Studying the Structure and Properties of Epoxy Composites Modified by Original and Functionalized with Hexamethylenediamine by Electrochemically Synthesized Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO-HMDA
2.3. Preparation of Epoxy Nanocomposites
2.4. Methods
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene Oxide: From Fundamentals to Applications. J. Phys. Condens. Matter 2014, 27, 013002. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Nishina, Y.; Bianco, A.; Ménard-Moyon, C. A Flexible Method for Covalent Double Functionalization of Graphene Oxide. Angew. Chem. Int. Ed. 2020, 59, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, Z.; Li, M.; Yang, B.; Xin, M.; Li, S.; Yin, Z. Yin Zongjie Surface Functional Modification of Graphene and Graphene Oxide. Acta Chim. Sin. 2016, 74, 789–799. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Keyte, J.; Pancholi, K.; Njuguna, J. Recent Developments in Graphene Oxide/Epoxy Carbon Fiber-Reinforced Composites. Front. Mater. 2019, 6, 1–30. [Google Scholar] [CrossRef]
- Ryu, S.H.; Sin, J.H.; Shanmugharaj, A.M. Study on the Effect of Hexamethylene Diamine Functionalized Graphene Oxide on the Curing Kinetics of Epoxy Nanocomposites. Eur. Polym. J. 2014, 52, 88–97. [Google Scholar] [CrossRef]
- Lee, D.-E.; Lee, G.H.; Son, N.R.; Zhang, H.-X.; Yoon, K.-B. Polyamide 6/MXene-Grafted Graphene Oxide Hybrid Nanocomposites. Iran. Polym. J. 2023, 32, 377–388. [Google Scholar] [CrossRef]
- Garkoti, C.; Shabir, J.; Mozumdar, S. Amine-Terminated Ionic Liquid Modified Magnetic Graphene Oxide (MGO-IL-NH2): A Highly Efficient and Reusable Nanocatalyst for the Synthesis of 3-Amino Alkylated Indoles. ChemistrySelect 2020, 5, 4337–4346. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Zhu, M. Amine-Terminated Ionic Liquid Modified Graphene Oxide/Copper Nanocomposite toward Efficient Lubrication. Appl. Surf. Sci. 2019, 491, 105–115. [Google Scholar] [CrossRef]
- Lee, H.-J.; Abdellah, A.; Ismail, F.M.; Gumeci, C.; Dale, N.; Parrondo, J.; Higgins, D.C. Understanding the Impact of Nitrogen Doping and/or Amine Functionalization of Reduced Graphene Oxide via Hydrothermal Routes for Supercapacitor Applications. Electrochim. Acta 2021, 397, 139241. [Google Scholar] [CrossRef]
- Hosseini, Y.; Najafi, M.; Khalili, S.; Jahanshahi, M.; Peyravi, M. Assembly of Amine-Functionalized Graphene Oxide for Efficient and Selective Adsorption of CO2. Mater. Chem. Phys. 2021, 270, 124788. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, Z.; He, H.; Shao, X.; Bai, R. Preparation and Characterization of Hexamethylenediamine-Modified Graphene Oxide/Co-Polyamide Nanocomposites. Polym. Polym. Compos. 2020, 28, 421–432. [Google Scholar] [CrossRef]
- Sierra, U.; Cuara, E.; Mercado, A.; Díaz-Barriga, E.; Bahena, A.; Cortés, A.; Martínez, J.P.; Solà, M.; Fernández, S. Efficient Synthesis of Amine-Functionalized Graphene Oxide by Ultrasound-Assisted Reactions and Density Functional Theory Mechanistic Insight. Appl. Nanosci. 2021, 11, 1637–1649. [Google Scholar] [CrossRef]
- Chakraborty, S.; Saha, S.; Dhanak, V.R.; Biswas, K.; Barbezat, M.; Terrasi, G.P.; Chakraborty, A.K. High Yield Synthesis of Amine Functionalized Graphene Oxide and Its Surface Properties. RSC Adv. 2016, 6, 67916–67924. [Google Scholar] [CrossRef]
- Caliman, C.C.; Mesquita, A.F.; Cipriano, D.F.; Freitas, J.C.C.; Cotta, A.A.C.; Macedo, W.A.A.; Porto, A.O. One-Pot Synthesis of Amine-Functionalized Graphene Oxide by Microwave-Assisted Reactions: An Outstanding Alternative for Supporting Materials in Supercapacitors. RSC Adv. 2018, 8, 6136–6145. [Google Scholar] [CrossRef]
- Aghili, M.; Yazdi, M.K.; Ranjbar, Z.; Jafari, S.H. Anticorrosion Performance of Electro-Deposited Epoxy/Amine Functionalized Graphene Oxide Nanocomposite Coatings. Corros. Sci. 2021, 179, 109143. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, Q.; Chen, H.; Chen, X.; Feng, S.; He, Y. Thermal Stability and Flame Retardancy Properties of Epoxy Resin Modified with Functionalized Graphene Oxide Containing Phosphorus and Silicon Elements. ACS Omega 2019, 4, 10975–10984. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical Properties of Epoxy Composites Filled with Silane-Functionalized Graphene Oxide. Compos. Part Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Zhu, R.; Gu, Y. Preparation and Characterization of Novel Multi-Branched Polymers in Situ Cured from Benzoxazine/Epoxy Resin/Primary Amines Blends. RSC Adv. 2016, 6, 15271–15278. [Google Scholar] [CrossRef]
- Lainioti, G.C.; Savva, P.; Druvari, D.; Avramidis, P.; Panagiotaras, D.; Karellou, E.I.E.; Kallitsis, J.K. Cross-Linking of Antimicrobial Polymers with Hexamethylene Diamine to Prevent Biofouling in Marine Applications. Prog. Org. Coat. 2021, 157, 106336. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ma, D.; Manes, A.; Oggioni, T.; Jiménez-Suárez, A.; Ureña, A.; Hamouda, A.M.S.; Sbarufatti, C. An Experimental and Numerical Investigation of Highly Strong and Tough Epoxy Based Nanocomposite by Addition of MWCNTs: Tensile and Mode I Fracture Tests. Compos. Struct. 2020, 252, 112692. [Google Scholar] [CrossRef]
- Zappalorto, M.; Pontefisso, A.; Fabrizi, A.; Quaresimin, M. Mechanical Behaviour of Epoxy/Silica Nanocomposites: Experiments and Modelling. Compos. Part Appl. Sci. Manuf. 2015, 72, 58–64. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Tastanova, L.; Akhmetova, M.; Apendina, A.; Orynbassar, R.; Lopukhova, M. The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites. J. Compos. Sci. 2023, 7, 482. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- ISO 178: 2019; Plastics—Determination of Flexural Properties. ISO Committee: Geneva, Switzerland, 2019.
