Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
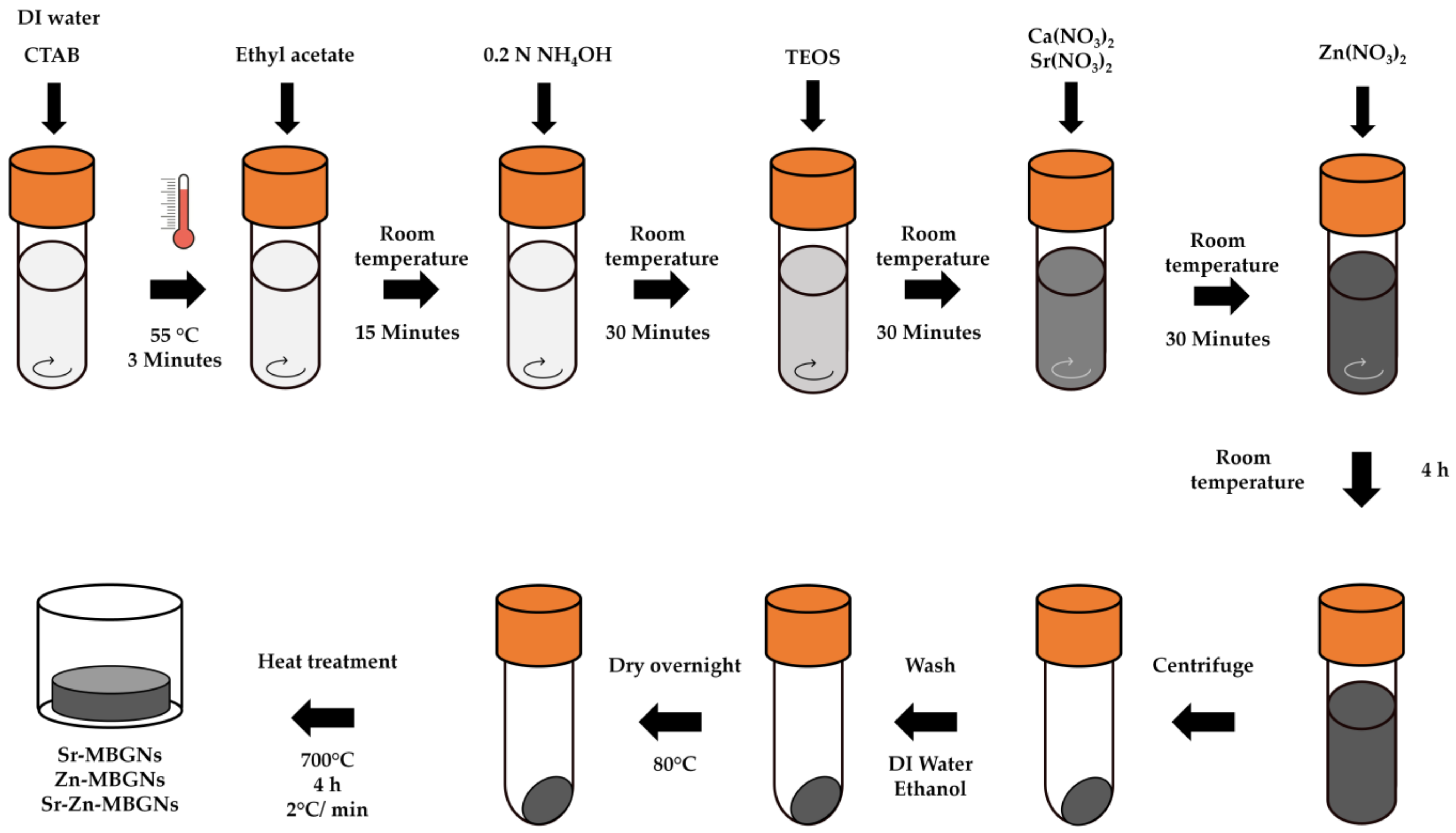
2.1. Mesoporous Bioactive Glass Nanoparticle (MBGN) Synthesis
2.2. Physicochemical Characterization of MBGNs
2.3. In Vitro Bioactivity Study
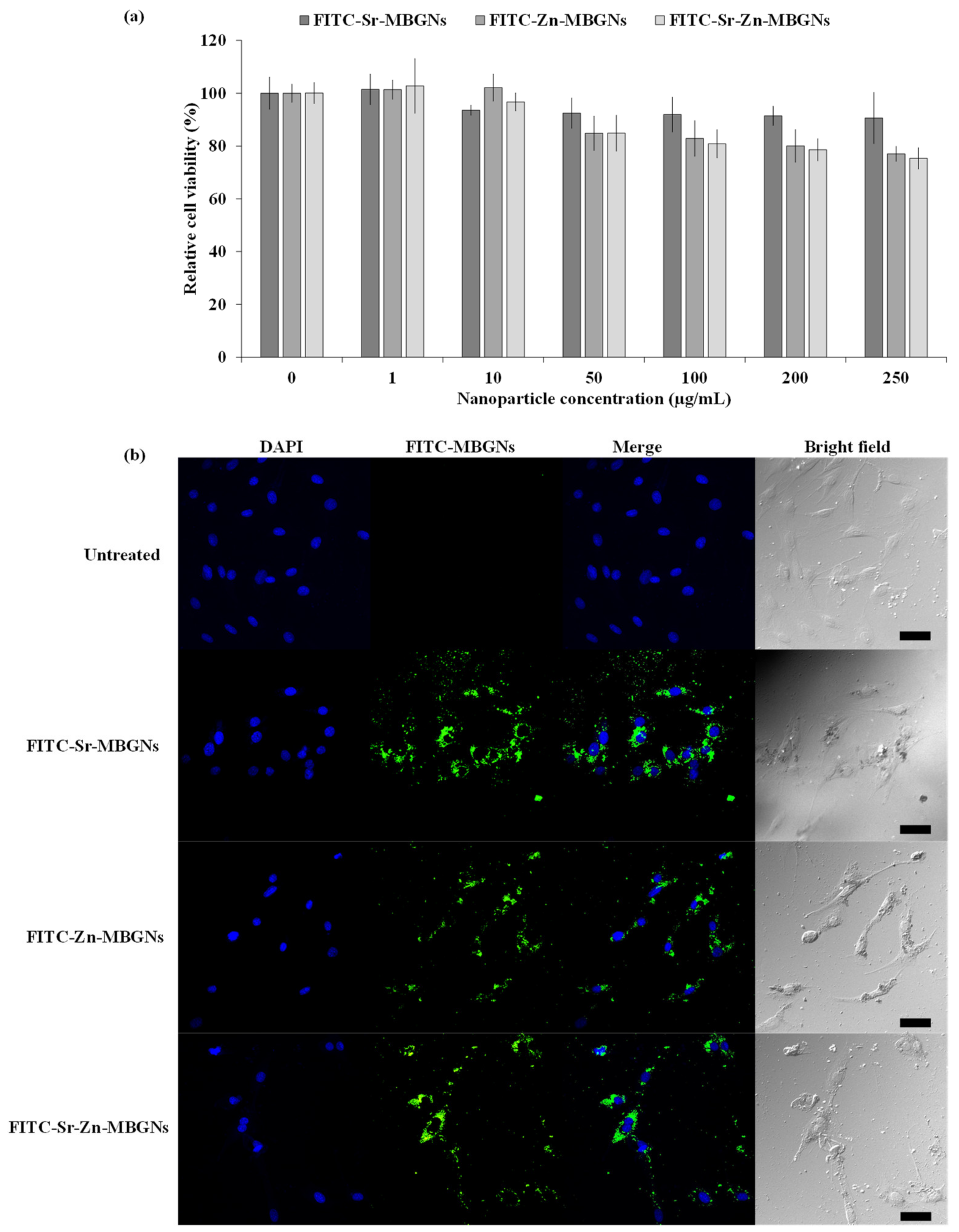
2.4. In Vitro Cell Viability
2.5. In Vitro Mineralization
2.6. Osteogenic Differentiation
2.7. Cellular Uptake
2.8. In Vitro Antibacterial Activity
2.9. Statistical Analysis
3. Results and Discussion
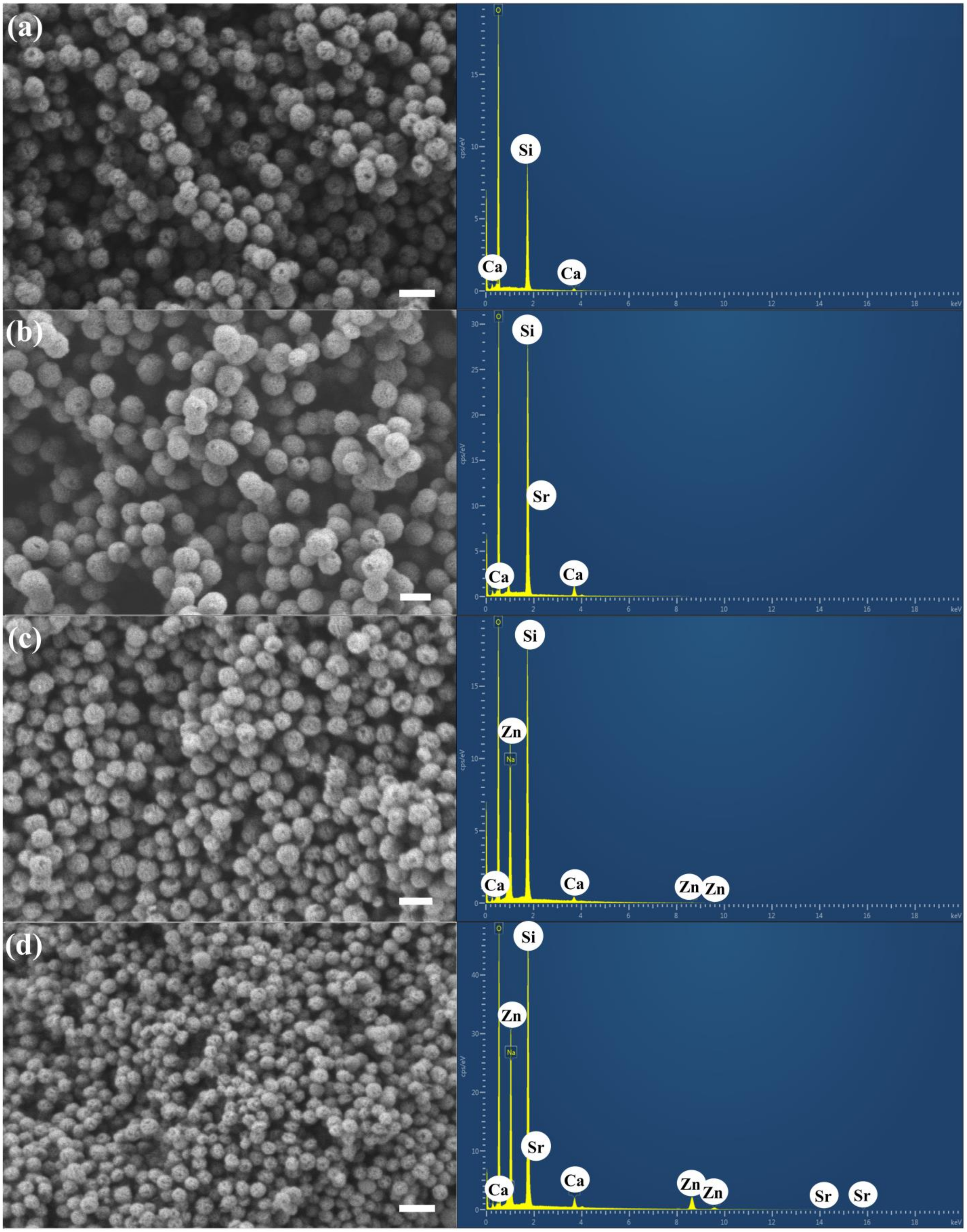
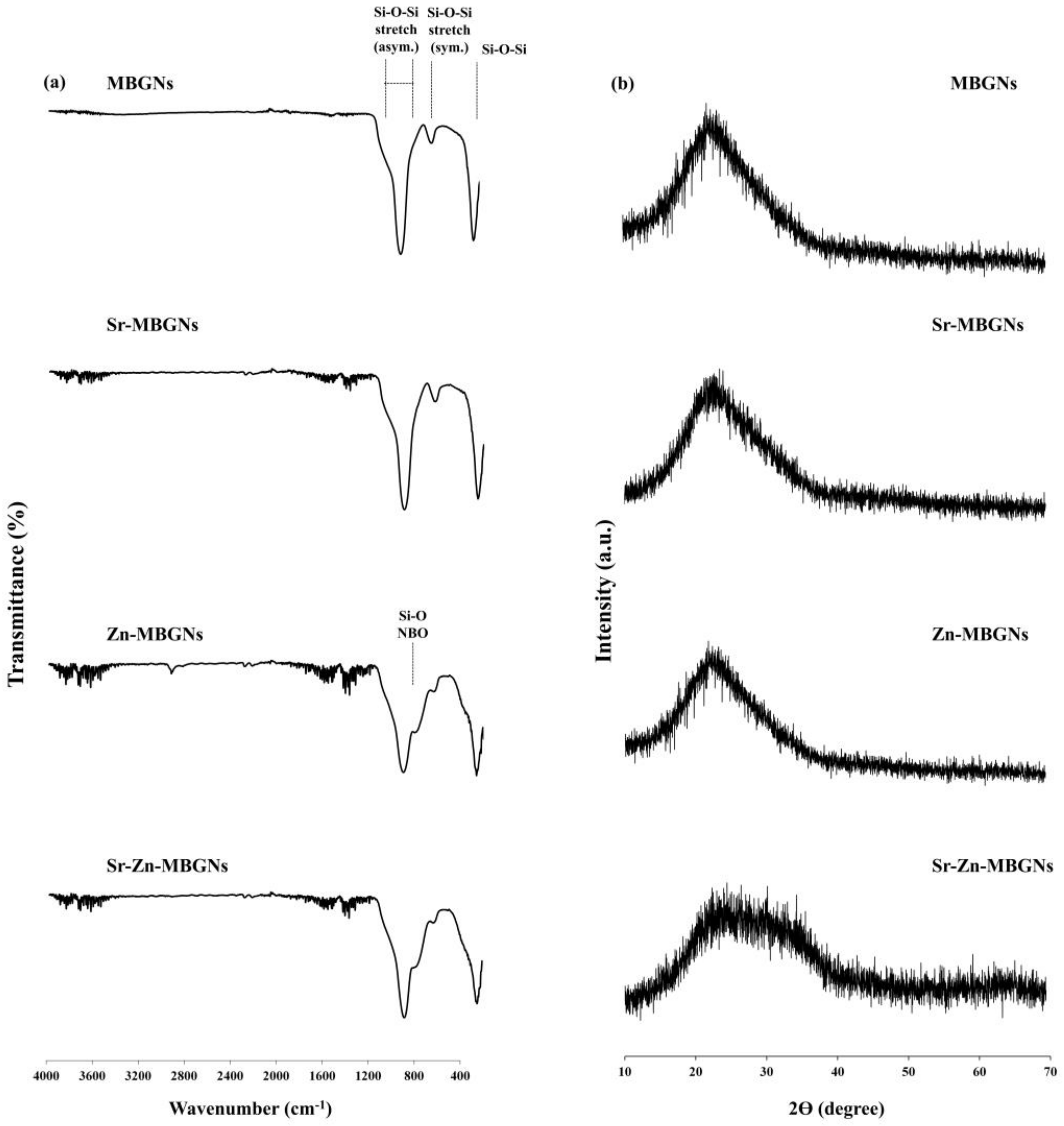
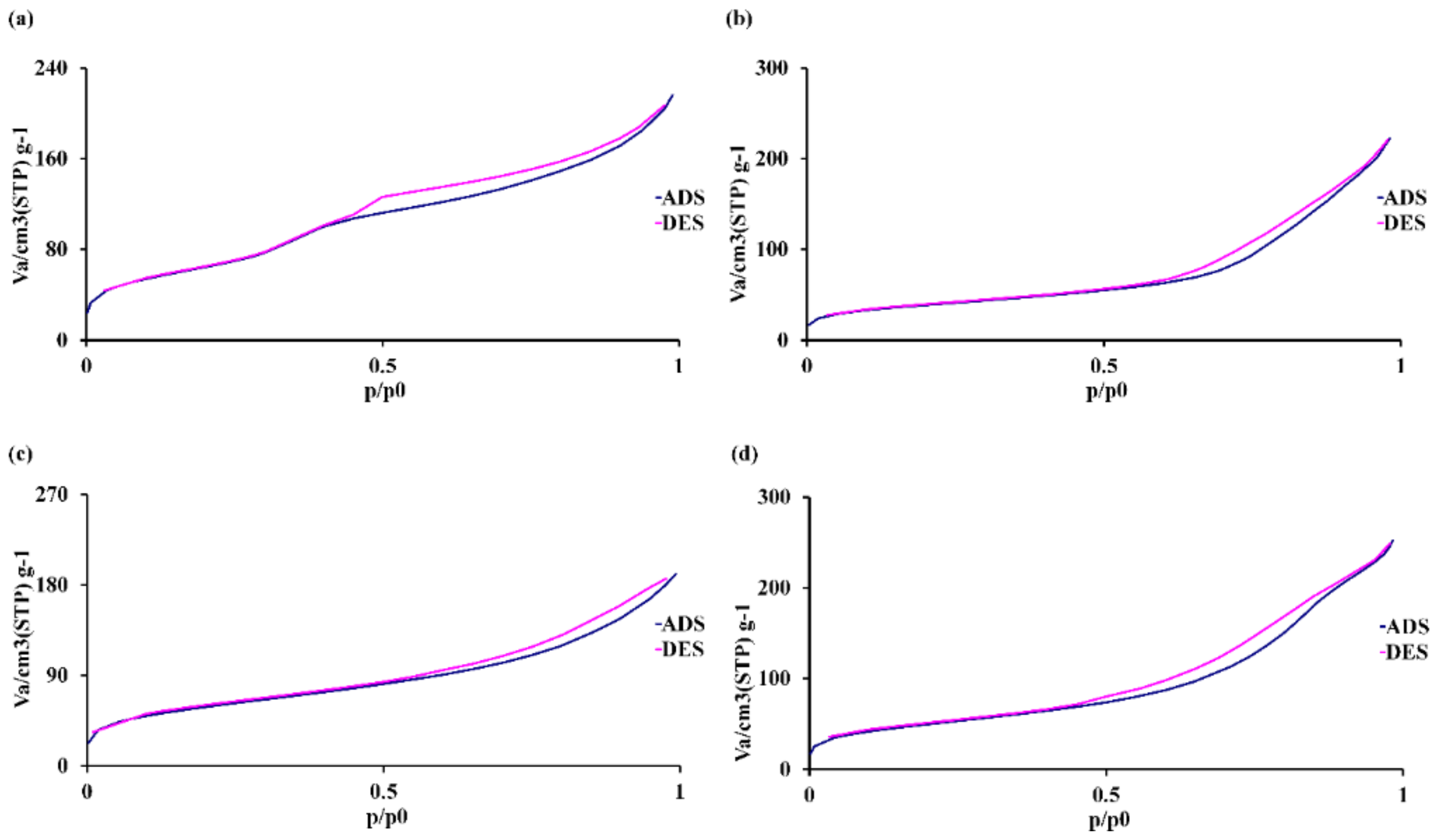
3.1. Characterization of MBGNs
3.2. In Vitro Bioactivity
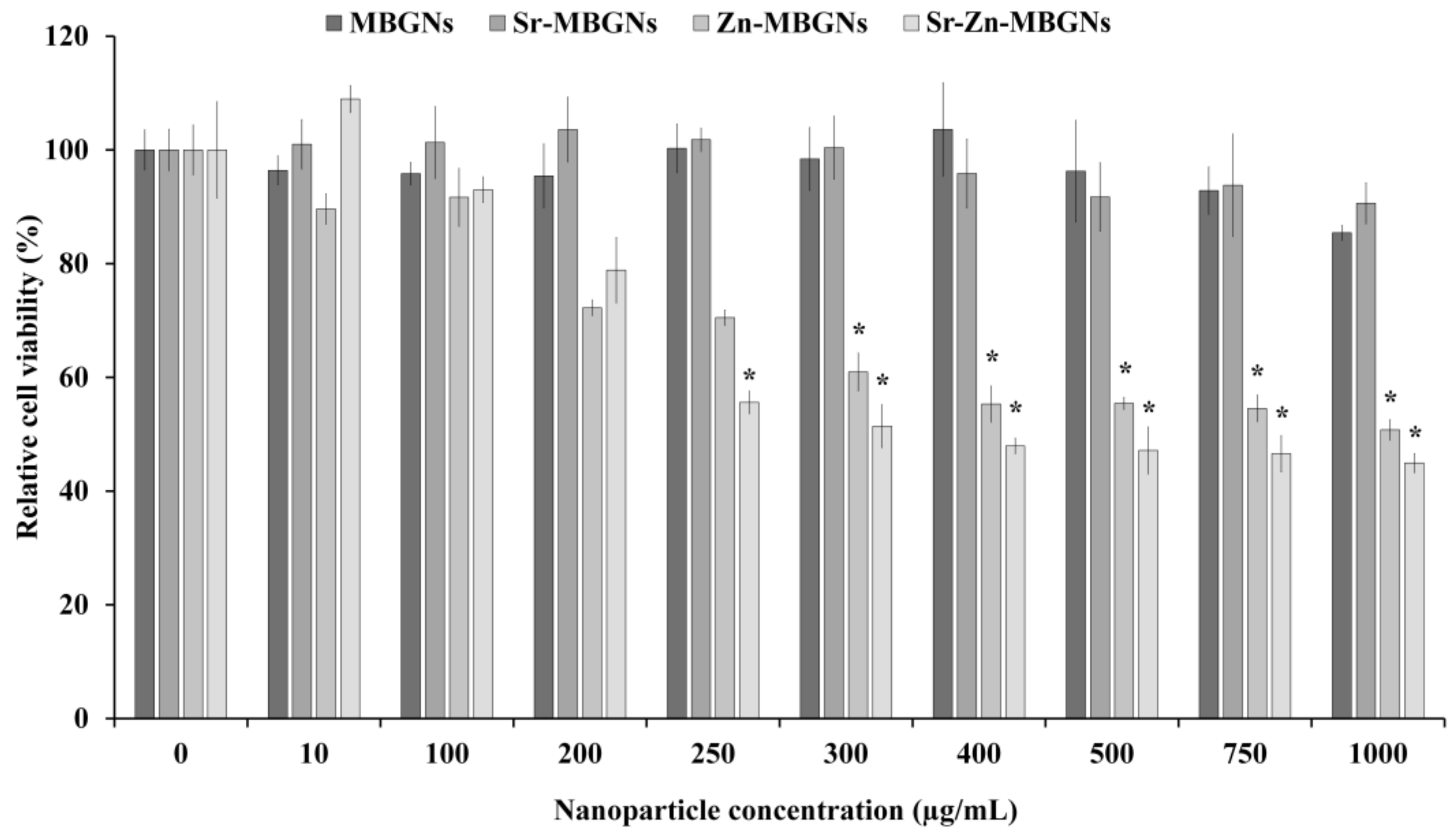
3.3. In Vitro Cytotoxicity
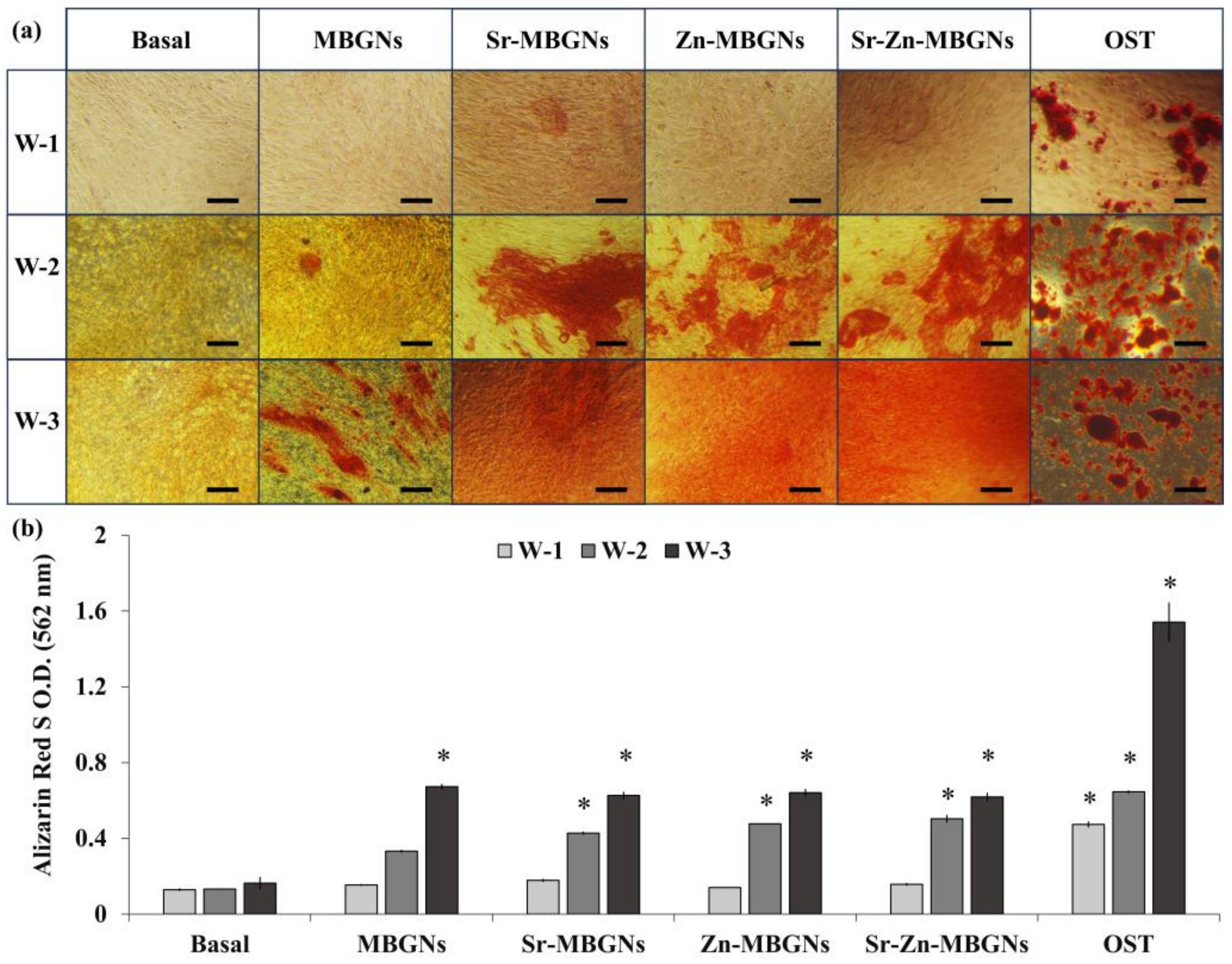
3.4. In Vitro Mineralization
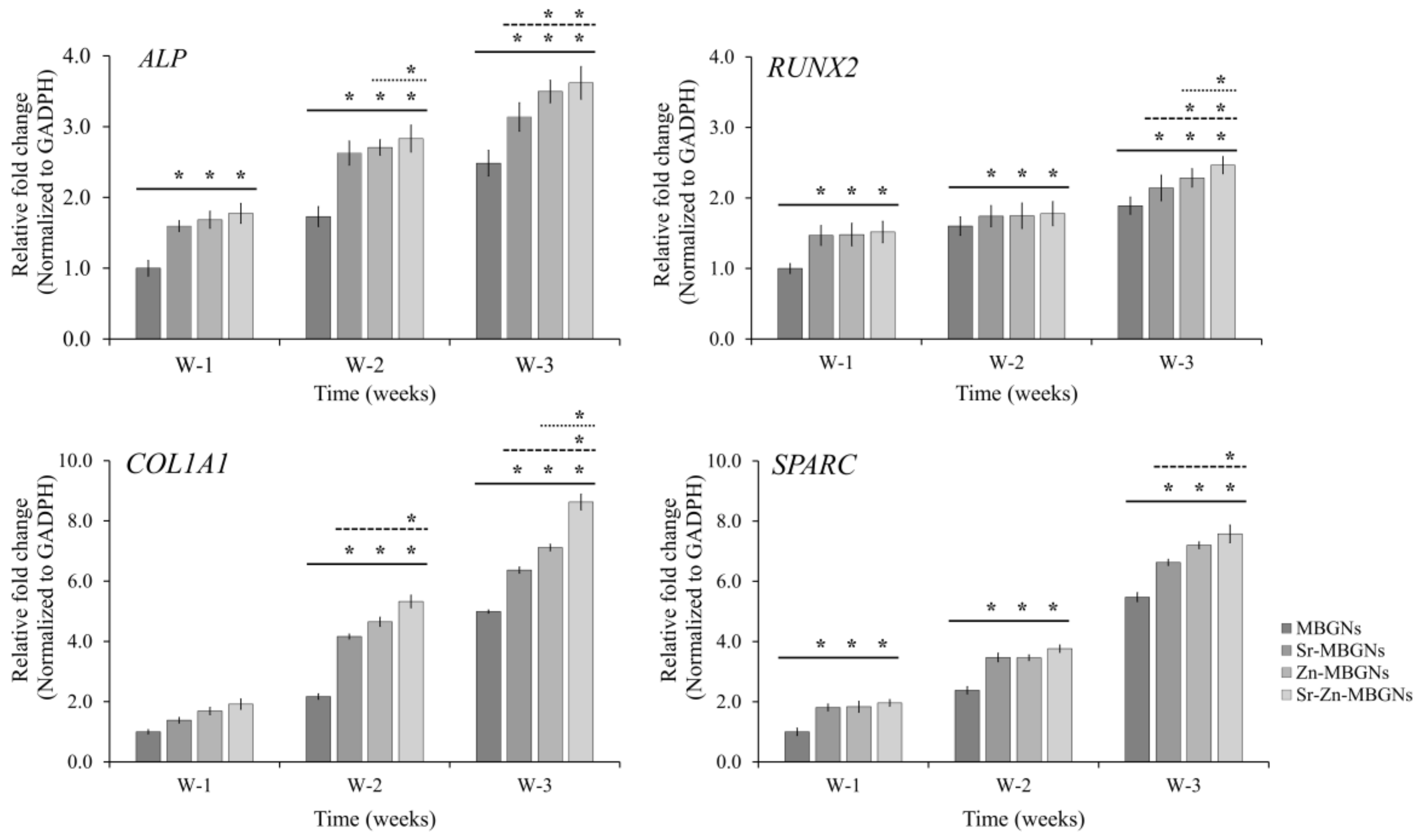
3.5. Osteogenic Differentiation
3.6. Cellular Uptake
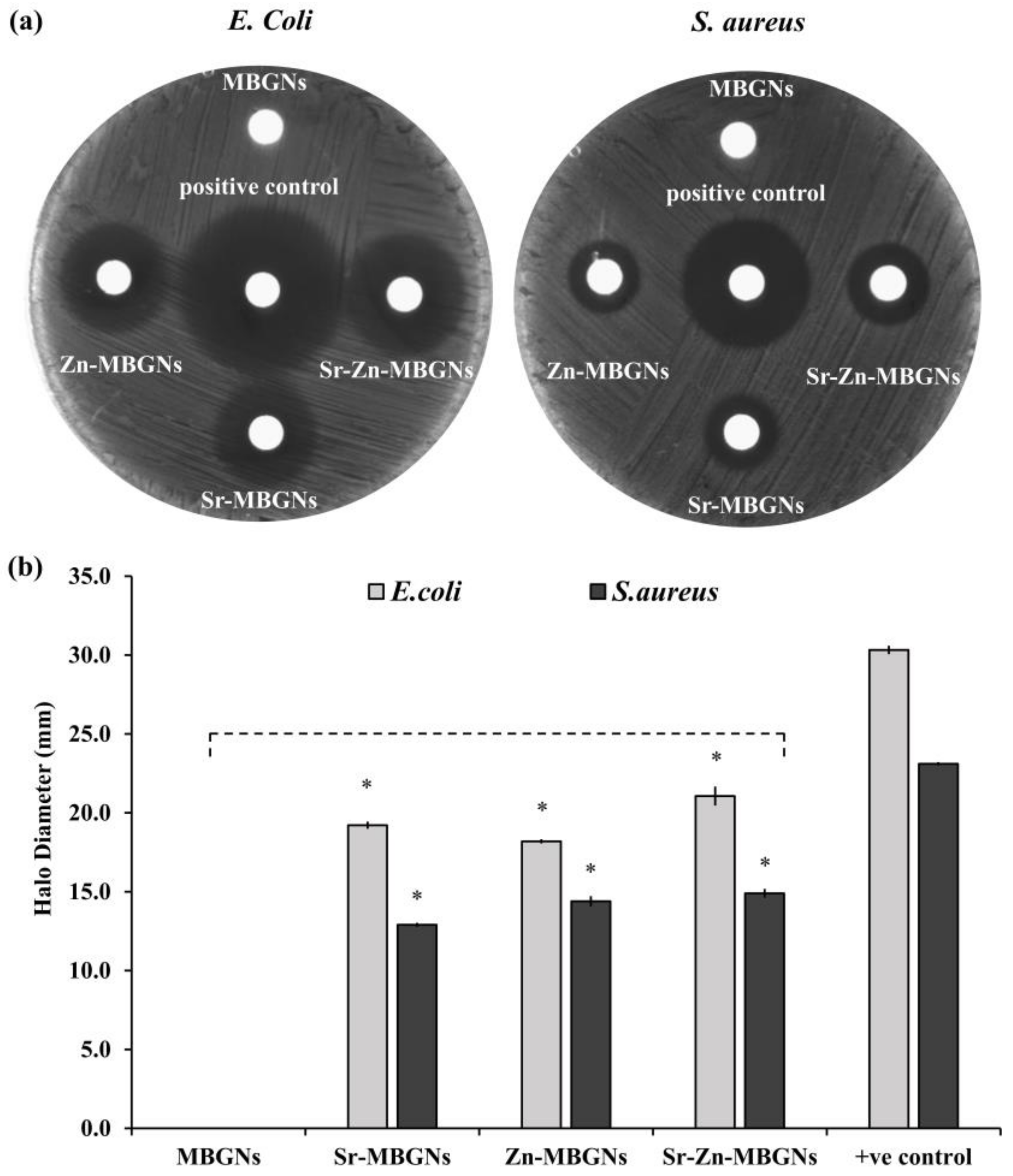
3.7. In Vitro Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Smith, A.A.; Liu, B.; Li, J.; Hunter, D.J.; Dhamdhere, G.; Salmon, B.; Jiang, J.; Cheng, D.; Johnson, C.A.; et al. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials 2015, 47, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Kim, G.K. Chapter 32—Bone Grafting. In High Yield Orthopaedics; Parvizi, J., Kim, G.K., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2010; pp. 64–65. [Google Scholar]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Stoffella, E.; Stanford, C.M. Chapter 5—Bone Grafts and Bone Substitute Materials. In Principles and Practice of Single Implant and Restorations; Torabinejad, M., Sabeti, M.A., Goodacre, C.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2014; pp. 75–86. [Google Scholar]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Zhang, X.; Chang, J.; Dai, K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 2018, 66, 81–92. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J. 1—Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–19. [Google Scholar]
- Wong, K.L.; Wong, C.T.; Liu, W.C.; Pan, H.B.; Fong, M.K.; Lam, W.M.; Cheung, W.L.; Tang, W.M.; Chiu, K.Y.; Luk, K.D.K.; et al. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials 2009, 30, 3810–3817. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From Bone Regeneration to Cancer Nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Chen, L.; Lin, X.; Huang, Y.; Dai, K.; Naoki, K.; Chen, G.; Chang, J. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012, 8, 341–349. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Bellucci, D.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C 2017, 79, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Q.; Rehman, M.A.U.; Burkovski, A.; Schmidt, J.; Beltrán, A.M.; Shahid, A.; Alber, N.K.; Peukert, W.; Boccaccini, A.R. Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2018, 29, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Greasley, S.L.; Ong, Z.Y.; Naruphontjirakul, P.; Page, S.J.; Hanna, J.V.; Redpath, A.N.; Tsigkou, O.; Rankin, S.; Ryan, M.P.; et al. Biodegradable zinc-containing mesoporous silica nanoparticles for cancer therapy. Mater. Today Adv. 2020, 6, 100066. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Kettiger, H. Chapter 1—Silica-based nanoparticles as drug delivery systems: Chances and challenges. In Inorganic Frameworks as Smart Nanomedicines; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–40. [Google Scholar]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Vazquez, N.I.; Gonzalez, Z.; Ferrari, B.; Castro, Y.J. Synthesis of mesoporous silica nanoparticles by sol–gel as nanocontainer for future drug delivery applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 139–145. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D.J. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.W.; Yamauchi, Y. Controlling physical features of mesoporous silica nanoparticles (MSNs) for emerging applications. J. Mater. Chem. 2012, 22, 1251–1256. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1573. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Chapter 1—Introduction. In Sol-Gel Science; Academic Press: San Diego, CA, USA, 1990; pp. 1–18. [Google Scholar]
- Hooshmand, S.; Mollazadeh, S.; Akrami, N.; Ghanad, M.; El-Fiqi, A.; Baino, F.; Nazarnezhad, S.; Kargozar, S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials 2021, 14, 3337. [Google Scholar] [CrossRef]
- Kurtuldu, F.; Mutlu, N.; Michálek, M.; Zheng, K.; Masar, M.; Liverani, L.; Chen, S.; Galusek, D.; Boccaccini, A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: Bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2021, 124, 112050. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Rajapantulu, A.; Bandyopadhyaya, R. Formation of Gold Nanoparticles in Water-in-Oil Microemulsions: Experiment, Mechanism, and Simulation. Langmuir 2021, 37, 6623–6631. [Google Scholar] [CrossRef]
