The Roles of Impurities and Surface Area on Thermal Stability and Oxidation Resistance of BN Nanoplatelets
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial BN Reference
2.2. Synthesis Method
2.3. Characterization Methods
3. Results and Discussion
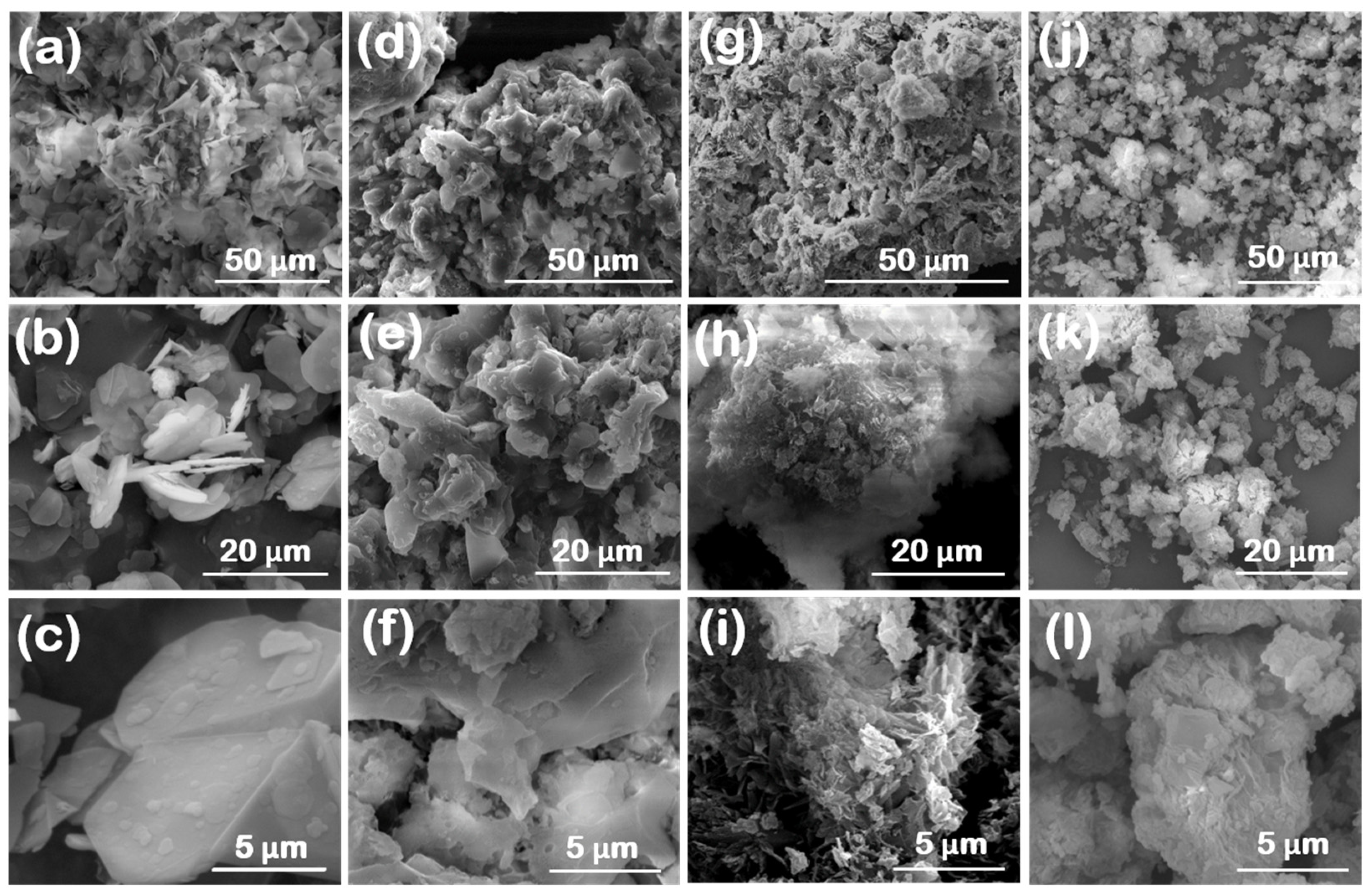
3.1. Surface Morphology
3.2. Microstructure and Purity
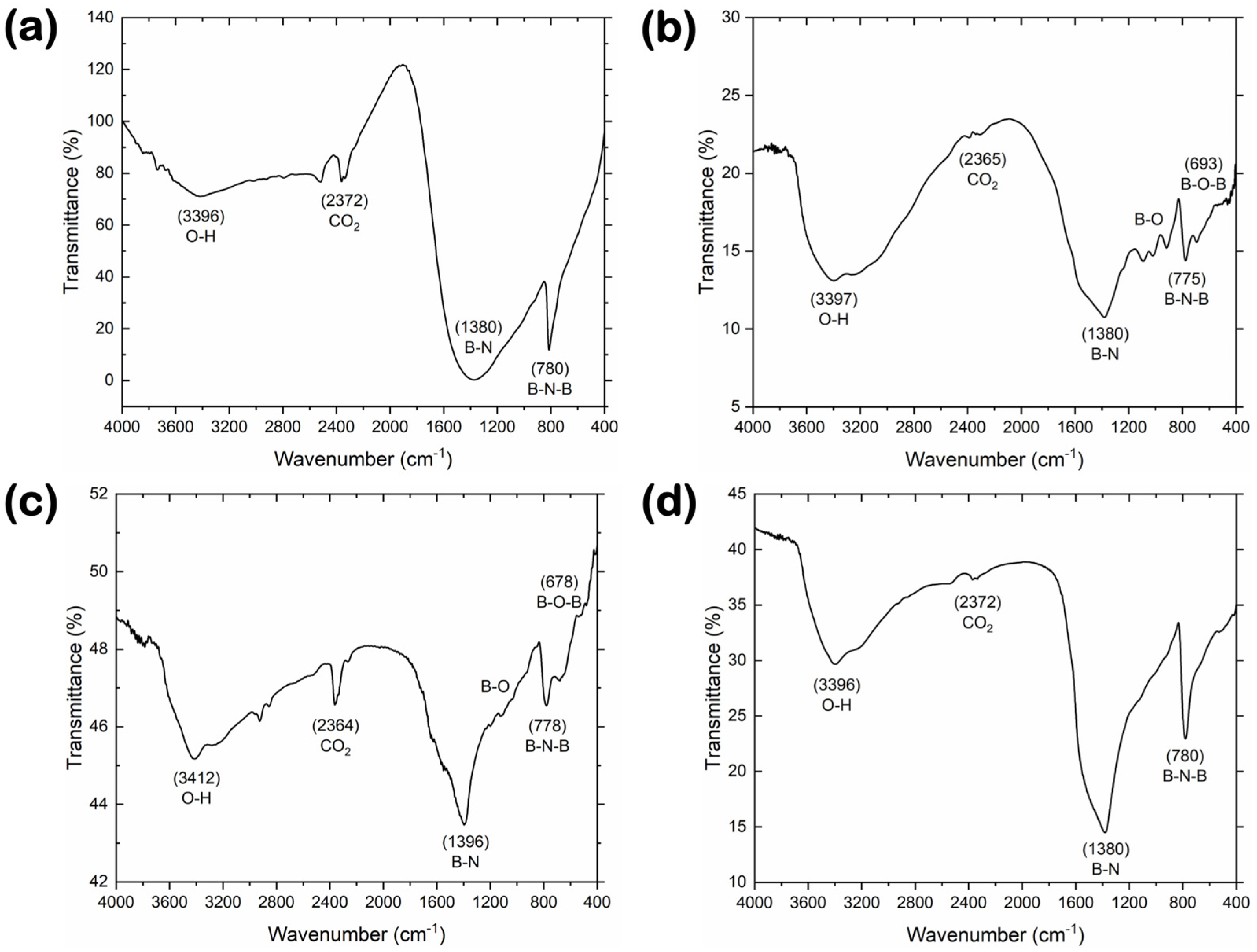
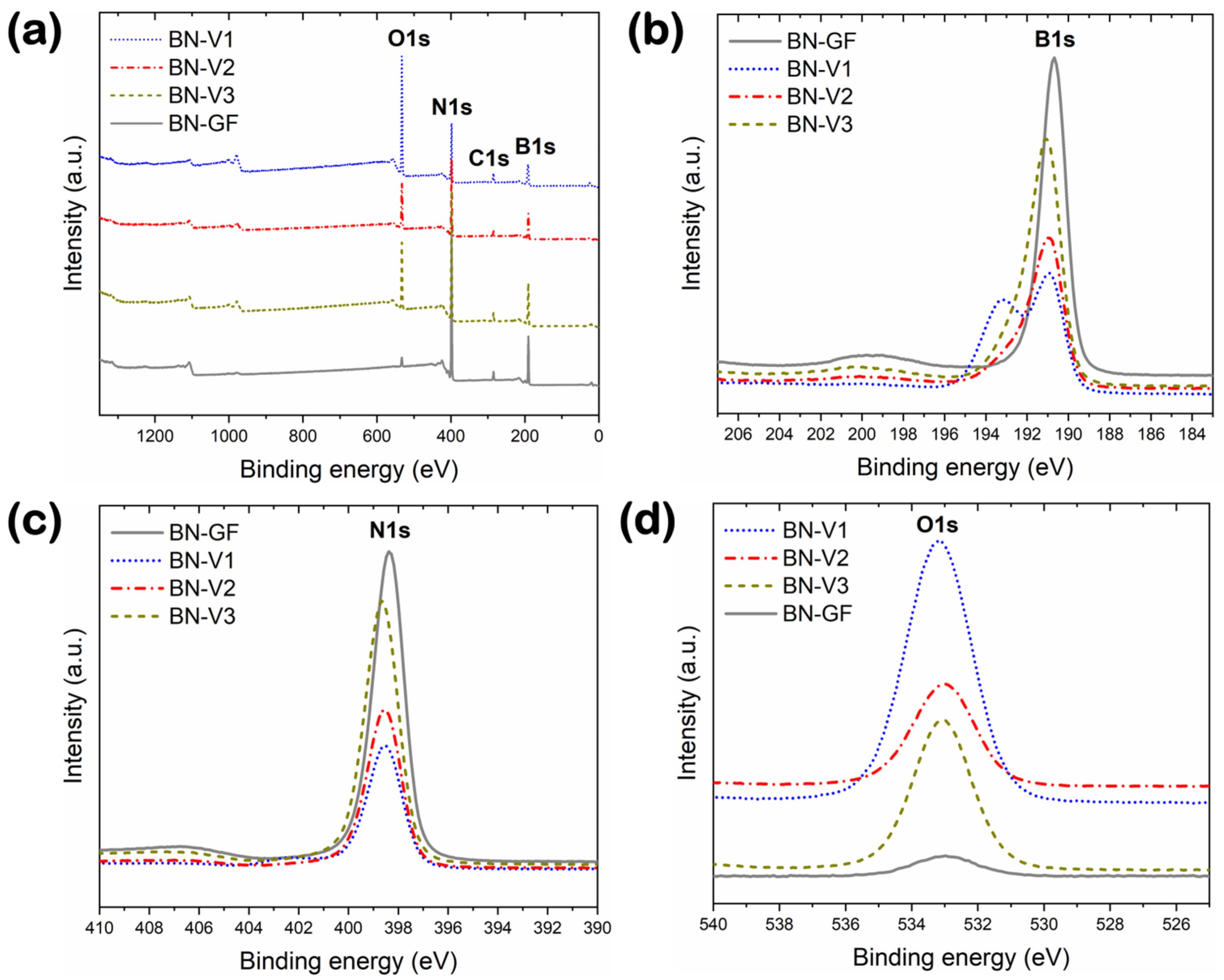
3.3. Surface Chemistry and Chemical Composition
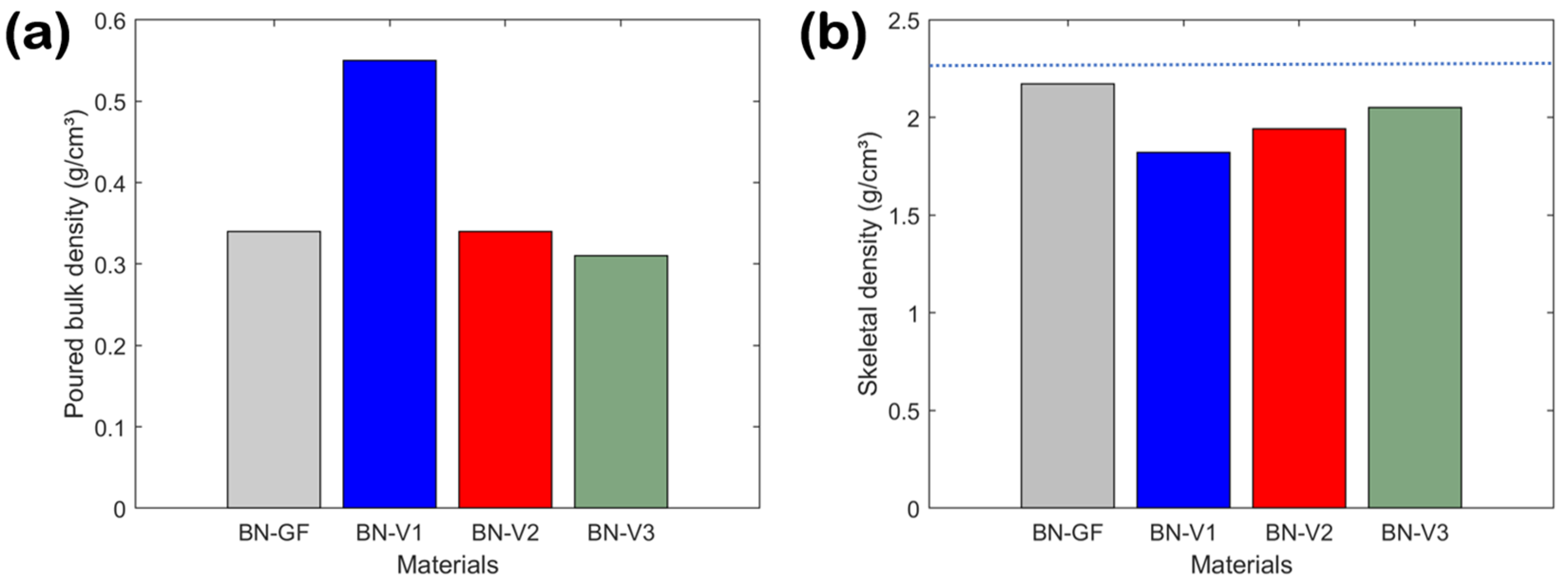
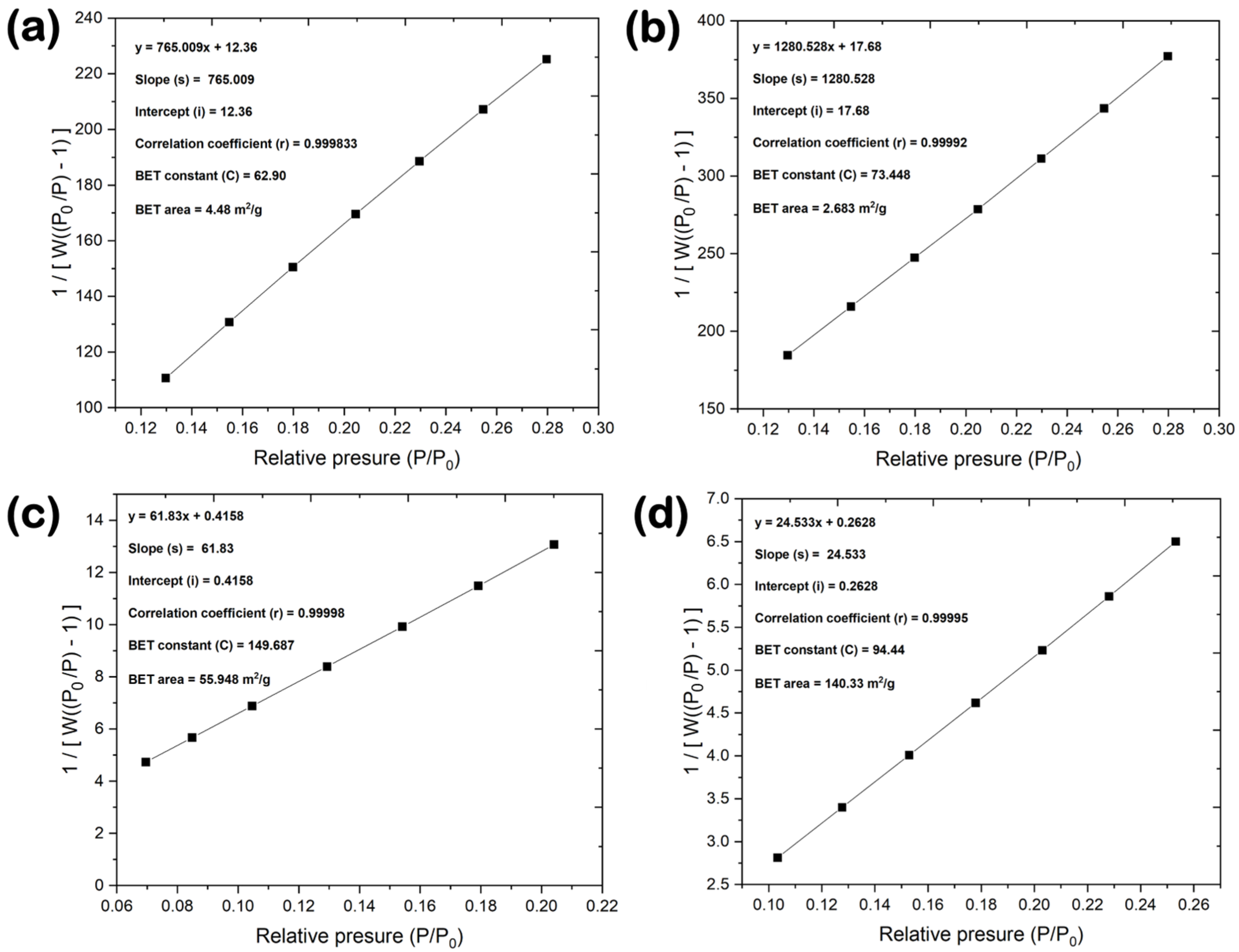
3.4. Powder Density, Pore Structure, and Surface Area
3.5. Thermal Stability and Oxidation Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Feng, Y.; Wang, F.; Yang, Z.; Wang, J. Two Dimensional Hexagonal Boron Nitride (2D-HBN): Synthesis, Properties and Applications. J. Mater. Chem. C 2017, 5, 11992–12022. