Optimizing the Structure and Optical Properties of Lanthanum Aluminate Perovskite through Nb5+ Doping
Abstract
1. Introduction
2. Experimental
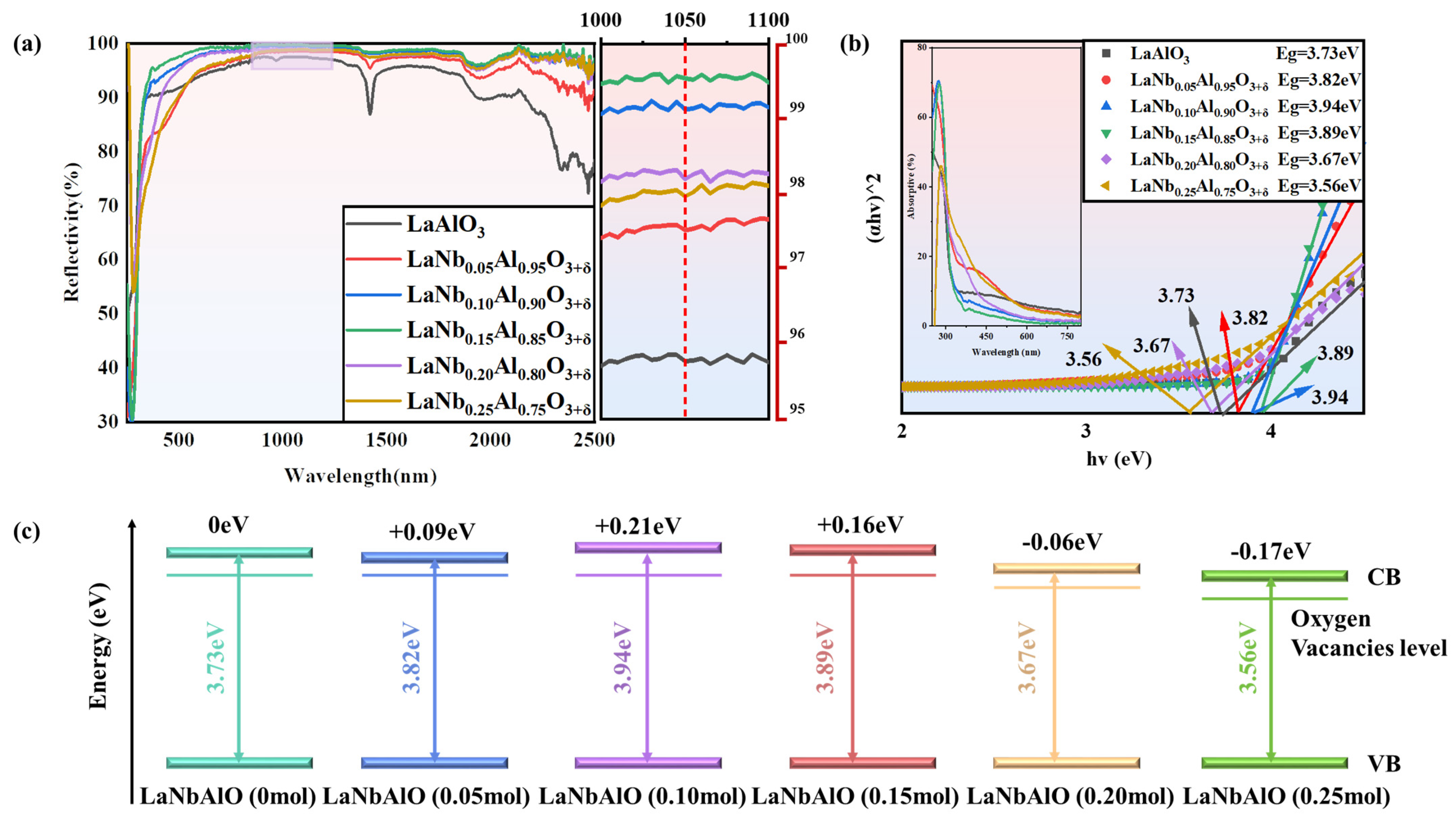
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dereń, P.J.; Mahiou, R.; Goldner, P. Multiphonon transitions in LaAlO3 doped with rare earth ions. Opt. Mater. 2009, 31, 465–469. [Google Scholar] [CrossRef]
- Dhahri, A.; Horchani-Naifer, K.; Benedetti, A.; Enrichi, F.; Ferid, M. Combustion synthesis and photoluminescence of Eu3+ doped LaAlO3 nanophosphors. Opt. Mater. 2012, 34, 1742–1746. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Wang, L.; Sun, Y.; Zhang, S. High emissivity coatings for high temperature application: Progress and prospect. Thin Solid Film. 2009, 517, 5120–5129. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Korili, S.A.; Gil, A. Progress and Recent Strategies in the Synthesis and Catalytic Applications of Perovskites Based on Lanthanum and Aluminum. Materials 2022, 15, 3288. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Janotti, A.; Van de Walle, C.G. Native point defects in LaAlO3: A hybrid functional study. Phys. Rev. B 2013, 88, 214117. [Google Scholar] [CrossRef]
- Han, Z.; Li, X.; Ye, J.; Kang, L.; Chen, Y.; Li, J.; Lin, Z.; Rohnke, M. Significantly Enhanced Infrared Emissivity of LaAlO3 by Co-Doping with Ca2+ and Cr3+ for Energy-Saving Applications. J. Am. Ceram. Soc. 2015, 98, 2336–2339. [Google Scholar] [CrossRef]