- Kanniyappan, H.; Venkatesan, M.; Panji, J.; Ramasamy, M.; Muthuvijayan, V. Evaluating the inherent osteogenic and angiogenic potential of mesoporous silica nanoparticles to augment vascularized bone tissue formation. Microporous Mesoporous Mater. 2021, 311, 110687. [Google Scholar] [CrossRef]
- Kumar, A.; Banrjee, S.; Roy, P.; Xu, H.; Mariappan, C.R. Osteogenic commitment of strontium nanoparticles doped mesoporous bioactive glass-ceramics. Mater. Sci. Eng. B 2022, 286, 116068. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh Beidokhti, S.; Baino, F.; Gholamzadeh-Virany, Z.; Mozafari, M.; Kargozar, S. Strontium-and cobalt-doped multicomponent mesoporous bioactive glasses (MBGS) for potential use in bone tissue engineering applications. Materials 2020, 13, 1348. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, K.; Zhou, T.; Boccaccini, A.R. Incorporation of Zinc into Binary SiO2-CaO Mesoporous Bioactive Glass Nanoparticles Enhances Anti-Inflammatory and Osteogenic Activities. Pharmaceutics 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Schmitz, S.I.; Moghaddam, A.; Boccaccini, A.R. Osteogenic properties of manganese-doped mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. Part A 2020, 108, 1806–1815. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Allam, R.; Kim, H.-W. Antioxidant cerium ions-containing mesoporous bioactive glass ultrasmall nanoparticles: Structural, physico-chemical, catalase-mimic and biological properties. Colloids Surf. B Biointerfaces 2021, 206, 111932. [Google Scholar] [CrossRef]
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Hohenbild, F.; Rehder, F.; Saur, M.; Fellenberg, J.; Moghaddam, A.; Ali, M.S.; Peukert, W.; et al. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. Part A 2021, 109, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80. [Google Scholar] [CrossRef]
- Schuhladen, K.; Stich, L.; Schmidt, J.; Steinkasserer, A.; Boccaccini, A.R.; Zinser, E. Cu, Zn doped borate bioactive glasses: Antibacterial efficacy and dose-dependent in vitro modulation of murine dendritic cells. Biomater. Sci. 2020, 8, 2143–2155. [Google Scholar] [CrossRef]
- Heras, C.; Jiménez-Holguín, J.; Doadrio, A.L.; Vallet-Regí, M.; Sánchez-Salcedo, S.; Salinas, A.J. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020, 114, 395–406. [Google Scholar] [CrossRef]
- Thanasrisuebwong, P.; Jones, J.R.; Eiamboonsert, S.; Ruangsawasdi, N.; Jirajariyavej, B.; Naruphontjirakul, P. Zinc-Containing Sol–Gel Glass Nanoparticles to Deliver Therapeutic Ions. Nanomaterials 2022, 12, 1691. [Google Scholar] [CrossRef]