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Geneva, Switzeland, 2012.
- ISO 179-1:2010; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. ISO: Geneva, Switzerland, 2010.
- ISO 306:2022; Plastics. Thermoplastic Materials. Determination of Vicat Softening Temperature (VST). ISO: Geneva, Switzerland, 2022.
- Mostovoy, A.; Bekeshev, A.; Shcherbakov, A.; Tastanova, L.; Akhmetova, M.; Apendina, A.; Lopukhova, M. Investigating the Structure and Properties of Epoxy Nanocomposites Containing Nanodiamonds Modified with Aminoacetic Acid. Polymers 2024, 16, 449. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Yakovleva, E.V.; Tseluikin, V.N.; Krasnov, V.V.; Mostovoy, A.S.; Vikulova, M.A.; Frolov, I.H.; Rakhmetulina, L.A. Synthesis of Multilayer Graphene Oxide in Electrochemical Graphite Dispersion in H2SO4. Russ. J. Appl. Chem. 2020, 93, 219–224. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Paton-Carrero, A.; Sanchez-Silva, L.; Valverde, J.L.; Romero, A. Influence of the Reduction Strategy in the Synthesis of Reduced Graphene Oxide. Adv. Powder Technol. 2017, 28, 3195–3203. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Goyat, M.S.; Hooda, A.; Gupta, T.K.; Kumar, K.; Halder, S.; Ghosh, P.K.; Dehiya, B.S. Role of Non-Functionalized Oxide Nanoparticles on Mechanical Properties and Toughening Mechanisms of Epoxy Nanocomposites. Ceram. Int. 2021, 47, 22316–22344. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, G.; Xiao, W.; Sui, G.; Yang, X. Effect of Amine Functionalized MWCNT-Epoxy Interfacial Interaction on MWCNT Dispersion and Mechanical Properties of Epoxy-Amine Composites. Polym. Compos. 2018, 39, E2552–E2561. [Google Scholar] [CrossRef]
- Sari, M.G.; Ramezanzadeh, B. Epoxy Composite Coating Corrosion Protection Properties Reinforcement through the Addition of Hydroxyl-Terminated Hyperbranched Polyamide Non-Covalently Assembled Graphene Oxide Platforms. Constr. Build. Mater. 2020, 234, 117421. [Google Scholar] [CrossRef]