- Ankoji, P.; Rudramadevi, B.H. Structural and luminescence properties of Eu3+ doped LaAlO3 nanophosphors by hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 30, 2750–2762. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, X.; Fu, Z.; Lu, Y. Tuning LaAlO3 lattice structure by growth rate at the picometer scale in LaAlO3 SrTiO3 heterostructures. J. Appl. Phys. 2018, 124, 125305. [Google Scholar] [CrossRef]
- Silveira, I.S.; Ferreira, N.S.; Souza, D.N. Structural, morphological and vibrational properties of LaAlO3 nanocrystals produced by four different methods. Ceram. Int. 2021, 47, 27748–27758. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, C.-L.; Mi, S.-B. Probing interface structure and cation segregation in (In, Nb) co-doped TiO2 thin films. Mater. Charact. 2022, 191, 112164. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, S.; Dong, B.; Zhang, Y.; Zhang, Q.; Du, P.; Wang, G. Preparation of environmentally friendly high-emissivity Ca2+-Fe3+ co-doped LaAlO3 ceramic. Int. J. Appl. Ceram. Technol. 2023, 20, 1785–1792. [Google Scholar] [CrossRef]
- Liu, H.; Sun, H.; Xie, R.; Zhang, X.; Zheng, K.; Peng, T.; Wu, X.; Zhang, Y. Substrate-dependent structural and CO sensing properties of LaCoO3 epitaxial films. Appl. Surf. Sci. 2018, 442, 742–749. [Google Scholar] [CrossRef]
- Du, J.-Y.; Ge, C.; Xing, J.; Li, J.-K.; Jin, K.-J.; Yang, J.-T.; Guo, H.-Z.; He, M.; Wang, C.; Lu, H.-B.; et al. Solar-blind ultraviolet photodetector based on (LaAlO3)0.3-(SrAl0.5Ta0.5O3)0.7 single crystal. AIP Adv. 2017, 7, 035302. [Google Scholar] [CrossRef]