- Kapoor, S.K.; Thiyam, R. Management of infection following reconstruction in bone tumors. J. Clin. Orthop. Trauma 2015, 6, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Stefani, S.; Venditti, M.; Di Domenico, E.G. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front. Microbiol. 2021, 12, 749685. [Google Scholar] [CrossRef] [PubMed]
- Khoeini, M.; Najafi, A.; Rastegar, H.; Amani, M. Improvement of hollow mesoporous silica nanoparticles synthesis by hard-templating method via CTAB surfactant. Ceram. Int. 2019, 45, 12700–12707. [Google Scholar] [CrossRef]
- Pinna, A.; Torki Baghbaderani, M.; Vigil Hernández, V.; Naruphontjirakul, P.; Li, S.; McFarlane, T.; Hachim, D.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Nanoceria provides antioxidant and osteogenic properties to mesoporous silica nanoparticles for osteoporosis treatment. Acta Biomater. 2021, 122, 365–376. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Hosseini, M.; Hassani Besheli, N.; Deng, D.; Lievens, C.; Zuo, Y.; Leeuwenburgh, S.C.G.; Yang, F. Facile post modification synthesis of copper-doped mesoporous bioactive glass with high antibacterial performance to fight bone infection. Biomater. Adv. 2023, 144, 213198. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Mubina, M.S.K.; Shailajha, S.; Sankaranarayanan, R.; Saranya, L. In vitro bioactivity, mechanical behavior and antibacterial properties of mesoporous SiO2-CaO-Na2O-P2O5 nano bioactive glass ceramics. J. Mech. Behav. Biomed. Mater. 2019, 100, 103379. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Nandhakumar, S.; Dhanaraju, M.D.; Sundar, V.D.; Heera, B. Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly(ε-caprolactone) nanoparticles. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 249–258. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Labbaf, S.; Karimzadeh, F.; Pinna, A.; Houreh, A.B.; Nasr-Esfahani, M.H. Mesoporous bioactive glasses for the combined application of osteosarcoma treatment and bone regeneration. Mater. Sci. Eng. C 2019, 104, 109994. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol–gel derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- Amudha, S.; Ramya, J.R.; Arul, K.T.; Deepika, A.; Sathiamurthi, P.; Mohana, B.; Asokan, K.; Dong, C.-L.; Kalkura, S.N. Enhanced mechanical and biocompatible properties of strontium ions doped mesoporous bioactive glass. Compos. Part B Eng. 2020, 196, 108099. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Greasley, S.L.; Chen, S.; Porter, A.E.; Jones, J.R. Monodispersed strontium containing bioactive glass nanoparticles and MC3T3-E1 cellular response. Biomed. Glass. 2016, 2, 72–81. [Google Scholar] [CrossRef]