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, Properties and Applications of Two-Dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Sohail, M.; Hamdy, M.S.; Taha, T.A.; AlSalem, H.S.; Alenad, A.M.; Amin, M.A.; Shah, R.; Palamanit, A.; Khan, J.; et al. Fabrication, Characteristics, and Applications of Boron Nitride and Their Composite Nanomaterials. Surf. Interfaces 2022, 29, 101725. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Matveev, A.T.; Permyakova, E.S.; Leybo, D.V.; Konopatsky, A.S.; Sorokin, P.B. Recent Progress in Fabrication and Application of BN Nanostructures and BN-Based Nanohybrids. Nanomaterials 2022, 12, 2810. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, N.; Polychronopoulou, K.; Rebholz, C. Thermal and Chemical Stability of Hexagonal Boron Nitride (h-BN) Nanoplatelets. Vacuum 2015, 112, 42–45. [Google Scholar] [CrossRef]
- Kostoglou, N.; Lukovic, J.; Babic, B.; Matovic, B.; Photiou, D.; Constantinides, G.; Polychronopoulou, K.; Ryzhkov, V.; Grossmann, B.; Mitterer, C.; et al. Few-Step Synthesis, Thermal Purification and Structural Characterization of Porous Boron Nitride Nanoplatelets. Mater. Des. 2016, 110, 540–548. [Google Scholar] [CrossRef]
- Matovic, B.; Babic, B.; Devecerski, A.; Radovic, M.; Minovic, A.; Miljkovic, M.; Boskovic, S. New Synthetic Route for Nanocrystalline Boron Nitride Powder. Mater. Lett. 2011, 65, 307–309. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Z.; Wen, Y.; Wang, Y. Facile Synthesis and Characterization of Hexagonal Boron Nitride Nanoplates by Two-Step Route. J. Am. Ceram. Soc. 2011, 94, 4496–4501. [Google Scholar] [CrossRef]
- Nersisyan, H.; Lee, T.H.; Lee, K.H.; Jeong, S.U.; Kang, K.S.; Bae, K.K.; Lee, J.H. Thermally Induced Formation of 2D Hexagonal BN Nanoplates with Tunable Characteristics. J. Solid State Chem. 2015, 225, 13–18. