Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HA/Peptide-Based Composite Coatings
2.3. Characterization
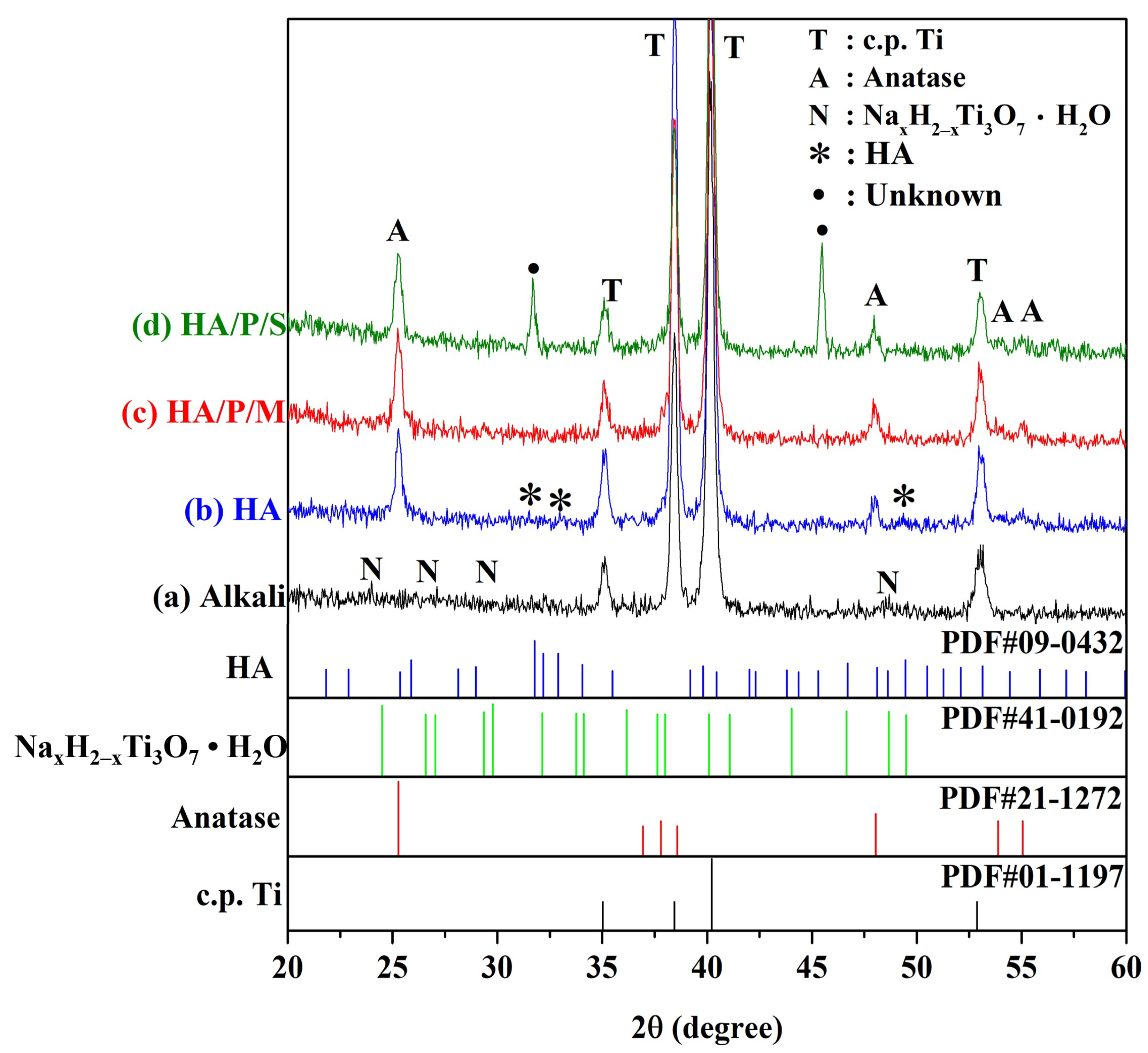
2.3.1. XRD
2.3.2. SEM
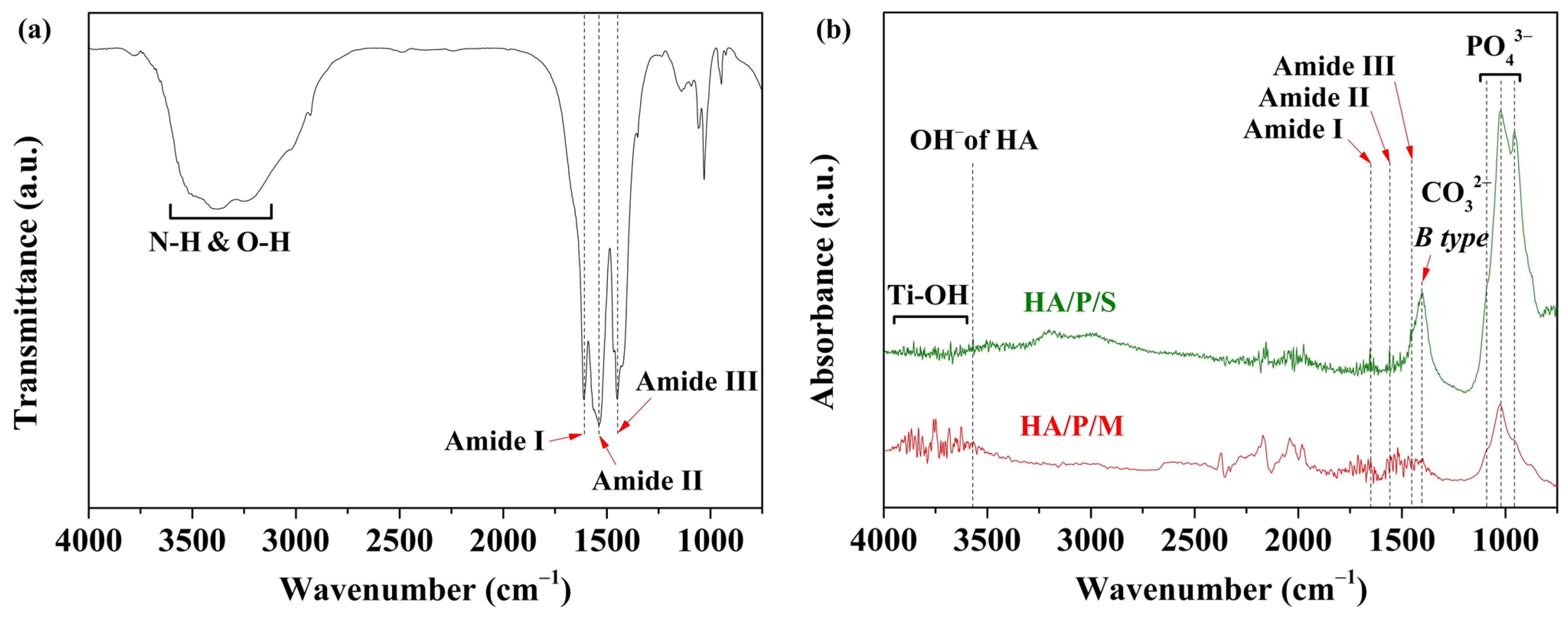
2.3.3. FTIR
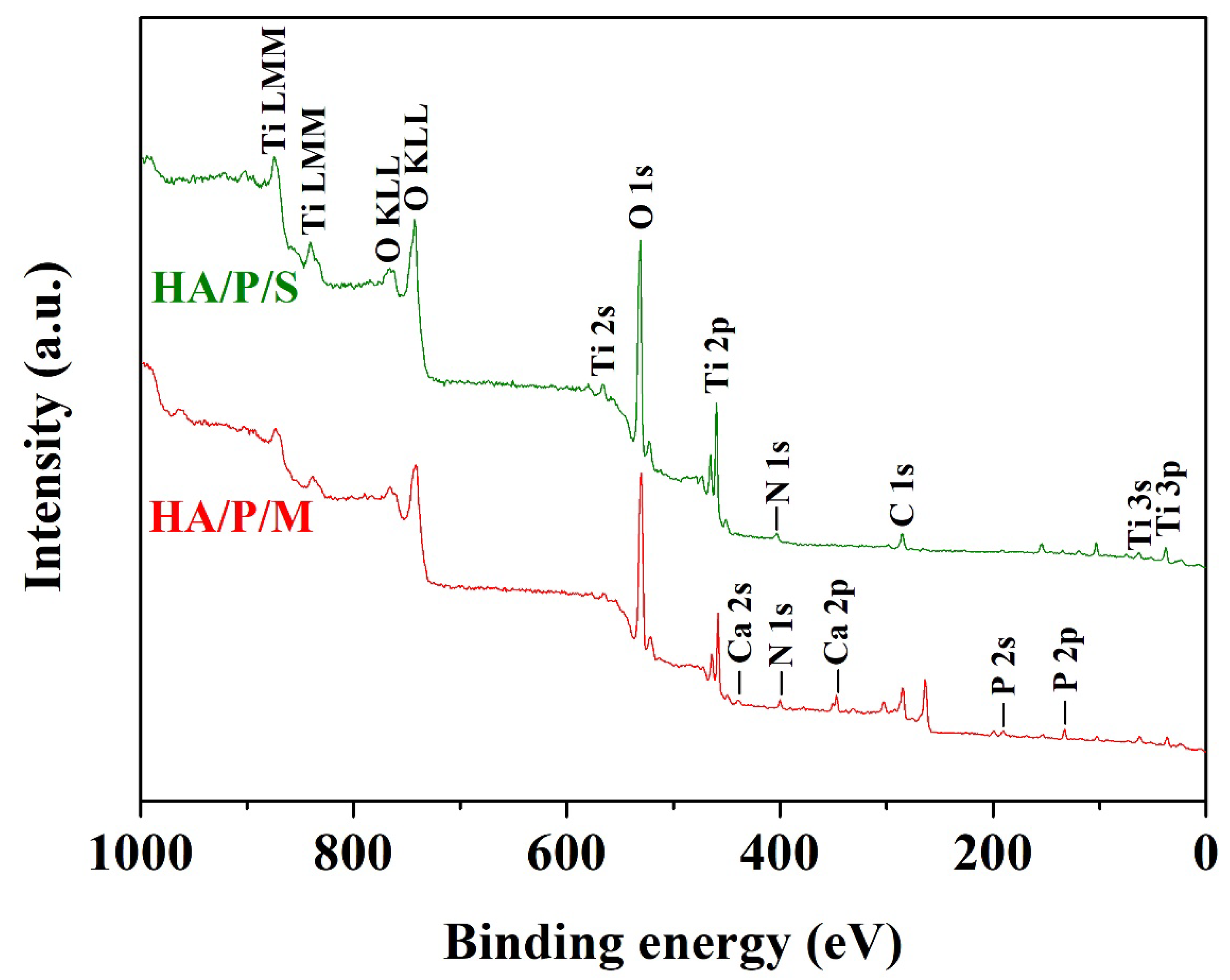