- Sun, X.; Wang, D.; Memon, M.H.; Zhu, S.; Yu, H.; Wang, H.; Fang, S.; Kang, Y.; Liu, X.; Luo, Y.; et al. Anisotropic photoresponse behavior of a LaAlO3 single-crystal-based vacuum-ultraviolet photodetector. Nanoscale 2022, 14, 16829–16836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Deng, M.; He, J.; Xu, Y.; Liu, Z.Q. A highly luminescent Mn4+ activated LaAlO3 far-red-emitting phosphor for plant growth LEDs: Charge compensation induced Mn4+ incorporation. Dalton Trans. 2019, 48, 6738–6745. [Google Scholar] [CrossRef] [PubMed]
- Visweswara Rao, T.K.; Satya Kamal, C.; Samuel, T.; Srinivasa Rao, V.; Srinivasa Rao, V.; Reddy, P.V.S.S.S.N.; Ramachandra Rao, K. Color tunable luminescence from LaAlO3:Bi3+, Ho3+ doped phosphors for field emission displays. J. Mater. Sci. Mater. Electron. 2017, 29, 1011–1017. [Google Scholar] [CrossRef]
- Dhahri, A.; Horchani-Naifer, K.; Benedetti, A.; Enrichi, F.; Férid, M.; Riello, P. Combustion synthesis and photoluminescence of Tb3+ doped LaAlO3 nanophosphors. Opt. Mater. 2013, 35, 1184–1188. [Google Scholar] [CrossRef]
- Huang, C.; Li, S.; Gong, Q.; Fang, Q.; Xu, M.; Tao, S.; Zhao, C.; Hang, Y. Optical properties of Nd,Th:LaAlO3 demonstrates its potential in high-energy pulsed laser. Opt. Laser Technol. 2022, 156, 108495. [Google Scholar] [CrossRef]
- Liu, G.; Fu, L.; Gao, Z.; Yang, X.; Fu, Z.; Wang, Z.; Yang, Y. Investigation into the temperature sensing behavior of Yb3+ sensitized Er3+ doped Y2O3, YAG and LaAlO3 phosphors. RSC Adv. 2015, 5, 51820–51827. [Google Scholar] [CrossRef]