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, G.; Ma, X.F.; Huang, X.; Zhao, J.; Luo, H.; Sun, D. Boron Nitride Nanoplatelets as Two-Dimensional Thermal Fillers in Epoxy Composites: New Scenarios at Very Low Filler Loadings. J. Polym. Eng. 2020, 40, 859–867. [Google Scholar] [CrossRef]
- Wang, L.; Hang, R.; Xu, Y.; Guo, C.; Qian, Y. From Ultrathin Nanosheets, Triangular Plates to Nanocrystals with Exposed (102) Facets, a Morphology and Phase Transformation of Sp2 Hybrid BN Nanomaterials. RSC Adv. 2014, 4, 14233–14240. [Google Scholar] [CrossRef]
- Zhu, H.L.; Han, Q.X.; Wu, J.; Meng, X.L.; Cui, H.Z. Large Scale Synthesis of Nanoporous BN Flake with High Surface Areas. J. Cryst. Growth 2016, 434, 19–24. [Google Scholar] [CrossRef]
- Matović, B.; Luković, J.; Nikolić, M.; Babić, B.; Stanković, N.; Jokić, B.; Jelenković, B. Synthesis and Characterization of Nanocrystaline Hexagonal Boron Nitride Powders: XRD and Luminescence Properties. Ceram. Int. 2016, 42, 16655–16658. [Google Scholar] [CrossRef]
- Epp, J. X-ray Diffraction (XRD) Techniques for Materials Characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Woodhead Publishing: Sawston, UK, 2016; pp. 81–124. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; Morilla Dos Santos, C.; Sasaki, J.M. The Scherrer Equation and the Dynamical Theory of X-ray Diffraction. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Weston, N.E.; O’connor, T.E. Turbostratic Boron Nitride, Thermal Transformation to Ordered-Layer-Lattice Boron Nitride. J. Am. Chem. Soc. 1962, 84, 4619–4622. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray Photoelectron Spectroscopy: Towards Reliable Binding Energy Referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- ASTM D7481-18; Standard Test Methods for Determining Loose and Tapped Bulk Densities of Powders Using a Graduated Cylinder. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018. Available online: https://www.astm.org/d7481-18.html (accessed on 12 March 2024).