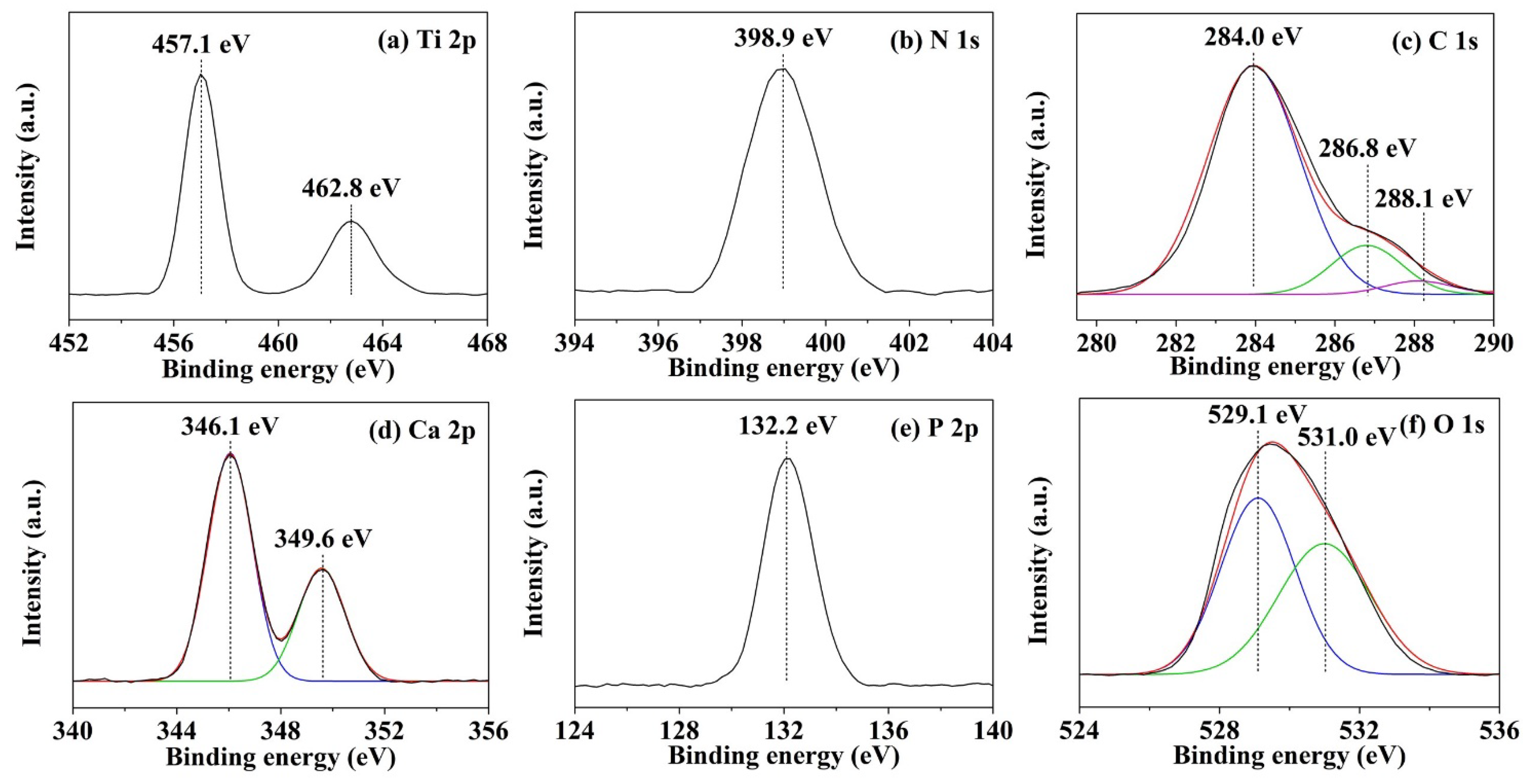
2.3.4. XPS
2.4. Contact Angle Measurement
2.5. Protein Absorption Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphology
3.2. Phase Composition
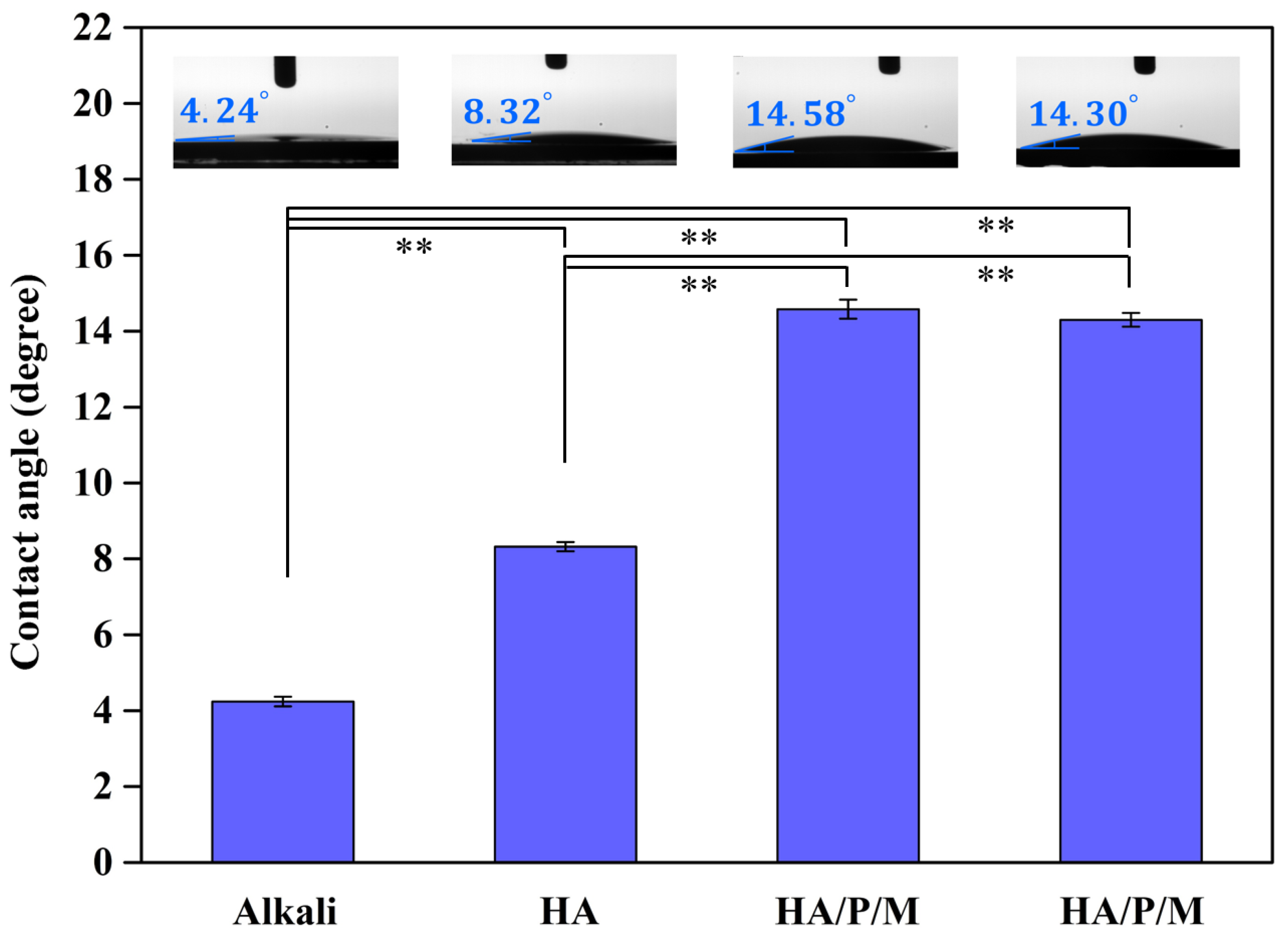
3.3. Wettability
3.4. Protein Absorption Test
4. Conclusions
- When pure titanium was treated with a 5 M NaOH solution at 60 °C for 24 h, a nanonetwork structure formed on the pure titanium surface. The HA on the surfaces of the HA/P/M and HA/P/S composite coatings were spherical nanoparticles.
- The XRD results indicated that the HA/P/M and HA/P/S composite coatings exhibited the presence of bioactive TiO2 anatase along with low crystallinity HA.
- FTIR analysis showed that the peptides extracted from oyster shells included characteristic amide I, II, and III functional groups.
- The HA coating was synthesized using the oyster shells, and The HA coating contained B-type carbonate; the structure of the B-type carbonate was similar to the inorganic mineral structure of human bone.
- The static contact angle measurements indicated that HA/P/M (14.58°) and HA/P/S (14.30°) exhibited excellent surface wettability.