- Shaik, E.B.; Pindiprolu, S.K.S.S.; Phanikumar, C.S.; Samuel, T.; Kumar, B.V.N.; Santhoshi, P.M.; Reddy, P.V.S.S.S.N.; Kumar, B.P.; Ramachandra, R.K. Optical emissions of chitosan modified LaAlO3: Bi3+, Tb3+ nanoparticles for bio labeling and drug delivery to breast cancer cells. Opt. Mater. 2020, 107, 110162. [Google Scholar] [CrossRef]
- Breckenfeld, E.; Wilson, R.B.; Martin, L.W. Effect of growth induced (non)stoichiometry on the thermal conductivity, permittivity, and dielectric loss of LaAlO3 films. Appl. Phys. Lett. 2013, 103, 082901. [Google Scholar] [CrossRef]
- Zahoor, A.; Isa, M.; Mahmood, T. Computational study of Be doped LaAlO3 perovskite. Phys. B Condens. Matter 2023, 652, 414631. [Google Scholar] [CrossRef]
- Xue, Y.; Deng, W.; Liu, Y.; Bai, X.; Chen, X.; Zhao, P.; Pan, Y.; Zhang, H.; Chang, A.; Xie, Y. Effect of Ce-doping on microstructure and electrical properties of LaAlO3 ceramics. Ceram. Int. 2023, 49, 5884–5892. [Google Scholar] [CrossRef]
- Wang, T.; de Oliveira, R.B.; Andreeta, M.R.B.; Wang, H.; Jia, Z.; Tao, X. Oriented Crystal Growth of La0.557Li0.330TiO3 in Bulk Ceramics Induced by LaAlO3 Single-Crystal Fibers. Cryst. Growth Des. 2021, 21, 2093–2100. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, P.; He, L.; Wang, D.; Luo, H.; Otsuka, K.; Wang, Y.; Ren, X. Tilt strain glass in Sr and Nb co-doped LaAlO3 ceramics. Acta Mater. 2019, 168, 250–260. [Google Scholar] [CrossRef]
- Gu, X.; Jin, S.; Guan, X.; Yu, X.; Yu, Z.; Yan, Y.; Wu, K.; Zhao, L.; Liu, X. Comparative study of La0.7Ca0.18Sr0.12MnO3 films with room-temperature TCR grown on SrTiO3, La0.3Sr0.7Al0.65Ta0.35O3 and LaAlO3 substrates. Ceram. Int. 2023, 49, 22952–22960. [Google Scholar] [CrossRef]