- Ali, F.; Ishfaq, N.; Said, A.; Nawaz, Z.; Ali, Z.; Ali, N.; Afzal, A.; Bilal, M. Fabrication, Characterization, Morphological and Thermal Investigations of Functionalized Multi-Walled Carbon Nanotubes Reinforced Epoxy Nanocomposites. Prog. Org. Coat. 2021, 150, 105962. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Sul, J.-H.; Prusty, B.G.; Crosky, A. Effect of the Addition of Multi-Walled Carbon Nanotubes on the Thermomechanical Properties of Epoxy Resin. Polym. Compos. 2017, 38, 1873–1880. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Plakunova, E.V.; Panova, L.G. New Epoxy Composites Based on Potassium Polytitanates. Int. Polym. Sci. Technol. 2013, 40, 49–51. [Google Scholar] [CrossRef]
- Hameed, A.; Islam, M.; Ahmad, I.; Mahmood, N.; Saeed, S.; Javed, H. Thermal and Mechanical Properties of Carbon Nanotube/Epoxy Nanocomposites Reinforced with Pristine and Functionalized Multiwalled Carbon Nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar] [CrossRef]
- Shahrestanaki, A.A.K.; Mehrshad, M.; Akhlaghi, S.H. Preparation and Non-Isothermal Cure Kinetics Study of Epoxy Resin Nanocomposites with Amine and Epoxy Functionalized Magnetic Nanoparticles. High Perform. Polym. 2021, 33, 1025–1034. [Google Scholar] [CrossRef]
- Chhetri, S.; Adak, N.C.; Samanta, P.; Murmu, N.C.; Kuila, T. Functionalized Reduced Graphene Oxide/Epoxy Composites with Enhanced Mechanical Properties and Thermal Stability. Polym. Test. 2017, 63, 1–11. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Yang, W.-H.; Yu, S.-H.; Sun, R.; Wong, C.-P.; Liao, W.-H. Covalent Polymer Functionalization of Graphene for Improved Dielectric Properties and Thermal Stability of Epoxy Composites. Compos. Sci. Technol. 2016, 122, 27–35. [Google Scholar] [CrossRef]
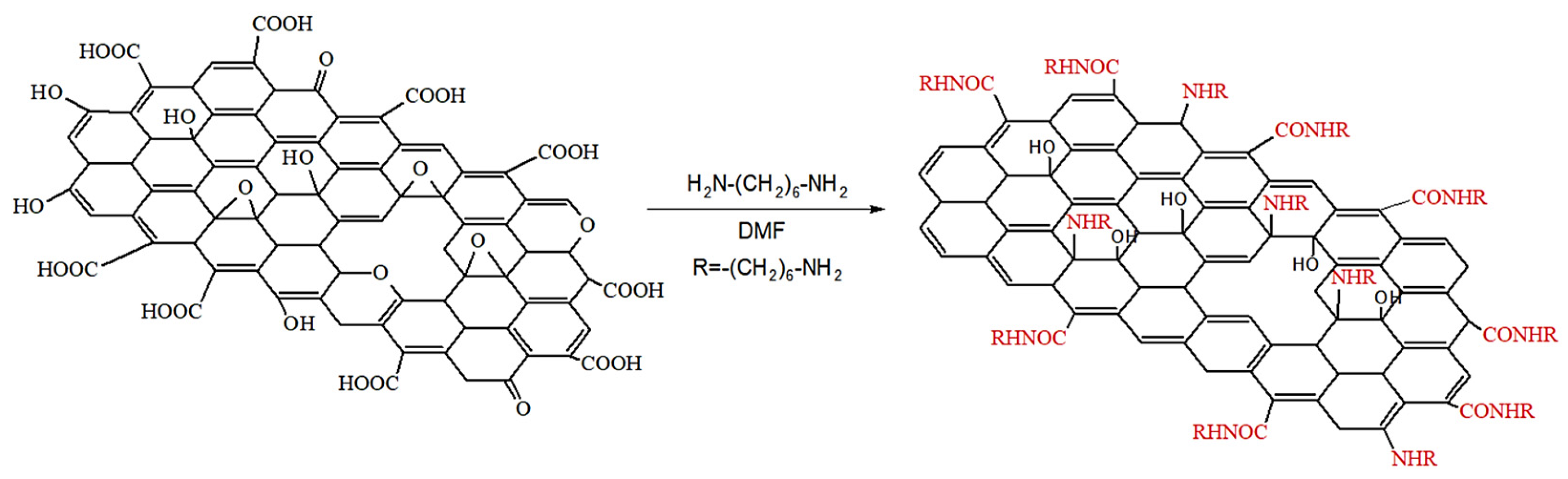

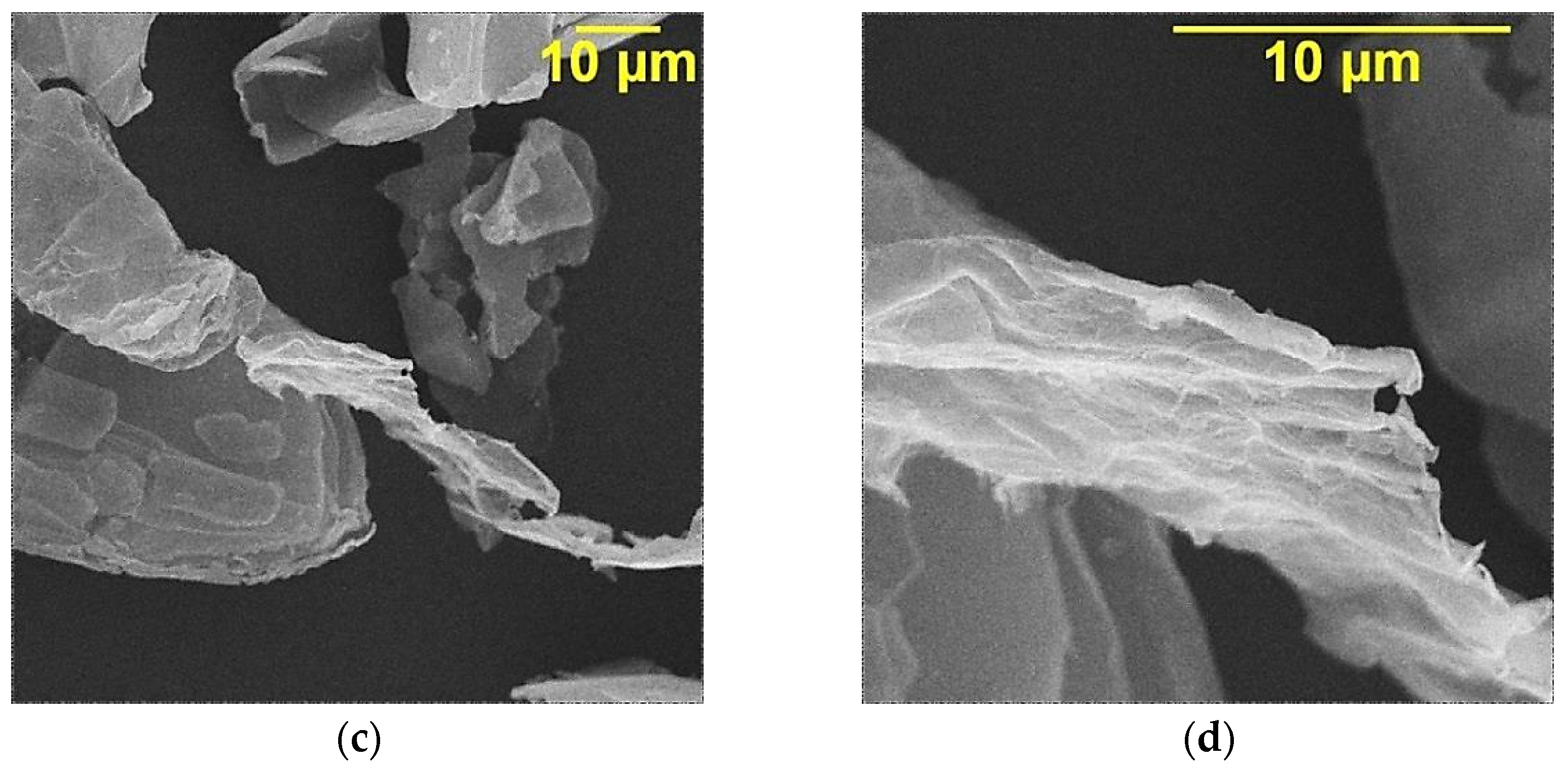
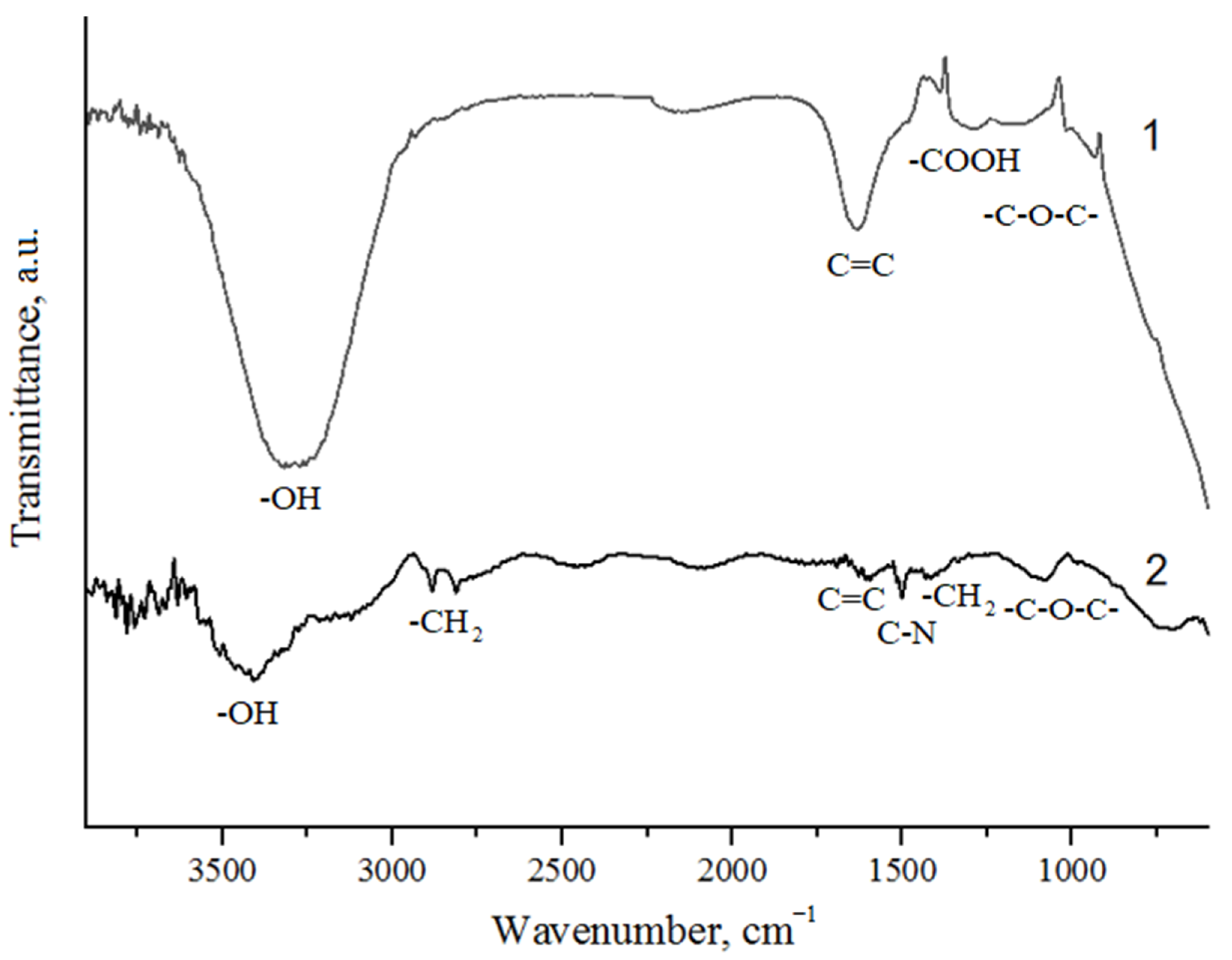
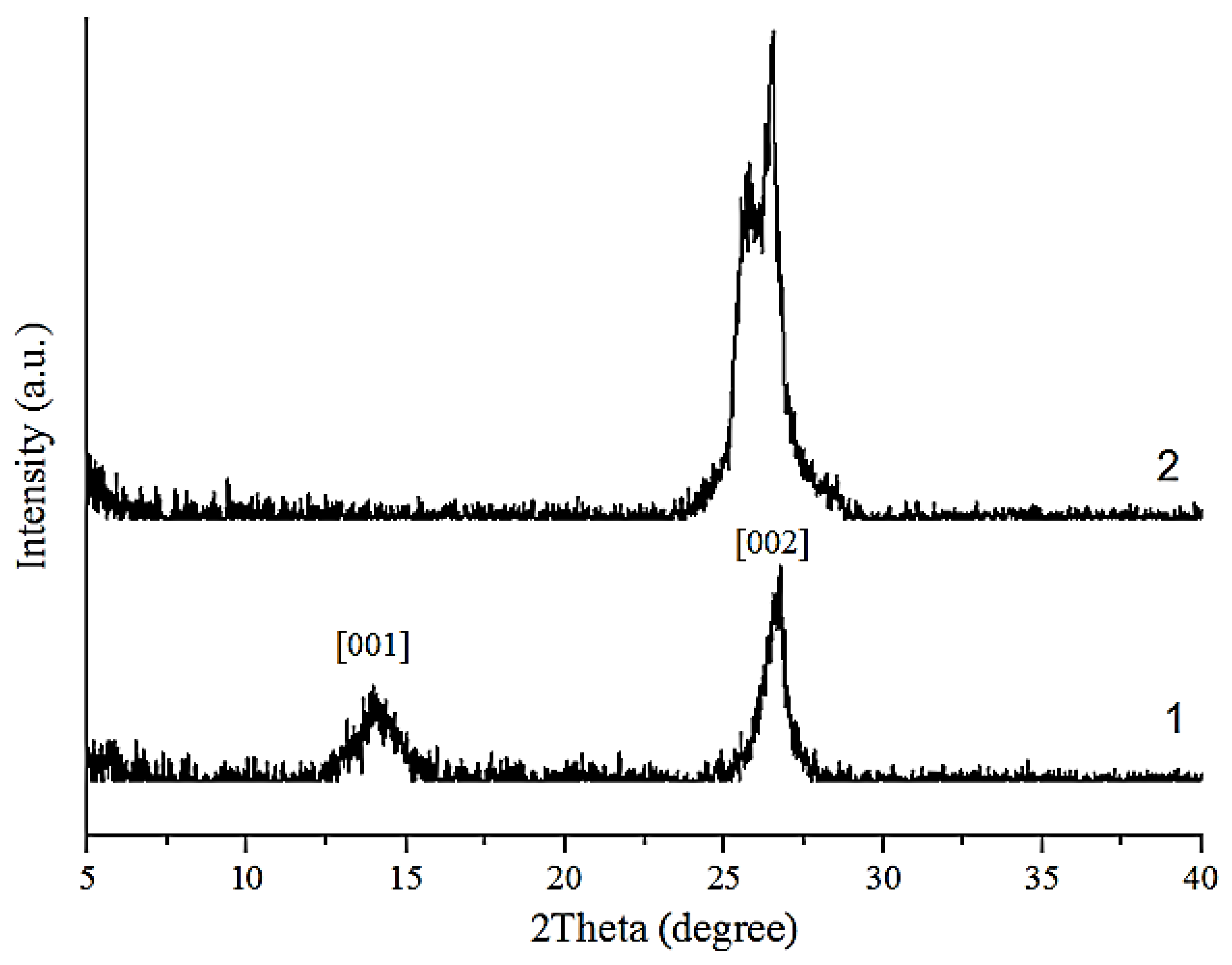
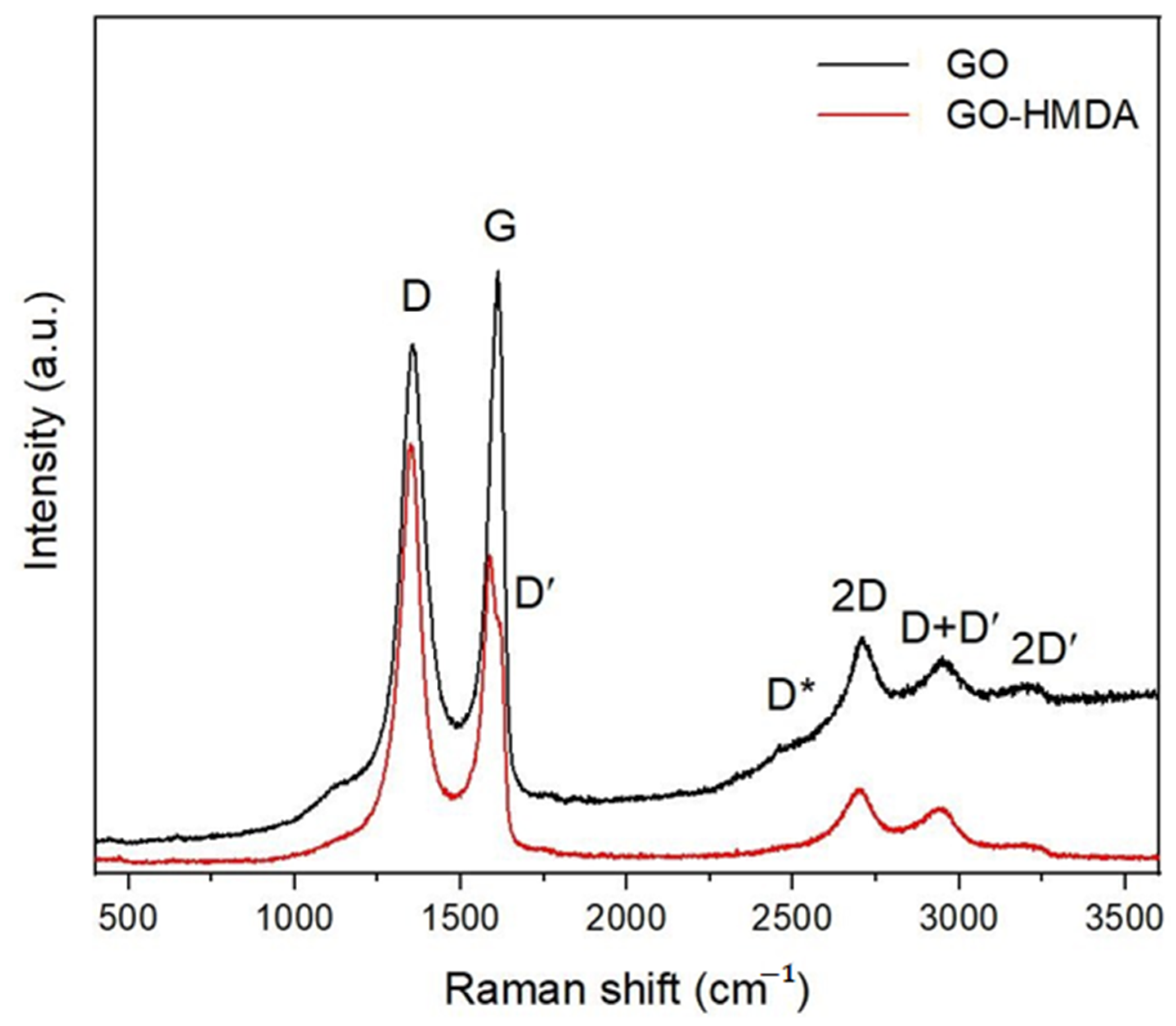
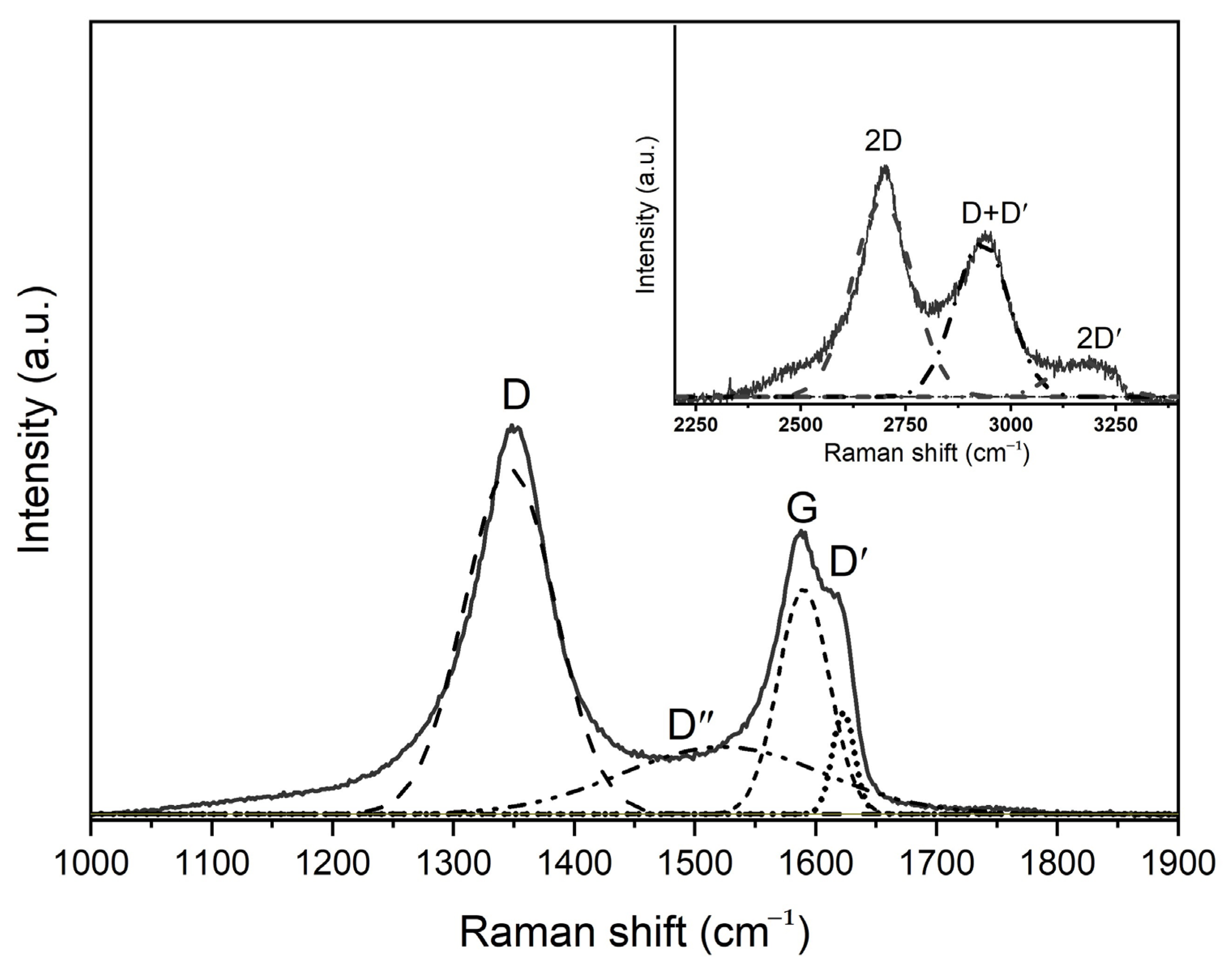
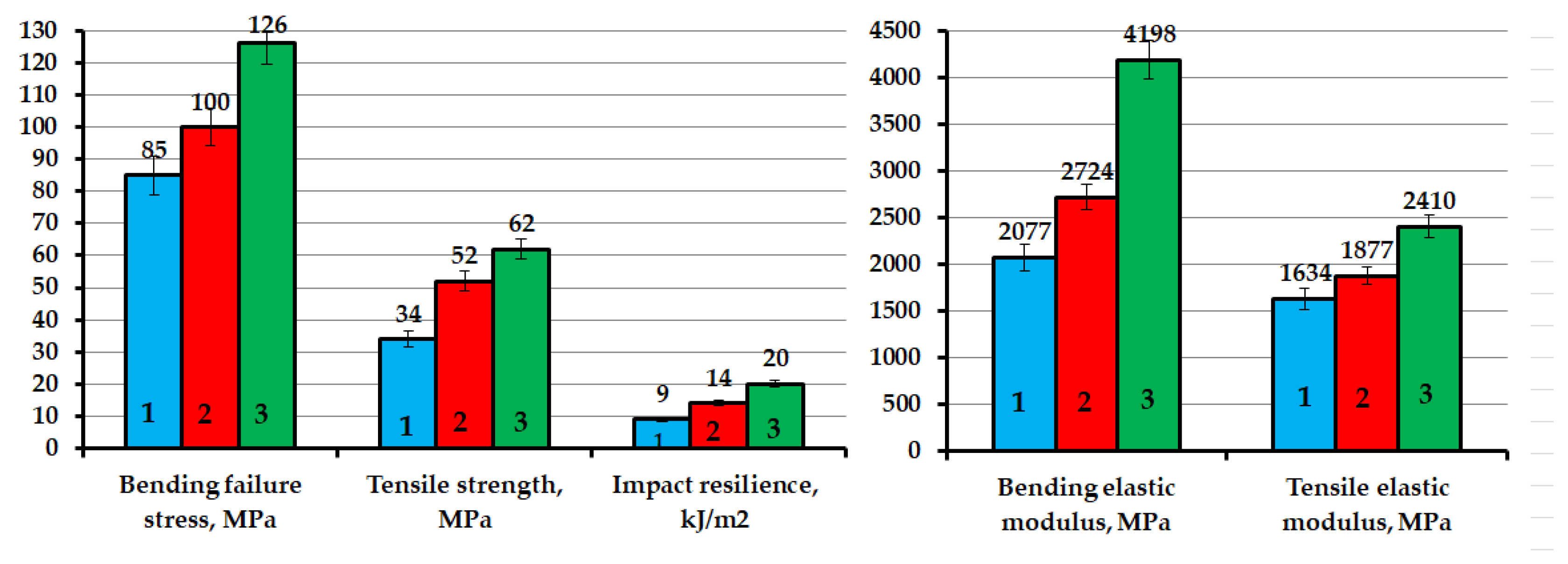