- ISO 9277:2022; Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Standard Organization (ISO): Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/71014.html (accessed on 12 March 2024).
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Mazhar, H.; Adamson, D.H.; Al-Harthi, M.A. Differently Oxidized Portions of Functionalized Hexagonal Boron Nitride. Mater. Chem. Phys. 2023, 308, 128243. [Google Scholar] [CrossRef]
- Hod, O. Graphite and Hexagonal Boron–Nitride Have the Same Interlayer Distance. Why? J. Chem. Theory Comput. 2012, 8, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Balint, M.G.; Petrescu, M.I. An Attempt to Identify the Presence of Polytype Stacking Faults in HBN Powders by Means of X-ray Diffraction. Diam. Relat. Mater. 2009, 18, 1157–1162. [Google Scholar] [CrossRef]
- Alkoy, S.; Toy, C.; Gdniil, T.; Tekin, A. Crystallization Behavior and Characterization of Turbostratic Boron Nitride. J. Eur. Ceram. Soc. 1997, 17, 1415–1422. [Google Scholar] [CrossRef]
- Harrison, H.; Lamb, J.T.; Nowlin, K.S.; Guenthner, A.J.; Ghiassi, K.B.; Kelkar, A.D.; Alston, J.R. Quantification of Hexagonal Boron Nitride Impurities in Boron Nitride Nanotubes: Via FTIR Spectroscopy. Nanoscale Adv. 2019, 1, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shao, C.; Wang, A. Chemical Exfoliating of Boron Nitride into Edge-Hydroxylated Nanosheets. J. Mater. Sci. 2023, 58, 4416–4427. [Google Scholar] [CrossRef]
- Pascuta, P.; Borodi, G.; Bosca, M.; Pop, L.; Rada, S.; Culea, E. Preparation and Structural Characterization of Some Fe2O3-B2O3-ZnO Glasses and Glass Ceramics. J. Phys. Conf. Ser. 2009, 182, 012072. [Google Scholar] [CrossRef]
- Elbeyli, I.Y. Production of Crystalline Boric Acid and Sodium Citrate from Borax Decahydrate. Hydrometallurgy 2015, 158, 19–26. [Google Scholar] [CrossRef]
- Baker, M.A.; Mollart, T.P.; Gibson, P.N.; Gissler, W. Combined X-Ray Photoelectron/Auger Electron Spectroscopy/Glancing Angle X-ray Diffraction/Extended X-ray Absorption Fine Structure Investigation of TiBxNy Coatings. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 284–291. [Google Scholar] [CrossRef]