- The results of the protein adsorption test revealed that the HA composite coating containing oyster shell peptides had an approximately 2.3 times higher concentration of adsorbed protein than the pure HA coating. The oyster shell peptides helped boost the adsorption of protein on the coatings’ surface, thereby enhancing the coatings’ biocompatibility. Hence, the HA/P/M and HA/P/S composite coatings fabricated on Ti surfaces in this study hold promise for potential applications as dental implants.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Senopati, G.; Rahman Rashid, R.A.; Kartika, I.; Palanisamy, S. Recent Development of Low-Cost β-Ti Alloys for Biomedical Applications: A Review. Metals 2023, 13, 194. [Google Scholar] [CrossRef]
- Wong, K.-K.; Hsu, H.-C.; Wu, S.-C.; Ho, W.-F. A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications. Materials 2023, 16, 7046. [Google Scholar] [CrossRef]
- Wei, G.; Tan, M.; Attarilar, S.; Li, J.; Uglov, V.V.; Wang, B.; Liu, J.; Lu, L.; Wang, L. An overview of surface modification, a way toward fabrication of nascent biomedical Ti–6Al–4V alloys. J. Mater. Res. Technol. 2023, 24, 5896–5921. [Google Scholar] [CrossRef]
- Camargo, W.A.; Takemoto, S.; Hoekstra, J.W.; Leewenburgh, S.C.; Jansen, J.A.; Van den Beucken, J.J.J.P.; Alghamdi, H.S. Effect of surface alkali-based treatment of titanium implants on ability to promote in vitro mineralization and in vivo bone formation. Acta Biomater. 2017, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.-H.; Hsu, H.-C.; Wu, S.-C.; Shih, Y.-C.; Yang, H.-W.; Ho, W.-F. Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials 2023, 13, 1296. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Hsu, H.-C.; Yu, H.-C.; Shen, C.-E.; Ho, W.-F. Preparation and evaluation of osteoinductive porous biphasic calcium phosphate granules obtained from eggshell for bone tissue engineering. Adv. Powder Technol. 2023, 34, 103909. [Google Scholar] [CrossRef]
- Adeleke, S.A.; Ramesh, S.; Bushroa, A.R.; Ching, Y.C.; Sopyan, I.; Maleque, M.A.; Krishnasamy, S.; Chandran, H.; Misran, H.; Sutharsini, U. The properties of hydroxyapatite ceramic coatings produced by plasma electrolytic oxidation. Ceram. Int. 2018, 44, 1802–1811. [Google Scholar] [CrossRef]
- Han, X.; Ma, J.; Tian, A.; Wang, Y.; Li, Y.; Dong, B.; Tong, X.; Ma, X. Surface modification techniques of titanium and titanium alloys for biomedical orthopaedics applications: A review. Colloids Surf. B Biointerfaces 2023, 227, 113339. [Google Scholar] [CrossRef] [PubMed]
- Rogowska-Tylman, J.; Locs, J.; Salma, I.; Woźniak, B.; Pilmane, M.; Zalite, V.; Wojnarowicz, J.; Kędzierska-Sar, A.; Chudoba, T.; Szlązak, K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng. C 2019, 99, 669–684. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, S.; Wang, Q.; Ramachandran, C.S. Effect of hydrothermal treatment on the surface characteristics and bioactivity of HAP based MAO coating on Ti–6Al–4V alloy. Surf. Coat. Technol. 2023, 464, 129566. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Jonasova, L.; Muller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar] [CrossRef]
- Arslan, Y.E.; Sezgin Arslan, T.; Derkus, B.; Emregul, E.; Emregul, K.C. Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductive biocomposite scaffolds for bone tissue engineering: From waste to regenerative medicine products. Colloids Surf. B Biointerfaces 2017, 154, 160–170. [Google Scholar] [CrossRef] [PubMed]