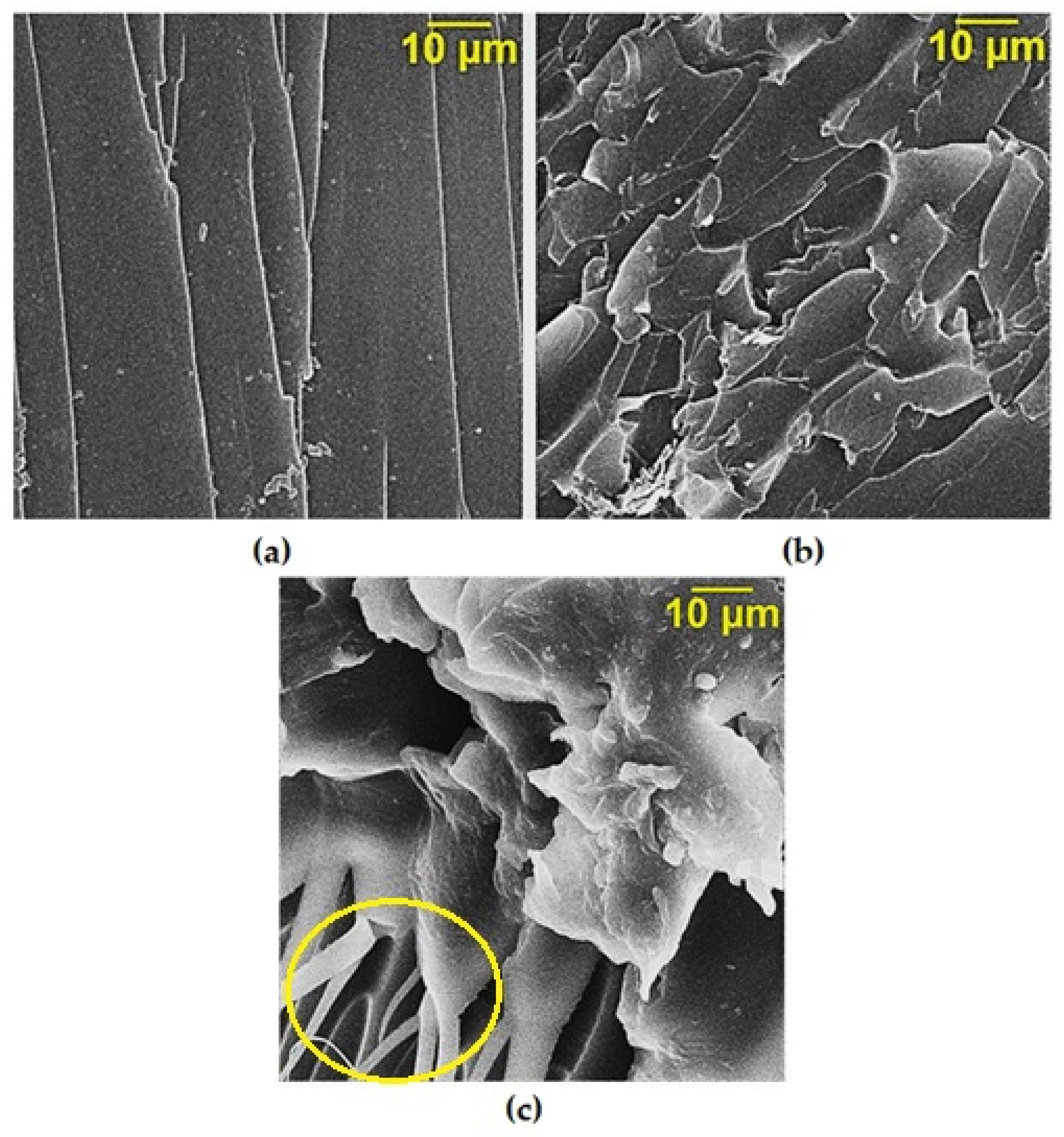

| Sample | Position of the Band (cm−1) | Pick Identification | ID/IG | I2D/IG | Crystalline Size La (nm) |
|---|---|---|---|---|---|
| GO | 1358 | D | 0.91 | 0.95 | 21.13 |
| 1612 | G | ||||
| 2708 | 2D | ||||
| 2953 | D + D′ | ||||
| 2477 | D* | ||||
| 3198 | 2D′ | ||||
| GO-HMDA | 1349 | D | 1.35 | 0.29 | 14.19 |
| 1587 | G | ||||
| 1616 | D′ | ||||
| 2692 | 2D | ||||
| 2945 | D + D′ | ||||
| 3191 | 2D′ |
| Composition | τgel, min | τres, min | Tmax, °C |
|---|---|---|---|
| Pristine epoxy composition without GO | 104 | 146 | 88 |
| Epoxy composition containing GO | 146 | 195 | 94 |
| Epoxy composition containing GO-HMDA | 68 | 105 | 116 |
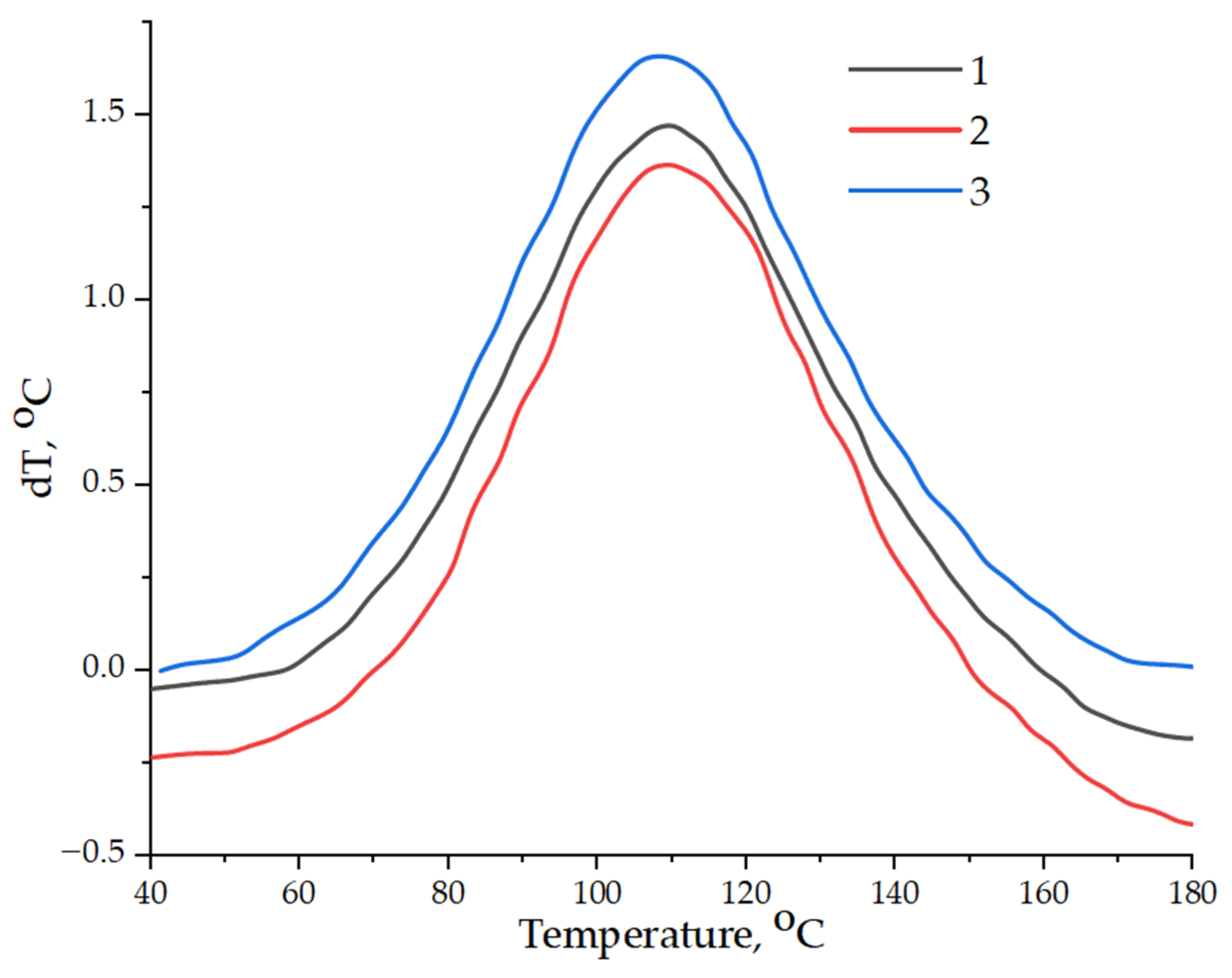
| Composition | Tstart–Tend Tmax °C | H, J/g |
|---|---|---|
| Pristine epoxy composition without GO | 58.9–159.5 108.4 | 535.7 |
| Epoxy composition containing GO | 70.7–150.4 107.8 | 446.4 |
| Epoxy composition containing GO-HMDA | 45.5–174.8 108.4 | 614.5 |