- Van Thiet, D.; Dung, D.D.; Nguyen, V.Q.; Duong, A.T.; Chung, N.X.; Hong, N.T.; Cho, S. Thermoelectric, Magnetic Properties and Re-entrant Spin-glass State in MBE Grown FeAs Film on LaAlO3(100) Substrate. ECS J. Solid State Sci. Technol. 2023, 12, 023005. [Google Scholar] [CrossRef]
- Choi, M. Hydrogen passivation of oxygen vacancies in LaAlO3. Curr. Appl. Phys. 2022, 39, 154–157. [Google Scholar] [CrossRef]
- Gupta, M.; Rambadey, O.V.; Shirbhate, S.C.; Acharya, S.; Sagdeo, A.; Sagdeo, P.R. Probing the Signature of Disordering and Delocalization of Oxygen Vacancies and Anti-site Defects in Doped LaAlO3 Solid Electrolytes. J. Phys. Chem. C 2022, 126, 20251–20262. [Google Scholar] [CrossRef]
- Hu, X.; Ren, R.; Xu, Y.; Maroof, Z. Effects of strain and oxygen vacancy on the electronic structure of (SrMnO3)2/(LaAlO3)2.5 (001) heterostructure. Phys. B Condens. Matter 2022, 624, 413310. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Y.; Wei, H.; Chen, X.; Zhang, X.; Cao, B. Tunable the kondo effect at LaAlO3/SrTiO3 interface by oxygen vacancies. Vacuum 2022, 204, 111372. [Google Scholar] [CrossRef]
- Ferrari, V.; Weissmann, M. Tuning the insulator-metal transition in oxide interfaces: An ab initio study exploring the role of oxygen vacancies and cation interdiffusion. Phys. Status Solidi (b) 2014, 251, 1601–1607. [Google Scholar] [CrossRef]
- Hossain, R.; Billah, A.; Ishizaki, M.; Kubota, S.; Hirose, F.; Ahmmad, B. Oxygen vacancy mediated room-temperature ferromagnetism and band gap narrowing in DyFe0.5Cr0.5O3 nanoparticles. Dalton Trans. 2021, 50, 9519–9528. [Google Scholar] [CrossRef] [PubMed]