- Mutlu, N.; Kurtuldu, F.; Unalan, I.; Neščáková, Z.; Kaňková, H.; Galusková, D.; Michálek, M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Effect of Zn and Ga doping on bioactivity, degradation, and antibacterial properties of borate 1393-B3 bioactive glass. Ceram. Int. 2022, 48, 16404–16417. [Google Scholar] [CrossRef]
- Aina, V.; Bonino, F.; Morterra, C.; Miola, M.; Bianchi, C.L.; Malavasi, G.; Marchetti, M.; Bolis, V. Influence of the Chemical Composition on Nature and Activity of the Surface Layer of Zn-Substituted Sol−Gel (Bioactive) Glasses. J. Phys. Chem. C 2011, 115, 2196–2210. [Google Scholar] [CrossRef]
- Zhao, R.; Shi, L.; Gu, L.; Qin, X.; Song, Z.; Fan, X.; Zhao, P.; Li, C.; Zheng, H.; Li, Z. Evaluation of bioactive glass scaffolds incorporating SrO or ZnO for bone repair: In vitro bioactivity and antibacterial activity. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040910. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol–gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Damian-Buda, A.-I.; Ghițulică, C.-D.; Cucuruz, A.; Voicu, G.; Culita, D.; Fruth-Oprișan, V.; Ciocan, L.T. Mesoporous Bioactive Glass Nanoparticles in the SiO2-P2O5-CaO-MO (M=Mg, Zn) System: Synthesis and Properties. J. Funct. Biomater. 2022, 13, 180. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Zhong, W.; Xiao, H.; Liu, X.; Yu, T.; Zhou, C. Tuning biodegradability and biocompatibility of mesoporous silica nanoparticles by doping strontium. Ceram. Int. 2020, 46, 11762–11769. [Google Scholar] [CrossRef]
- Fei, Y.-C.; Chen, L.-G.; Kuo, C.-K.; Chou, Y.-J. Influence of strontium dopant on bioactivity and osteoblast activity of spray pyrolyzed strontium-doped mesoporous bioactive glasses. J. Asian Ceram. Soc. 2021, 9, 221–228. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kawachi, G.; Kikuta, K.; Cho, S.B.; Kamitakahara, M.; Ohtsuki, C. Preparation of bioactive spherical particles in the CaO–SiO2 system through sol–gel processing under coexistence of poly(ethylene glycol). J. Eur. Ceram. Soc. 2008, 28, 1595–1602. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive Glasses—Structure and Properties. Angew. Chem. Int. Ed. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Tabia, Z.; El Mabrouk, K.; Bricha, M.; Nouneh, K. Mesoporous bioactive glass nanoparticles doped with magnesium: Drug delivery and acellular in vitro bioactivity. RSC Adv. 2019, 9, 12232–12246. [Google Scholar] [CrossRef] [PubMed]