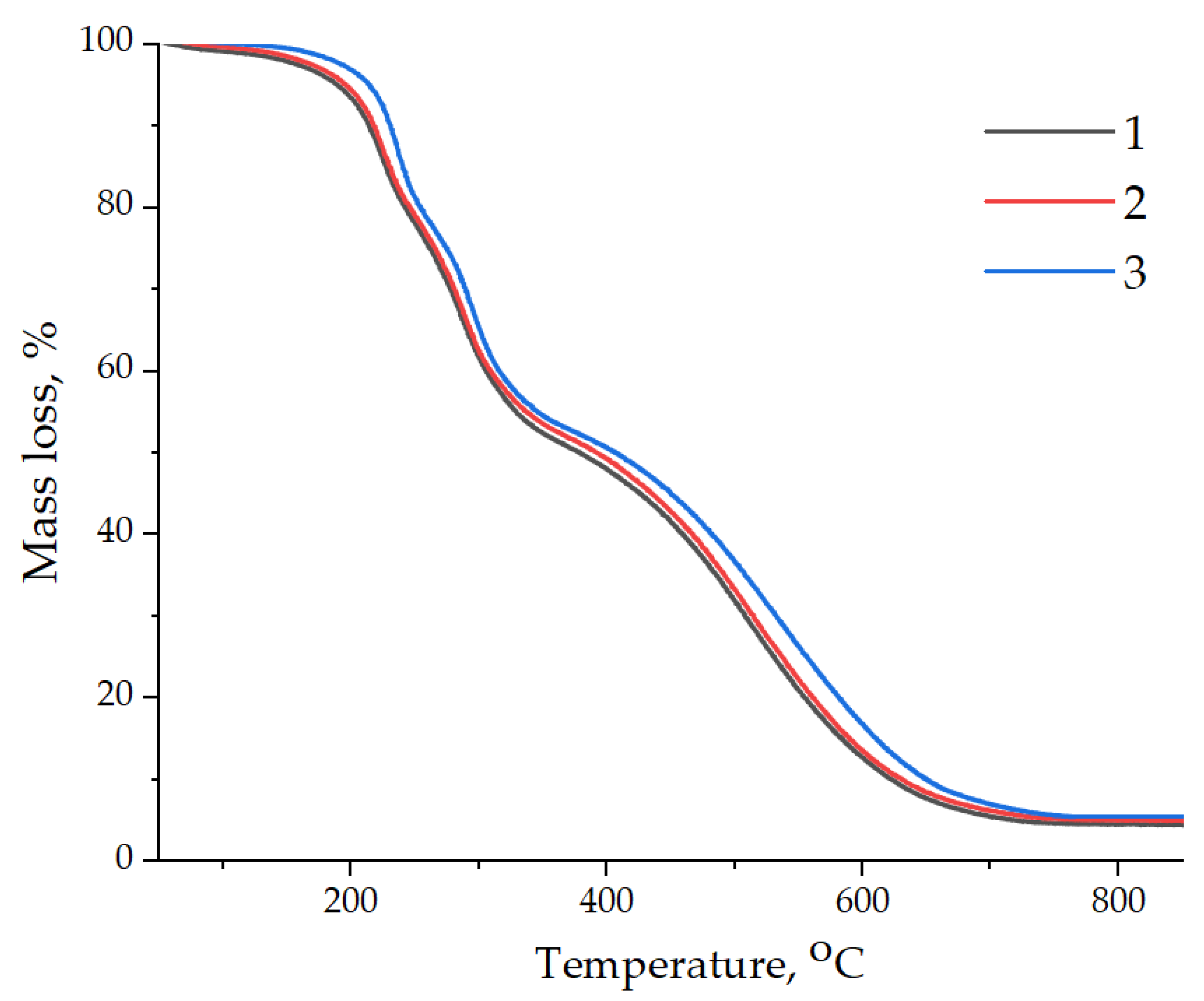
| Samples | T5%, °C | T10%, °C | T30%, °C | T50%, °C | T60%, °C | T80%, °C | Residues at 900 °C, wt.% |
|---|---|---|---|---|---|---|---|
| EP | 190 | 214 | 279 | 385 | 460 | 558 | 4.45 |
| EP/GO | 195 | 217 | 281 | 392 | 467 | 562 | 4.85 |
| EP/GO-HMDA | 214 | 231 | 290 | 410 | 486 | 584 | 5.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostovoy, A.; Bekeshev, A.; Brudnik, S.; Yakovlev, A.; Shcherbakov, A.; Zhanturina, N.; Zhumabekova, A.; Yakovleva, E.; Tseluikin, V.; Lopukhova, M. Studying the Structure and Properties of Epoxy Composites Modified by Original and Functionalized with Hexamethylenediamine by Electrochemically Synthesized Graphene Oxide. Nanomaterials 2024, 14, 602. https://doi.org/10.3390/nano14070602
Mostovoy A, Bekeshev A, Brudnik S, Yakovlev A, Shcherbakov A, Zhanturina N, Zhumabekova A, Yakovleva E, Tseluikin V, Lopukhova M. Studying the Structure and Properties of Epoxy Composites Modified by Original and Functionalized with Hexamethylenediamine by Electrochemically Synthesized Graphene Oxide. Nanomaterials. 2024; 14(7):602. https://doi.org/10.3390/nano14070602
Chicago/Turabian StyleMostovoy, Anton, Amirbek Bekeshev, Sergey Brudnik, Andrey Yakovlev, Andrey Shcherbakov, Nurgul Zhanturina, Arai Zhumabekova, Elena Yakovleva, Vitaly Tseluikin, and Marina Lopukhova. 2024. "Studying the Structure and Properties of Epoxy Composites Modified by Original and Functionalized with Hexamethylenediamine by Electrochemically Synthesized Graphene Oxide" Nanomaterials 14, no. 7: 602. https://doi.org/10.3390/nano14070602
APA StyleMostovoy, A., Bekeshev, A., Brudnik, S., Yakovlev, A., Shcherbakov, A., Zhanturina, N., Zhumabekova, A., Yakovleva, E., Tseluikin, V., & Lopukhova, M. (2024). Studying the Structure and Properties of Epoxy Composites Modified by Original and Functionalized with Hexamethylenediamine by Electrochemically Synthesized Graphene Oxide. Nanomaterials, 14(7), 602. https://doi.org/10.3390/nano14070602








