]
- Ekar, J.; Panjan, P.; Drev, S.; Kovač, J. ToF-SIMS Depth Profiling of Metal, Metal Oxide, and Alloy Multilayers in Atmospheres of H2, C2H2, CO, and O2. J. Am. Soc. Mass Spectrom. 2022, 33, 31–44. [Google Scholar] [CrossRef]
- Terlier, T.; Maindron, T.; Barnes, J.-P.; Léonard, D. Characterization of advanced ALD-based thin film barriers for organic electronics using ToF-SIMS analysis. Org. Electron. 2018, 59, 21–26. [Google Scholar] [CrossRef]
- Zhang, A.C.; Maguire, S.M.; Ford, J.T.; Composto, R.J. Using Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry to Depth Profile Nanoparticles in Polymer Nanocomposites. Microsc. Microanal. 2023, 29, 1557–1565. [Google Scholar] [CrossRef]
- Písařík, P.; Jelínek, M.; Remsa, J.; Mikšovský, J.; Zemek, J.; Jurek, K.; Kubinová, Š.; Lukeš, J.; Šepitka, J. Antibacterial, mechanical and surface properties of Ag-DLC films prepared by dual PLD for medical applications. Mater. Sci. Eng. C 2017, 77, 955–962. [Google Scholar] [CrossRef]
- Domínguez-Meister, S.; Rojas, T.C.; Frías, J.E.; Sánchez-López, J.C. Silver effect on the tribological and antibacterial properties of a-C:Ag coatings. Tribol. Int. 2019, 140, 125837. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J. Mechanisms of Plasma Polymerization—Reviewed from a Chemical Point of View. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Farella, I.; Altamura, D.; Valentini, A.; Quinto, M.; Sabbatini, L.; Zambonin, P.G. The swelling of vapor-sensitive fluoropolymers modified with metal nanoparticles: Interpretation of the material–vapor interaction mechanism. Sens. Actuators B 2004, 100, 9–16. [Google Scholar] [CrossRef]
- Cioffi, N.; Losito, I.; Torsi, L.; Farella, I.; Valentini, A.; Sabbatini, L.; Zambonin, P.G.; Bleve-Zacheo, T. Analysis of the Surface Chemical Composition and Morphological Structure of Vapor-Sensing Gold-Fluoropolymer Nanocomposites. Chem. Mater. 2002, 14, 804–811. [Google Scholar] [CrossRef]
- Chevallier, P.; Holvoet, S.; Turgeon, S.; Horny, P.; Pireaux, J.J.; Mantovani, D. Annealing and Ultraviolet Treatment of Plasma Fluorocarbon Films for Enhanced Cohesion and Stability. J. Appl. Polym. Sci. 2010, 118, 3176–3186. [Google Scholar] [CrossRef]
- Hubert, J.; Mertens, J.; Dufour, T.; Vandencasteele, N.; Reniers, F.; Viville, P.; Lazzaroni, R.; Raes, M.; Terryn, H. Synthesis and texturization processes of (super)-hydrophobic fluorinated surfaces by atmospheric plasma. J. Mater. Res. 2015, 30, 3177–3191. [Google Scholar] [CrossRef]
- Petlin, D.; Tverdokhlebov, S.; Anissimov, Y. Plasma treatment as an efficient tool for controlled drug release from polymeric materials: A review. J. Control. Release 2017, 266, 57–74. [Google Scholar] [CrossRef]
- Cloutier, M.; Turgeon, S.; Chevallier, P.; Mantovani, D. On the Interface between Plasma Fluorocarbon Films and 316L Stainless Steel Substrates for Advanced Coated Stents. Adv. Mater. Res. 2012, 409, 117–122. [Google Scholar] [CrossRef]
- Dorri, M.M.; Chevallier, P.; Turgeon, S.; Mantovani, D. Enhancing the barrier properties of a fluorocarbon plasma-deposited coating by producing an Interface of amorphous oxide layer on 316L stainless steel for stent applications. Surf. Coat. Technol. 2018, 347, 209–216. [Google Scholar] [CrossRef]