- Linderalv, C.; Lindman, A.; Erhart, P. A Unifying Perspective on Oxygen Vacancies in Wide Band Gap Oxides. J. Phys. Chem. Lett. 2018, 9, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Robertson, J.; Clark, S.J. Electronic defects in LaAlO3. Microelectron. Eng. 2008, 85, 65–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, F.; Liu, C.; Chen, X. Band gap and oxygen vacancy diffusion of anatase (101) surface: The effect of strain. Theor. Chem. Acc. 2016, 135, 171. [Google Scholar] [CrossRef]
- Sarkar, S.; Saha, S.; Motapothula, M.R.; Patra, A.; Cao, B.C.; Prakash, S.; Cong, C.X.; Mathew, S.; Ghosh, S.; Yu, T.; et al. Magneto-Optical Study of Defect Induced Sharp Photoluminescence in LaAlO3 and SrTiO3. Sci. Rep. 2016, 6, 33145. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Gupta, I.; Kumar, V.; Singh, D. Preparation and luminescence behaviour of perovskite LaAlO3:Tb3+ nanophosphors for innovative displays. Optik 2022, 267, 169709. [Google Scholar] [CrossRef]
- Chen, B.; Li, C.; Deng, D.; Ruan, F.; Wu, M.; Wang, L.; Zhu, Y.; Xu, S. Temperature sensitive properties of Eu2+/Eu3+ dual-emitting LaAlO3 phosphors. J. Alloys Compd. 2019, 792, 702–712. [Google Scholar] [CrossRef]
- Jusza, A.; Lipińska, L.; Baran, M.; Polis, P.; Olszyna, A.; Piramidowicz, R. Short wavelength emission properties of Tm3+ and Tm3++Yb3+ doped LaAlO3 nanocrystals and polymer composites. Opt. Mater. 2019, 97, 109365. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Wang, H.; Ma, Z.; Gao, L.; Liu, Y.; Liu, Y.; Shu, Y.; He, J. Enhanced optical reflectivity and electrical properties in perovskite functional ceramics by inhibiting oxygen vacancy formation. Ceram. Int. 2021, 47, 5549–5558. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Luo, W. Oxygen vacancy regulation and its high frequency response mechanism in microwave ceramics. J. Mater. Chem. C 2018, 6, 11023–11034. [Google Scholar] [CrossRef]
- Mikhailov, M. Possibilities of replacing electromagnetic radiation of the Sun by accelerated electrons in testing space technology materials. J. Adv. Mater.-Covina 1996, 6, 465–470. [Google Scholar]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S.J.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, M.; Moheb, A.; Joudaki, E.J.O.C. High surface area nano-sized La0.6Ca0.4MnO3 perovskite powder prepared by low temperature pyrolysis of a modified citrate gel. Open Chem. 2009, 7, 809–817. [Google Scholar] [CrossRef]
- Bao, W.; Ma, F.; Zhang, Y.; Hao, X.; Deng, Z.; Zou, X.; Gao, W. Synthesis and characterization of Fe3+ doped Co0.5Mg0.5Al2O4 inorganic pigments with high near-infrared reflectance. Powder Technol. 2016, 292, 7–13. [Google Scholar] [CrossRef]
- Nepomniashchaia, N.; Vetokhina, V.; Chvostova, D.; Bryknar, Z.; Dejneka, A.; Tyunina, M. Low-temperature NIR-VUV optical constants of (001) LaAlO3 crystal. Opt. Mater. Express 2022, 12, 3081–3089. [Google Scholar] [CrossRef]
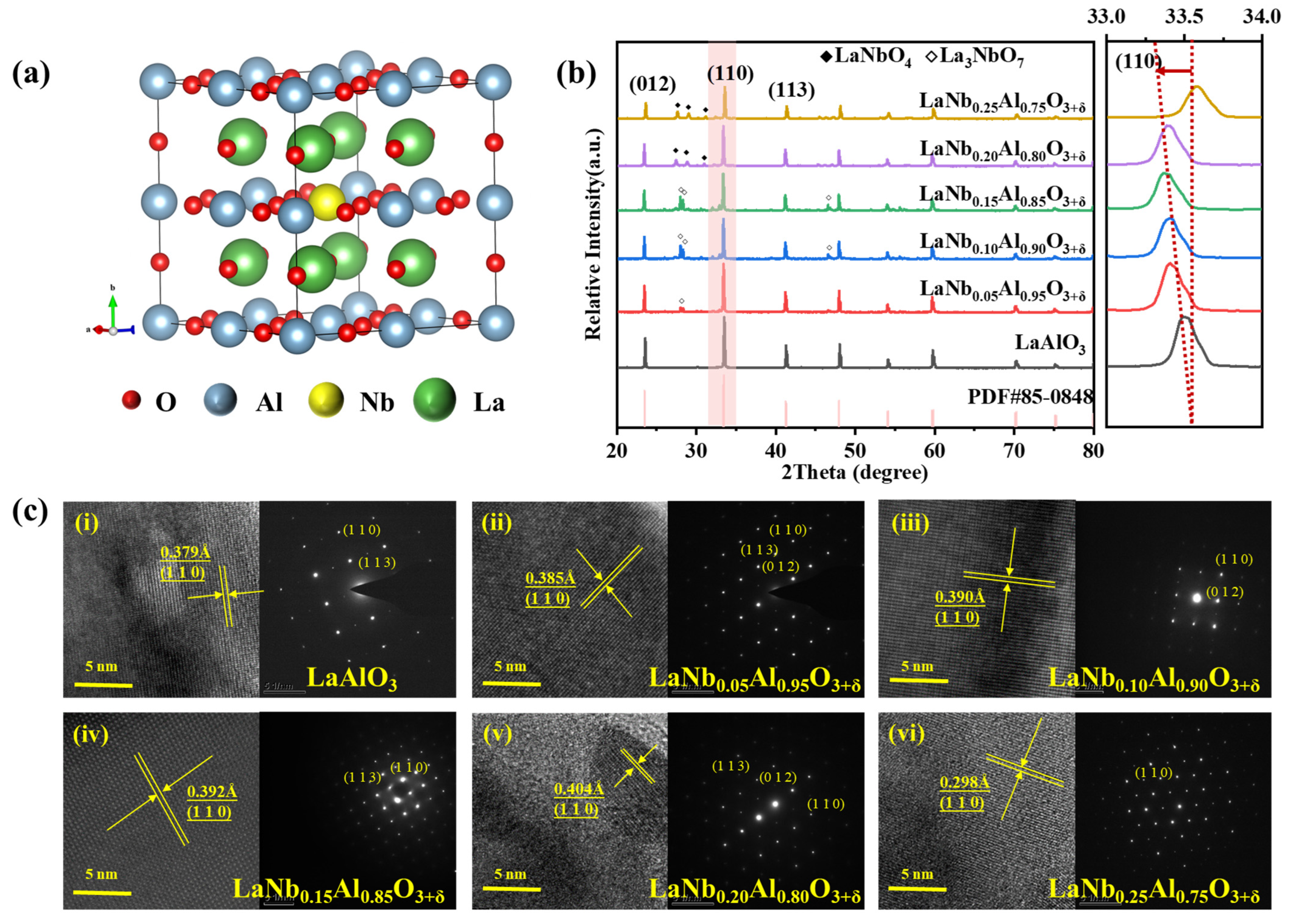
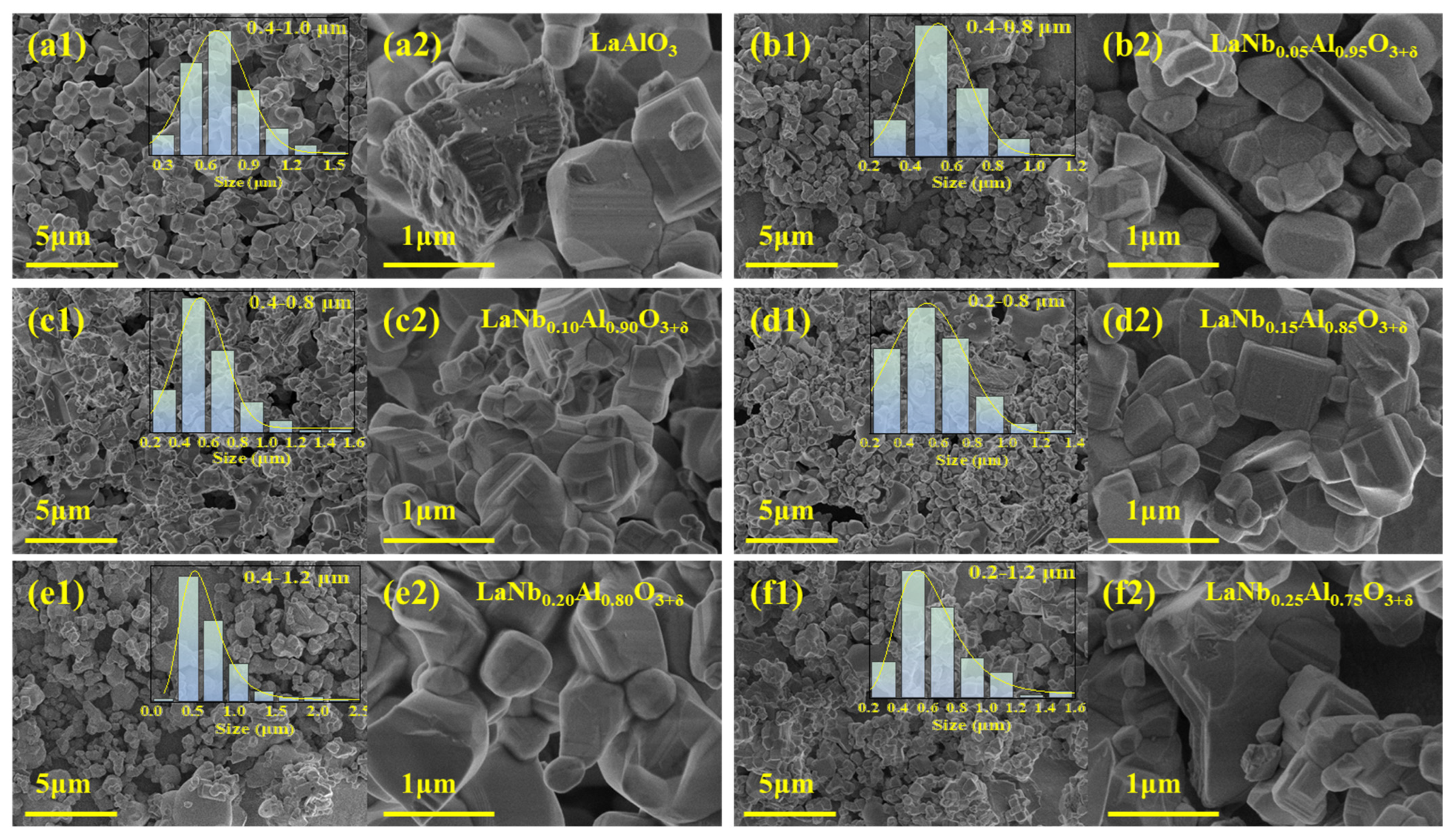
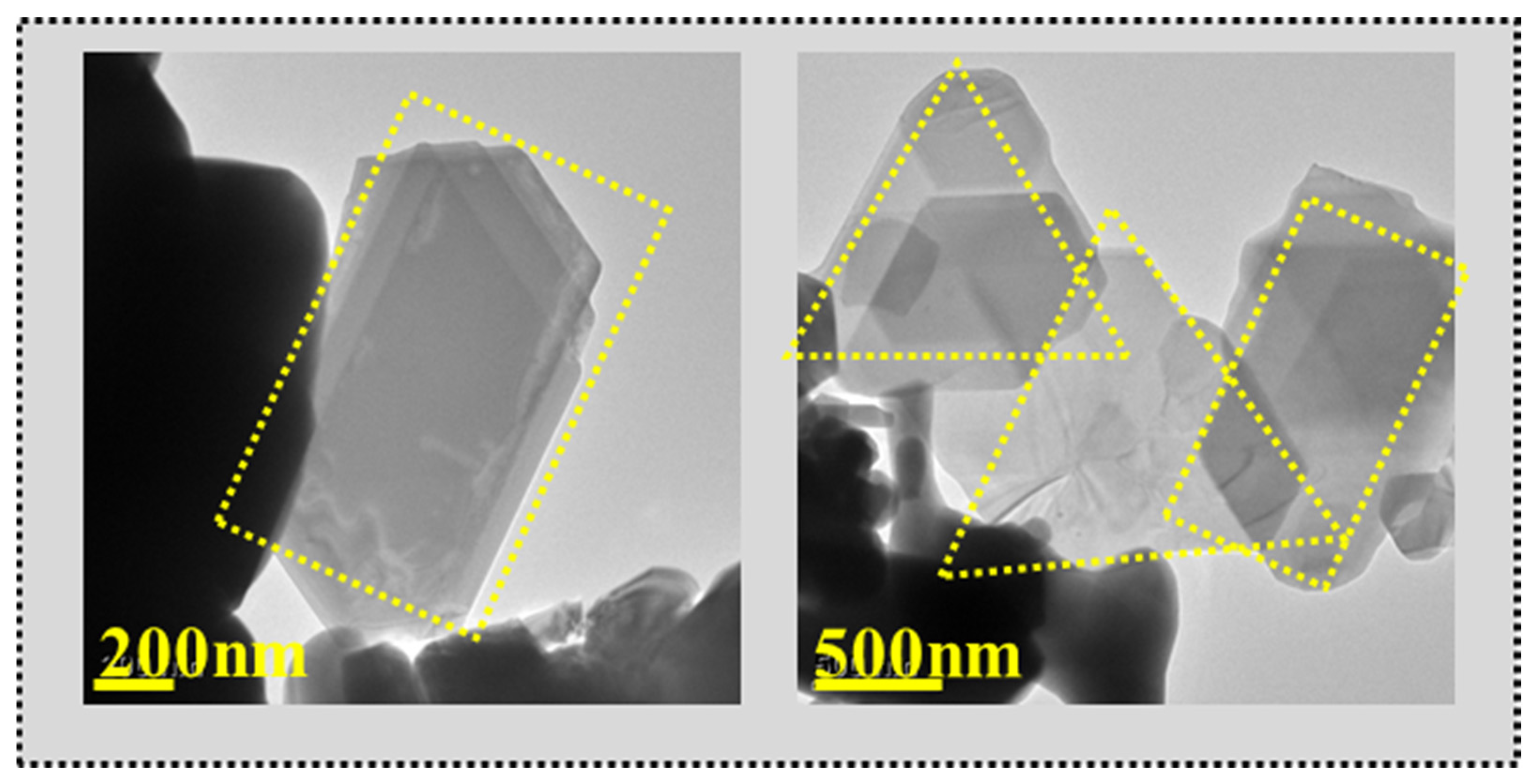
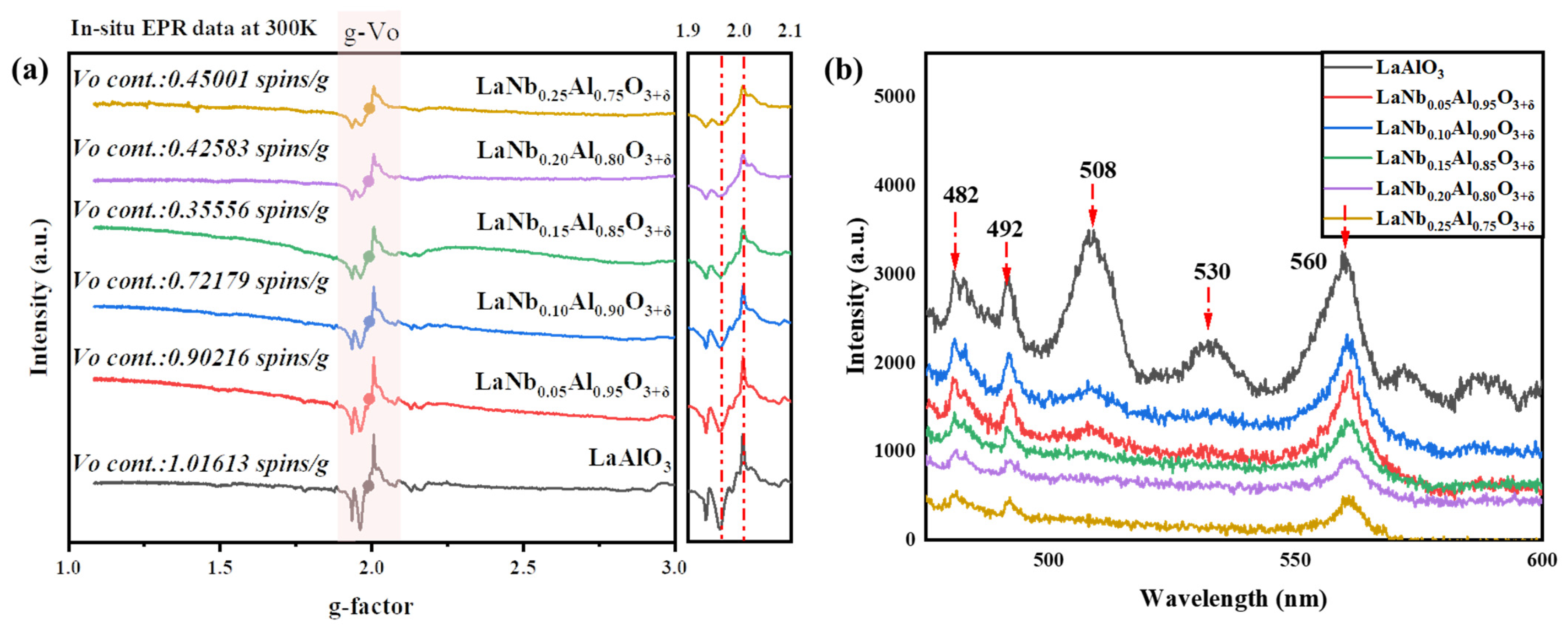

| Lattice Parameters (Refined) | |||||||