- Schreifels, J.A.; Maybury, P.C.; Swartz, W.E. X-Ray Photoelectron Spectroscopy of Nickel Boride Catalysts: Correlation of Surface States with Reaction Products in the Hydrogenation of Acrylonitrile. J. Catal. 1980, 65, 195–206. [Google Scholar] [CrossRef]
- Paine, R.T.; Narula, C.K. Synthetic Routes to Boron Nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical Adsorption Characterization of Nanoporous Materials: Progress and Challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Kostoglou, N.; Tampaxis, C.; Charalambopoulou, G.; Constantinides, G.; Ryzhkov, V.; Doumanidis, C.; Matovic, B.; Mitterer, C.; Rebholz, C. Boron Nitride Nanotubes versus Carbon Nanotubes: A Thermal Stability and Oxidation Behavior Study. Nanomaterials 2020, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pakdel, A.; Zhang, J.; Weng, Q.; Zhai, T.; Zhi, C.; Golberg, D.; Bando, Y. Large-Surface-Area BN Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Dielectric Properties. Nanoscale Res. Lett. 2012, 7, 662. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.; Farmer, S.; Moore, A.; Sayir, H. High-Temperature Oxidation of Boron Nitride: I, Monolithic Boron Nitride. J. Am. Ceram. Soc. 1999, 82, 393–398. [Google Scholar] [CrossRef]
- Balci, S.; Sezgi, N.A.; Eren, E. Boron Oxide Production Kinetics Using Boric Acid as Raw Material. Ind. Eng. Chem. Res. 2012, 51, 11091–11096. [Google Scholar] [CrossRef]
- Salles, V.; Bernard, S.; Chiriac, R.; Miele, P. Structural and Thermal Properties of Boron Nitride Nanoparticles. J. Eur. Ceram. Soc. 2012, 32, 1867–1871. [Google Scholar] [CrossRef]
- Lavrenko, V.A.; Alexeev, A.F. High-Temperature Oxidation of Boron Nitride. Ceram. Int. 1986, 12, 25–31. [Google Scholar] [CrossRef]
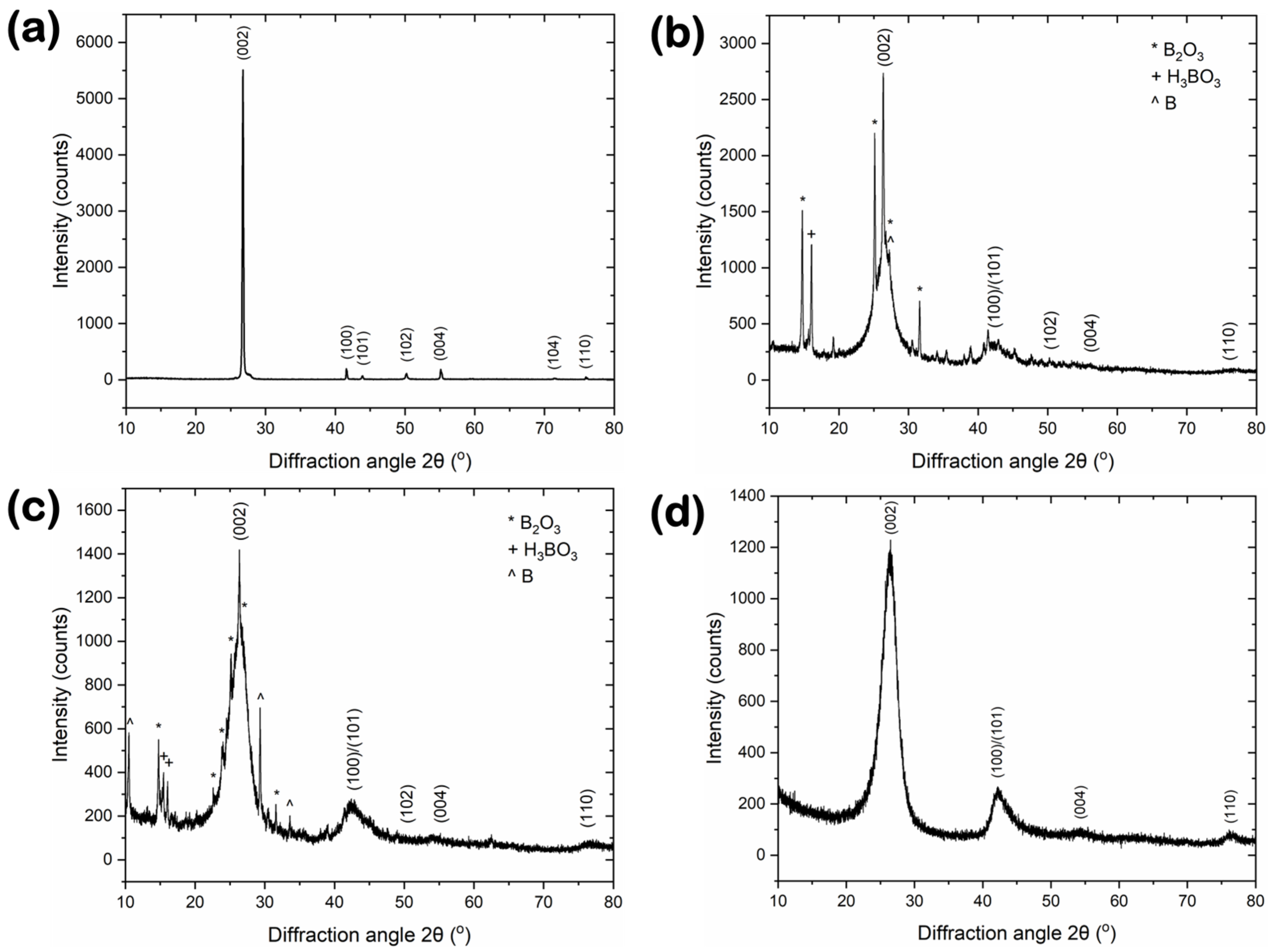


| Material | 2θ(002) [°] | 2θ(100) [°] | FWHM(002) [°] | FWHM(100) [°] | d(002) [nm] | Lc(002) [nm] | La(100) [nm] | n [-] | GI [-] |
|---|---|---|---|---|---|---|---|---|---|
| BN-GF | 26.74 | 41.63 | 0.195 | 0.083 | 0.334 | 41.3 | 210 | 125 | 1.64 |
| BN-V1 | 26.38 | 41.41 | 0.62 | 0.348 | 0.339 | 13 | 50 | 39 | 16 |
| BN-V2 | 26.34 | 41.5 | 1.45 | 0.457 | 0.339 | 5.6 | 38 | 18 | 60 |