- Shirtcliffe, N.; Thiemann, P.; Stratmann, M.; Grundmeier, G. Chemical structure and morphology of thin, organo-silicon plasma-polymer films as a function of process parameters. Surf. Coat. Technol. 2001, 142, 1121–1128. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Zhang, Y.; Yin, M.; Shi, C.-o. Radio frequency plasma polymers of n-butyl methacrylate and their controlled drug release characteristics: I. Effect of the oxygen gas. Surf. Coat. Technol. 2007, 201, 6861–6864. [Google Scholar] [CrossRef]
- Ellinas, K.; Kefallinou, D.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Is There a Threshold in the Antibacterial Action of Superhydrophobic Surfaces? ACS Appl. Mater. Interfaces 2017, 9, 39781–39789. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]

| Parameters | Polymerization |
|---|---|
| C2F6 + H2 | |
| Peak power (W) | 150 |
| Duty cycles | 5.3% (ton = 5 ms, toff = 90 ms) |
| 10% (ton = 5 ms, toff = 45 ms) | |
| 20% (ton = 5 ms, toff = 20 ms) | |
| Gas pressure (mTorr) | 700 |
| Gas flow rate (sccm) | 19.0 for C2F6 and 1.2 for H2 |
| Distance to the powered antenna (cm) | 11 |
| Treatment time (min) | 5 |
| Sample | Proportion (%) | |||
|---|---|---|---|---|
| Component | Duty Cycle | |||
| 5.3% | 10% | 20% | ||
| CFx a-C:H | C-C/C-H | 32 ± 5 | 32 ± 1 | 39 ± 5 |
| C-CF | 15 ± 5 | 19.0 ± 0.7 | 16 ± 3 | |
| CF | 8 ± 3 | 14.2 ± 0.4 | 13 ± 3 | |
| CF2 | 35 ± 4 | 28 ± 1 | 26.0 ± 0.6 | |
| CF3 | 9.6 ± 0.4 | 6 ± 1 | 5.8 ± 0.7 | |
| CFx a-C:H:Ag | C-C/C-H | 33 ± 2 | 47 ± 2 | 32 ± 8 |
| C-CF | 22 ± 3 | 22 ± 7 | 28 ± 7 | |
| CF | 17 ± 2 | 15 ± 5 | 19 ± 1 | |
| CF2 | 23 ± 4 | 12 ± 2 | 16.3 ± 0.6 | |
| CF3 | 4.5 ± 0.8 | 4 ± 1 | 5 ± 1 | |
| CFx a-C:H:AgO | C-C/C-H | 41 ± 7 | 24 ± 7 | 14 ± 6 |
| C-CF | 18 ± 3 | 28 ± 5 | 17 ± 5 | |
| CF | 11 ± 1 | 18 ± 2 | 16 ± 5 | |
| CF2 | 22 ± 5 | 23 ± 2 | 43 ± 8 | |
| CF3 | 8 ± 4 | 6 ± 3 | 10 ± 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla-Gameros, L.; Chevallier, P.; Delvaux, X.; Yáñez-Hernández, L.A.; Houssiau, L.; Minne, X.; Houde, V.P.; Sarkissian, A.; Mantovani, D. Fluorocarbon Plasma-Polymerized Layer Increases the Release Time of Silver Ions and the Antibacterial Activity of Silver-Based Coatings. Nanomaterials 2024, 14, 609. https://doi.org/10.3390/nano14070609
Bonilla-Gameros L, Chevallier P, Delvaux X, Yáñez-Hernández LA, Houssiau L, Minne X, Houde VP, Sarkissian A, Mantovani D. Fluorocarbon Plasma-Polymerized Layer Increases the Release Time of Silver Ions and the Antibacterial Activity of Silver-Based Coatings. Nanomaterials. 2024; 14(7):609. https://doi.org/10.3390/nano14070609
Chicago/Turabian StyleBonilla-Gameros, Linda, Pascale Chevallier, Xavier Delvaux, L. Astrid Yáñez-Hernández, Laurent Houssiau, Xavier Minne, Vanessa P. Houde, Andranik Sarkissian, and Diego Mantovani. 2024. "Fluorocarbon Plasma-Polymerized Layer Increases the Release Time of Silver Ions and the Antibacterial Activity of Silver-Based Coatings" Nanomaterials 14, no. 7: 609. https://doi.org/10.3390/nano14070609
APA StyleBonilla-Gameros, L., Chevallier, P., Delvaux, X., Yáñez-Hernández, L. A., Houssiau, L., Minne, X., Houde, V. P., Sarkissian, A., & Mantovani, D. (2024). Fluorocarbon Plasma-Polymerized Layer Increases the Release Time of Silver Ions and the Antibacterial Activity of Silver-Based Coatings. Nanomaterials, 14(7), 609. https://doi.org/10.3390/nano14070609