|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (deg) | β (deg) | γ (deg) | V (Å3) | |
| x = 0 | 3.78274 | 3.78274 | 3.78274 | 89.9629 | 89.9629 | 89.9629 | 54.13 |
| x = 0.05 | 3.78338 | 3.78338 | 3.78338 | 89.9568 | 89.9568 | 89.9568 | 54.16 |
| x = 0.10 | 3.78628 | 3.78628 | 3.78628 | 89.9597 | 89.9597 | 89.9597 | 54.28 |
| x = 0.15 | 3.79118 | 3.79118 | 3.79118 | 89.9788 | 89.9788 | 89.9788 | 54.49 |
| x = 0.20 | 3.79239 | 3.79239 | 3.79239 | 89.9566 | 89.9566 | 89.9566 | 54.54 |
| x = 0.25 | 3.79321 | 3.79321 | 3.79321 | 89.9814 | 89.9814 | 89.9814 | 54.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zou, Y.; Chen, Y.; Lei, Z.; Zhao, L.; Song, L. Optimizing the Structure and Optical Properties of Lanthanum Aluminate Perovskite through Nb5+ Doping. Nanomaterials 2024, 14, 608. https://doi.org/10.3390/nano14070608
Liu W, Zou Y, Chen Y, Lei Z, Zhao L, Song L. Optimizing the Structure and Optical Properties of Lanthanum Aluminate Perovskite through Nb5+ Doping. Nanomaterials. 2024; 14(7):608. https://doi.org/10.3390/nano14070608
Chicago/Turabian StyleLiu, Wei, Yang Zou, Yuang Chen, Zijian Lei, Lili Zhao, and Lixin Song. 2024. "Optimizing the Structure and Optical Properties of Lanthanum Aluminate Perovskite through Nb5+ Doping" Nanomaterials 14, no. 7: 608. https://doi.org/10.3390/nano14070608
APA StyleLiu, W., Zou, Y., Chen, Y., Lei, Z., Zhao, L., & Song, L. (2024). Optimizing the Structure and Optical Properties of Lanthanum Aluminate Perovskite through Nb5+ Doping. Nanomaterials, 14(7), 608. https://doi.org/10.3390/nano14070608