- El Rhilassi, A.; Mourabet, M.; Bennani-Ziatni, M.; El Hamri, R.; Taitai, A. Interaction of some essential amino acids with synthesized poorly crystalline hydroxyapatite. J. Saudi Chem. Soc. 2016, 20 (Suppl. S1), S632–S640. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wu, S.-C.; Lin, C.-Y.; Ho, W.-F. Characterization of Hydroxyapatite/Chitosan Composite Coating Obtained from Crab Shells on Low-Modulus Ti–25Nb–8Sn Alloy through Hydrothermal Treatment. Coatings 2023, 13, 228. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Z.; Toftdal, M.S.; Møller, A.C.; Dagnaes-Hansen, F.; Dong, M.; Thomsen, J.S.; Brüel, A.; Chen, M. Synchronous delivery of hydroxyapatite and connective tissue growth factor derived osteoinductive peptide enhanced osteogenesis. J. Control. Release 2019, 301, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Nagata, F.; Kugimiya, S.; Kato, K. Synthesis of peptide-containing calcium phosphate nanoparticles exhibiting highly selective adsorption of various proteins. Appl. Surf. Sci. 2018, 458, 438–445. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N.; Motelica-Heino, M. Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure. Coatings 2019, 9, 156. [Google Scholar] [CrossRef]
- Predoi, D.; Popa, C.L.; Chapon, P.; Groza, A.; Iconaru, S.L. Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films. Materials 2016, 9, 778. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Balandin, S.V.; Belogurova-Ovchinnikova, O.Y.; Ovchinnikova, T.V. Marine Invertebrate Antimicrobial Peptides and Their Potential as Novel Peptide Antibiotics. Mar. Drugs 2023, 21, 503. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.B.; Dos Santos Machado, L.; Meneguetti, B.T.; Nogueira, M.L.; Carvalho, C.M.E.; Roel, A.R.; Franco, O.L. Utilization of antimicrobial peptides, analogues and mimics in creating antimicrobial surfaces and bio-materials. Biochem. Eng. J. 2019, 150, 107237. [Google Scholar] [CrossRef]
- Seo, J.-K.; Lee, M.J.; Go, H.-J.; Kim, G.D.; Jeong, H.D.; Nam, B.-H.; Park, N.G. Purification and antimicrobial function of ubiquitin isolated from the gill of Pacific oyster, Crassostrea gigas. Mol. Immunol. 2013, 53, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Brakemi, E.; Michael, K.; Tan, S.P.; Helen, H. Antimicrobial activity of natural mollusc shells: A review. Process Biochem. 2023, 137, 122–133. [Google Scholar] [CrossRef]
- Upadhyay, A.; Thiyagarajan, V.; Tong, Y. Proteomic characterization of oyster shell organic matrix proteins (OMP). Bioinformation 2016, 12, 266–278. [Google Scholar] [CrossRef][Green Version]
- Lamghari, M.; Almeida, M.J.; Berland, S.; Huet, H.; Laurent, A.; Milet, C.; Lopez, E. Stimulation of bone marrow cells and bone formation by nacre- in vivo and in vitro studies. Bone 1999, 25, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Chen, J.; Wang, H.; Zhong, S.; Hu, Y.; Wang, Z.; Zhang, Q. Abalone water-soluble matrix for self-healing biomineralization of tooth defects. Mater. Sci. Eng. C 2016, 67, 182–187. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, M.; Chang, L.; Liu, Q.; Wang, C.; Hu, L.; Zhang, Z.; Ding, W.; Chen, L.; Guo, S.; et al. Overview of structure, function and integrated utilization of marine shell. Sci. Total Environ. 2023, 870, 161950. [Google Scholar] [CrossRef]
- Bédouet, L.; Duplat, D.; Marie, A.; Dubost, L.; Berland, S.; Rousseau, M.; Milet, C.; Lopez, E. Heterogeneity of proteinase inhibitors in the water-soluble organic matrix from the oyster nacre. Mar. Biotechnol. 2007, 9, 437–449. [Google Scholar] [CrossRef]
- ASTM D 5725; Standard Test Method for Surface Wettability and Adsorbency of Sheeted Materials Using an Automated Contact Angle Tester. ASTM: West Conshohocken, PA, USA, 2008.