- Vichery, C.; Nedelec, J.-M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Liang, Q.; Hu, Q.; Miao, G.; Yuan, B.; Chen, X. A facile synthesis of novel mesoporous bioactive glass nanoparticles with various morphologies and tunable mesostructure by sacrificial liquid template method. Mater. Lett. 2015, 148, 45–49. [Google Scholar] [CrossRef]
- Vergnaud, F.; Mekonnen, B.; El Abbassi, A.; Vichery, C.; Nedelec, J.M. Correlating the Effect of Composition and Textural Properties on Bioactivity for Pristine and Copper-Doped Binary Mesoporous Bioactive Glass Nanoparticles. Materials 2023, 16, 6690. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Wessely-Szponder, J.; Palka, K.; Barylyak, A.; Zinchenko, V.; Przekora, A. Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. Int. J. Mol. Sci. 2023, 24, 5576. [Google Scholar] [CrossRef]
- Scott, R.; Kendall, C.; Stone, N.; Rogers, K. Elemental vs. phase composition of breast calcifications. Sci. Rep. 2017, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Reedwan Bin Zafar, A.; Namon, H.; Upsorn, B. Three-Dimensionally Ordered Macroporous-Mesoporous Bioactive Glass Ceramics for Drug Delivery Capacity and Evaluation of Drug Release. In Advanced Ceramic Materials; Mohsen, M., Ed.; Intech Open: Rijeka, Croatia, 2020; Chapter 11. [Google Scholar]
- Moghanian, A.; Zohourfazeli, M.; Mahdi Tajer, M.H.; Miri, Z.; Hosseini, S.; Rashvand, A. Preparation, characterization and in vitro biological response of simultaneous co-substitution of Zr+4/Sr+2 58S bioactive glass powder. Ceram. Int. 2021, 47, 23762–23769. [Google Scholar] [CrossRef]
- Aina, V.; Perardi, A.; Bergandi, L.; Malavasi, G.; Menabue, L.; Morterra, C.; Ghigo, D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem. Biol. Interact. 2007, 167, 207–218. [Google Scholar] [CrossRef]
- Aina, V.; Malavasi, G.; Fiorio Pla, A.; Munaron, L.; Morterra, C. Zinc-containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells. Acta Biomater. 2009, 5, 1211–1222. [Google Scholar] [CrossRef]