| BN-V3 | 26.38 | 41.5 | 2.5 | 1.29 | 0.339 | 3.2 | 13.5 | 10 | 69 |
| Material | Element (at.%) | |||||
|---|---|---|---|---|---|---|
| B | N | O | C | Si | Ca | |
| BN-GF | 44.3 | 49.1 | 2.7 | 3.8 | - | 0.1 |
| BN-V1 | 35.1 | 23 | 36.2 | 5.5 | 0.2 | - |
| BN-V2 | 40.2 | 37.6 | 18.3 | 3.9 | - | - |
| BN-V3 | 40.3 | 39.1 | 16.2 | 4.4 | - | - |
| Material | SBET [m2/g] | Smicro [m2/g] | Sext [m2/g] | Vmicro [cm³/g] | Smicro/SBET [%] | Sext/SBET [%] |
|---|---|---|---|---|---|---|
| BN-GF | 4 | 0 | 4 | 0 | 0 | 100 |
| BN-V1 | 3 | 0 | 3 | 0 | 0 | 100 |
| BN-V2 | 56 | 10 | 46 | 0.005 | 17.86 | 82.14 |
| BN-V3 | 140 | 18 | 122 | 0.010 | 12.86 | 87.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostoglou, N.; Stock, S.; Solomi, A.; Holzapfel, D.M.; Hinder, S.; Baker, M.; Constantinides, G.; Ryzhkov, V.; Maletaskic, J.; Matovic, B.; et al. The Roles of Impurities and Surface Area on Thermal Stability and Oxidation Resistance of BN Nanoplatelets. Nanomaterials 2024, 14, 601. https://doi.org/10.3390/nano14070601
Kostoglou N, Stock S, Solomi A, Holzapfel DM, Hinder S, Baker M, Constantinides G, Ryzhkov V, Maletaskic J, Matovic B, et al. The Roles of Impurities and Surface Area on Thermal Stability and Oxidation Resistance of BN Nanoplatelets. Nanomaterials. 2024; 14(7):601. https://doi.org/10.3390/nano14070601
Chicago/Turabian StyleKostoglou, Nikolaos, Sebastian Stock, Angelos Solomi, Damian M. Holzapfel, Steven Hinder, Mark Baker, Georgios Constantinides, Vladislav Ryzhkov, Jelena Maletaskic, Branko Matovic, and et al. 2024. "The Roles of Impurities and Surface Area on Thermal Stability and Oxidation Resistance of BN Nanoplatelets" Nanomaterials 14, no. 7: 601. https://doi.org/10.3390/nano14070601
APA StyleKostoglou, N., Stock, S., Solomi, A., Holzapfel, D. M., Hinder, S., Baker, M., Constantinides, G., Ryzhkov, V., Maletaskic, J., Matovic, B., Schneider, J. M., Rebholz, C., & Mitterer, C. (2024). The Roles of Impurities and Surface Area on Thermal Stability and Oxidation Resistance of BN Nanoplatelets. Nanomaterials, 14(7), 601. https://doi.org/10.3390/nano14070601