- An, L.; Li, W.; Xu, Y.; Zeng, D.; Cheng, Y.; Wang, G. Controlled additive-free hydrothermal synthesis and characterization of uniform hydroxyapatite nanobelts. Ceram. Int. 2016, 42, 3104–3112. [Google Scholar] [CrossRef]
- Fu, T.; Fan, J.T.; Shen, Y.G.; Sun, J.M. Hydrothermal calcification of alkali treated titanium in CaHPO4 solution. Mater. Chem. Phys. 2017, 189, 105–110. [Google Scholar] [CrossRef]
- Krukowski, S.; Lysenko, N.; Kolodziejski, W. Synthesis and characterization of nanocrystalline composites containing calcium hydroxyapatite and glycine. J. Solid State Chem. 2018, 264, 59–67. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K.; Venkatesh, M.; Karunakaran, G.; Kolesnikov, E.; Kuznetsov, D. One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceram. Int. 2017, 43, 3457–3461. [Google Scholar] [CrossRef]
- Pisarek, M.; Roguska, A.; Andrzejczuk, M.; Marcon, L.; Szunerits, S.; Lewandowska, M.; Janik-Czachor, M. Effect of two-step functionalization of Ti by chemical processes on protein adsorption. Appl. Surf. Sci. 2011, 257, 8196–8204. [Google Scholar] [CrossRef]
- Polzonetti, G.; Battocchio, C.; Iucci, G.; Dettin, M.; Gambaretto, R.; Di Bello, C.; Carravetta, V. Thin films of a self-assembling peptide on TiO2 and Au studied by NEXAFS, XPS and IR spectroscopies. Mater. Sci. Eng. C 2006, 26, 929–934. [Google Scholar] [CrossRef]
- Vanea, E.; Simon, V. XPS study of protein adsorption onto nanocrystalline aluminosilicate microparticles. Appl. Surf. Sci. 2011, 257, 2346–2352. [Google Scholar] [CrossRef]
- Kong, W.; Zhao, K.; Gao, C.; Zhu, P. Synthesis and characterization of carbonated hydroxyapatite with layered structure. Mater. Lett. 2019, 255, 126552. [Google Scholar] [CrossRef]
- Lai, M.; Jin, Z.; Su, Z. Surface modification of TiO2 nanotubes with osteogenic growth peptide to enhance osteoblast differentiation. Mater. Sci. Eng. C 2017, 73, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Fok, A.; Aparicio, C. Hydrophobic and antimicrobial dentin: A peptide-based 2-tier protective system for dental resin composite restorations. Acta Biomater. 2019, 88, 251–265. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nanoscale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Bartomeu Garcia, C.; Hameed, P.; Manivasagam, G.; Webster, T.J. Hydrothermal treatment of etched titanium: A potential surface nano-modification technique for enhanced biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102016. [Google Scholar] [CrossRef] [PubMed]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, K.-H.; Hsu, H.-C.; Kao, Y.-L.; Wu, S.-C.; Yang, T.-Y.; Ho, W.-F. Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells. Nanomaterials 2024, 14, 577. https://doi.org/10.3390/nano14070577
Hsieh K-H, Hsu H-C, Kao Y-L, Wu S-C, Yang T-Y, Ho W-F. Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells. Nanomaterials. 2024; 14(7):577. https://doi.org/10.3390/nano14070577
Chicago/Turabian StyleHsieh, Kuan-Hsiang, Hsueh-Chuan Hsu, Yu-Lin Kao, Shih-Ching Wu, Tzu-Yen Yang, and Wen-Fu Ho. 2024. "Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells" Nanomaterials 14, no. 7: 577. https://doi.org/10.3390/nano14070577
APA StyleHsieh, K.-H., Hsu, H.-C., Kao, Y.-L., Wu, S.-C., Yang, T.-Y., & Ho, W.-F. (2024). Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells. Nanomaterials, 14(7), 577. https://doi.org/10.3390/nano14070577