- Lee, N.H.; Kang, M.S.; Kim, T.H.; Yoon, D.S.; Mandakhbayar, N.; Jo, S.B.; Kim, H.S.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials 2021, 276, 121025. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration—A Review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 282. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Kanchanadumkerng, P.; Ruenraroengsak, P. Multifunctional Zn and Ag co-doped bioactive glass nanoparticles for bone therapeutic and regeneration. Sci. Rep. 2023, 13, 6775. [Google Scholar] [CrossRef]
- Leite, Á.J.; Gonçalves, A.I.; Rodrigues, M.T.; Gomes, M.E.; Mano, J.F. Strontium-Doped Bioactive Glass Nanoparticles in Osteogenic Commitment. ACS Appl. Mater. Interfaces 2018, 10, 23311–23320. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of osteoblast differentiation by cytokine networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Bhonde, R.; Gupta, P.K.; Totey, S. Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells. Differentiation 2012, 84, 185–192. [Google Scholar] [CrossRef]
- Zhu, Y.-S.; Gu, Y.; Jiang, C.; Chen, L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J. Cell. Physiol. 2020, 235, 2220–2231. [Google Scholar] [CrossRef]
- Gan, Q.; Dai, D.; Yuan, Y.; Qian, J.; Sha, S.; Shi, J.; Liu, C. Effect of size on the cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomed. Microdevices 2012, 14, 259–270. [Google Scholar] [CrossRef]
- Hassani Besheli, N.; Verbakel, J.; Hosseini, M.; Andrée, L.; Joosten, B.; Walboomers, X.F.; Cambi, A.; Yang, F.; Leeuwenburgh, S.C.G. Cellular Uptake of Modified Mesoporous Bioactive Glass Nanoparticles for Effective Intracellular Delivery of Therapeutic Agents. Int. J. Nanomed. 2023, 18, 1599–1612. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Stone, W.; Schemitsch, E.H.; Zalzal, P.; Papini, M.; Waldman, S.D.; Towler, M.R. Antibacterial and osteo-stimulatory effects of a borate-based glass series doped with strontium ions. J. Biomater. Appl. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneira, J.; Gorustovich, A. Bioactive glasses as delivery systems for antimicrobial agents. J. Appl. Microbiol. 2017, 122, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Wu, S.; Zhou, R.; Zhang, K.; Zhang, Z.; Liu, J.; Qin, S.; Shi, J. Nanomaterial-Based Zinc Ion Interference Therapy to Combat Bacterial Infections. Front. Immunol. 2022, 13, 899992. [Google Scholar] [CrossRef]
- Chausov, D.; Burmistrov, D.; Kurilov, A.; Bunkin, N.; Astashev, M.; Simakin, A.; Vedunova, M.; Gudkov, S. New Organosilicon Composite Based on Borosiloxane and Zinc Oxide Nanoparticles Inhibits Bacterial Growth, but Does Not Have a Toxic Effect on the Development of Animal Eukaryotic Cells. Materials 2021, 14, 6281. [Google Scholar] [CrossRef]
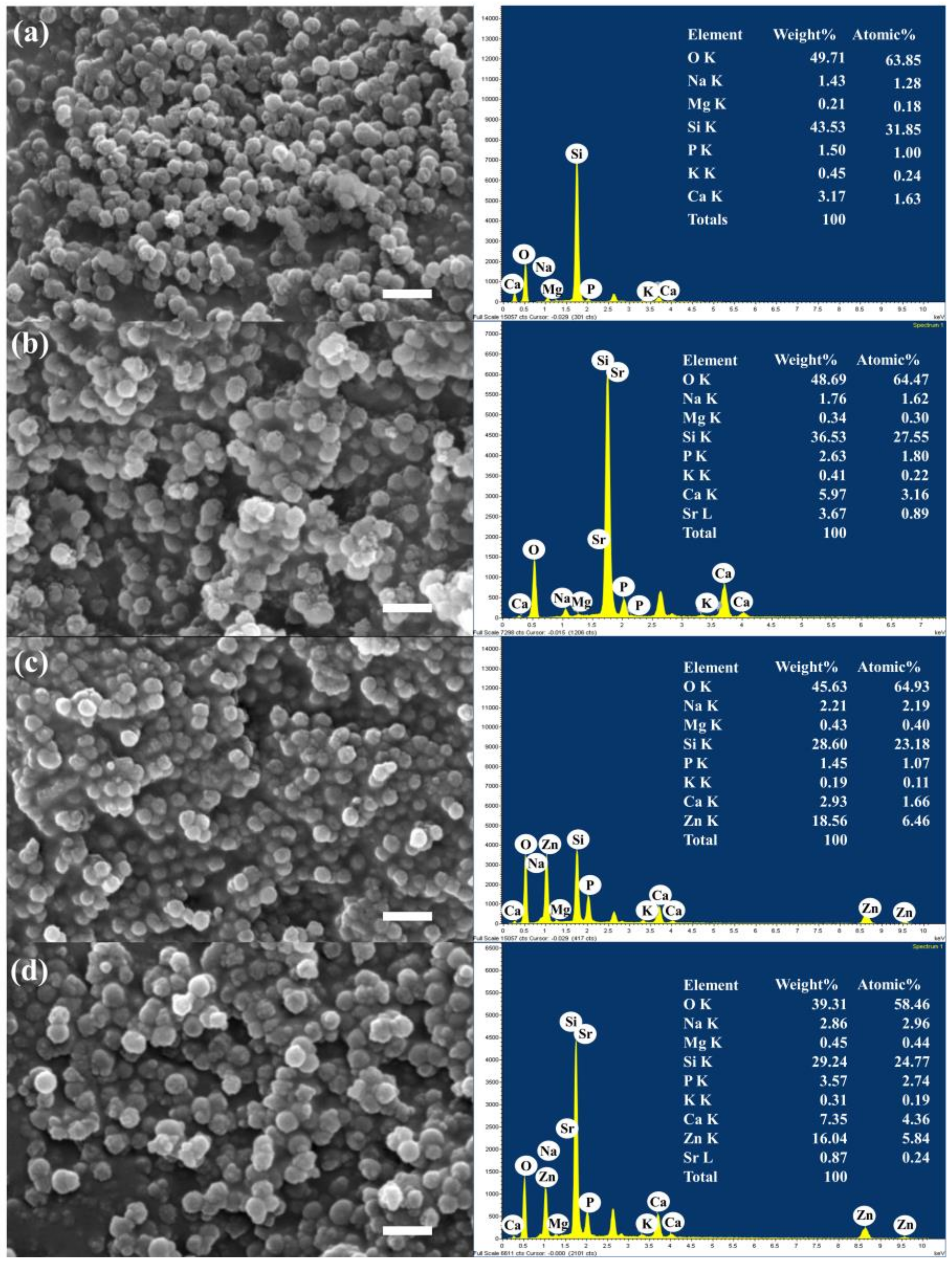
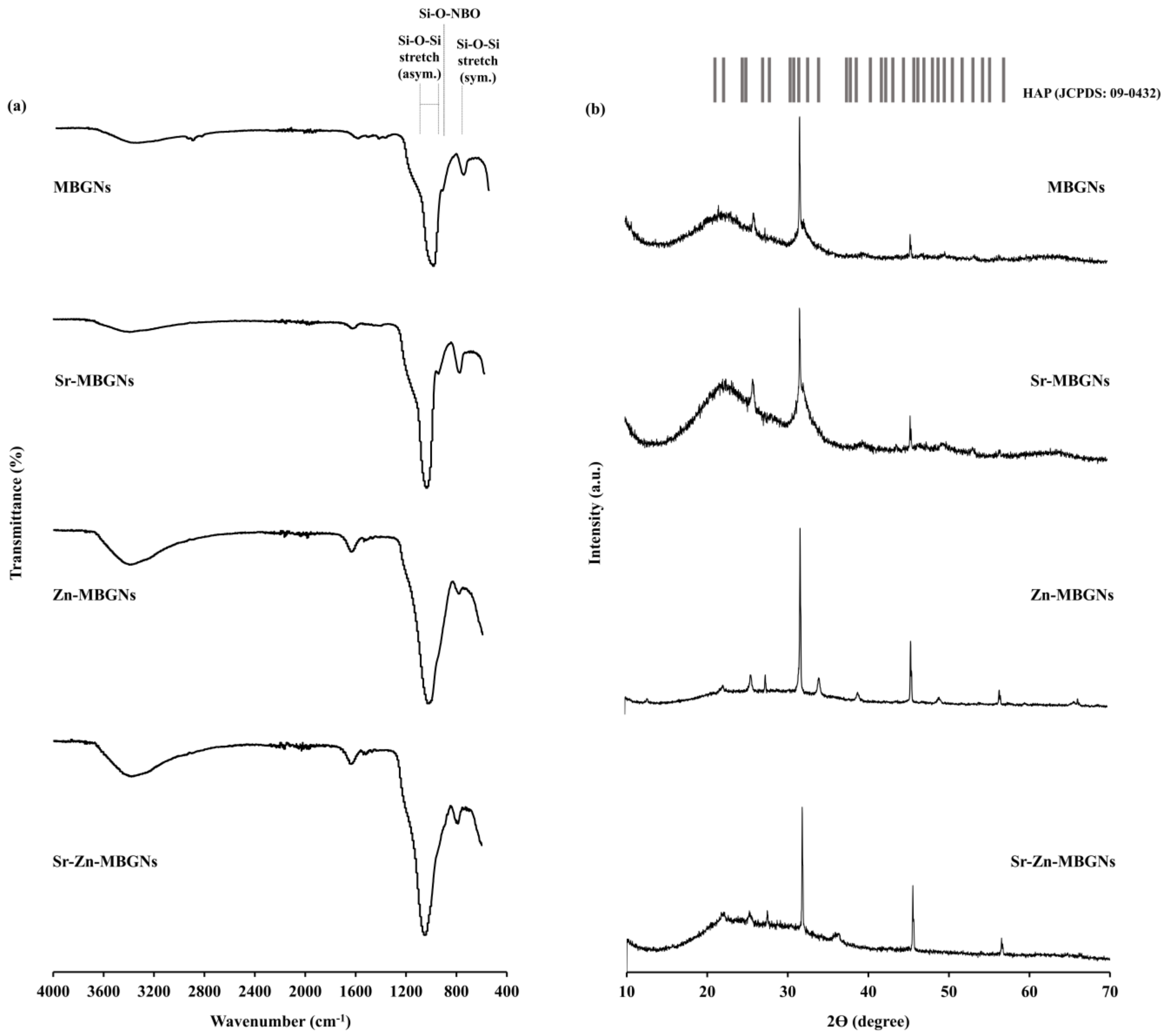
| Sample Group | %mol | |||
|---|---|---|---|---|
| SiO2 | CaO | SrO | ZnO | |
| MBGNs | 60 | 40 | ||
| Sr-MBGNs | 60 | 20 | 20 | |
| Zn-MBGNs | 60 | 20 | 20 | |
| Sr-Zn-MBGNs | 60 | 20 | 10 | 10 |
| Gene | Primer Sequences | Amplicon Size |
|---|---|---|
| RUNX2 | Fw: 5′ gta gat gga cct cgg gaa cc 3′ Rw: 5′ gag gcg gtc aga gaa caa ac 3′ | 78 bp |
| ALP | Fw: 5′ gga act cct gac cct tga cc 3′ Rw: 5′ tcc tgt tca gct cgt act gc 3′ | 86 bp |
| COL1A1 | Fw: 5′ gag tgc tgt ccc gtc tgc 3′ Rw: 5′ ttt ctt ggt cgg tgg gtg 3′ | 52 bp |
| SPARC | Fw: 5′ gag gaa acc gaa gag gag g 3′ Rw: 5′ ggg gtg ttg ttc tca tcc ag 3′ | 95 bp |
| Sample Group | Hydrodynamic Diameter Size (nm) | PI | Zeta Potential (mV) |
|---|---|---|---|
| MBGNs | 142.8 ± 6.7 | 0.441 ± 0.095 | −41.2 ± 0.6 |
| Sr-MBGNs | 161.1 ± 3.2 | 0.340 ± 0.037 | −40.4 ± 2.1 |
| Zn-MBGNs | 188.1 ± 5.2 | 0.288 ± 0.007 | −42.3 ± 1.9 |
| Sr-Zn-MBGNs | 181.1 ± 0.9 | 0.166 ± 0.025 | −41.9 ± 0.8 |
| Sample Group | %mol | |||
|---|---|---|---|---|
| SiO2 | CaO | SrO | ZnO | |
| MBGNs | 83.1 ± 0.8 | 16.9 ± 0.7 | ||
| Sr-MBGNs | 78.3 ± 0.6 | 10.0 ± 0.4 | 11.7 ± 0.7 | |
| Zn-MBGNs | 77.0 ± 0.7 | 5.8 ± 0.1 | 17.2 ± 0.7 | |
| Sr-Zn-MBGNs | 78.5 ± 0.8 | 4.4 ± 0.2 | 1.8 ± 0.3 | 15.3 ± 0.4 |
| Sample Group | [m2 g−1] | [cm3 g−1] | [nm] |
|---|---|---|---|
| as,BET | Pore Volume | Pore Diameter | |
| MBGNs | 120 | 0.28 | 9.4 |
| Sr-MBGNs | 137 | 0.34 | 10.0 |
| Zn-MBGNs | 210 | 0.29 | 8.6 |
| Sr-Zn-MBGNs | 180 | 0.39 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naruphontjirakul, P.; Li, M.; Boccaccini, A.R. Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications. Nanomaterials 2024, 14, 575. https://doi.org/10.3390/nano14070575
Naruphontjirakul P, Li M, Boccaccini AR. Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications. Nanomaterials. 2024; 14(7):575. https://doi.org/10.3390/nano14070575
Chicago/Turabian StyleNaruphontjirakul, Parichart, Meng Li, and Aldo R. Boccaccini. 2024. "Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications" Nanomaterials 14, no. 7: 575. https://doi.org/10.3390/nano14070575
APA StyleNaruphontjirakul, P., Li, M., & Boccaccini, A. R. (2024). Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications. Nanomaterials, 14(7), 575. https://doi.org/10.3390/nano14070575







