Ni,Fe,Co-LDH Coated Porous Transport Layers for Zero-Gap Alkaline Water Electrolyzers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ni,Fe,Co-LDH Synthesis
2.2. PTLs Characterization
2.3. Electrochemical Characterization
2.4. Stability Test
3. Results and Discussion
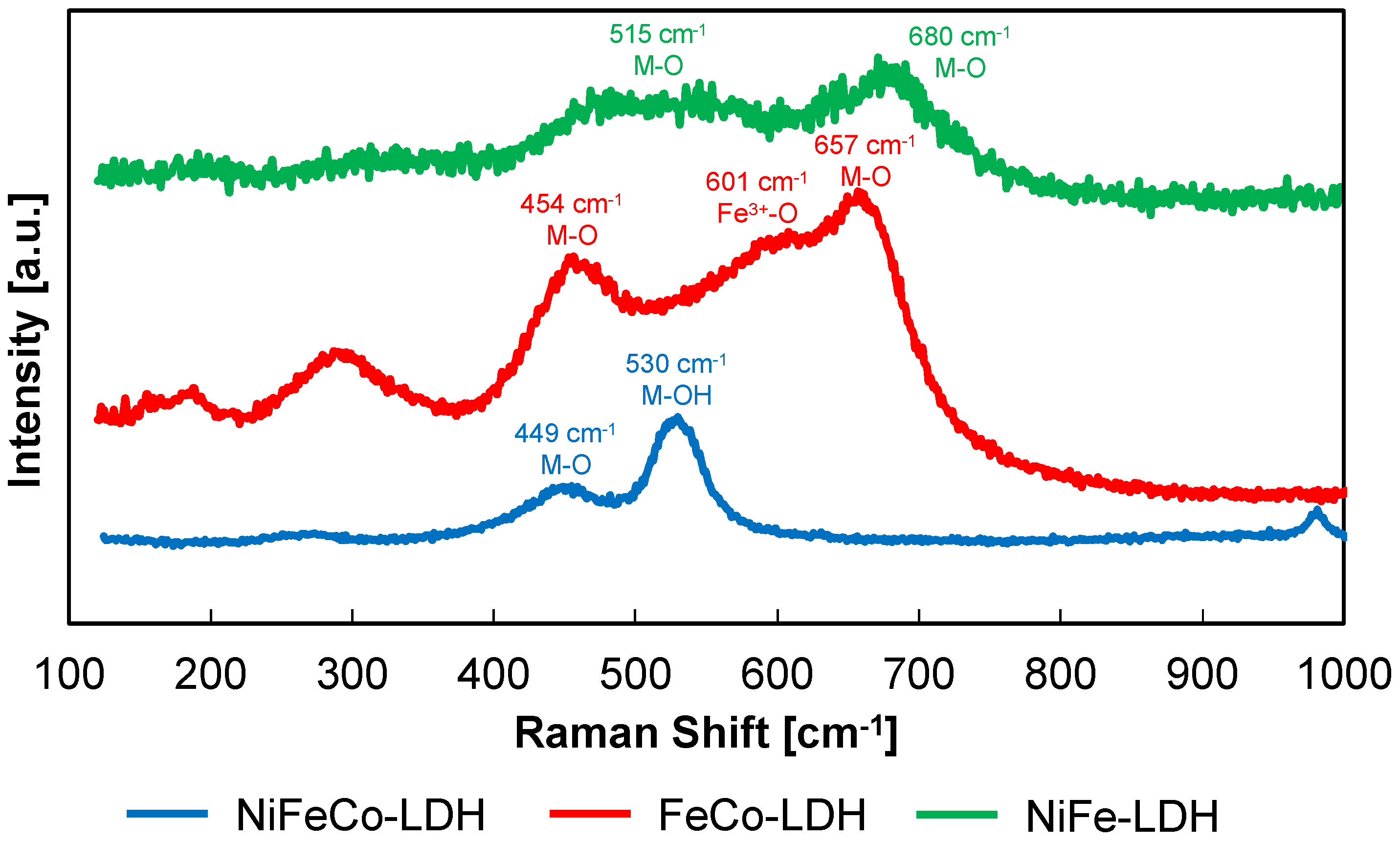
3.1. Ni,Fe,Co-LDH Electrodeposition
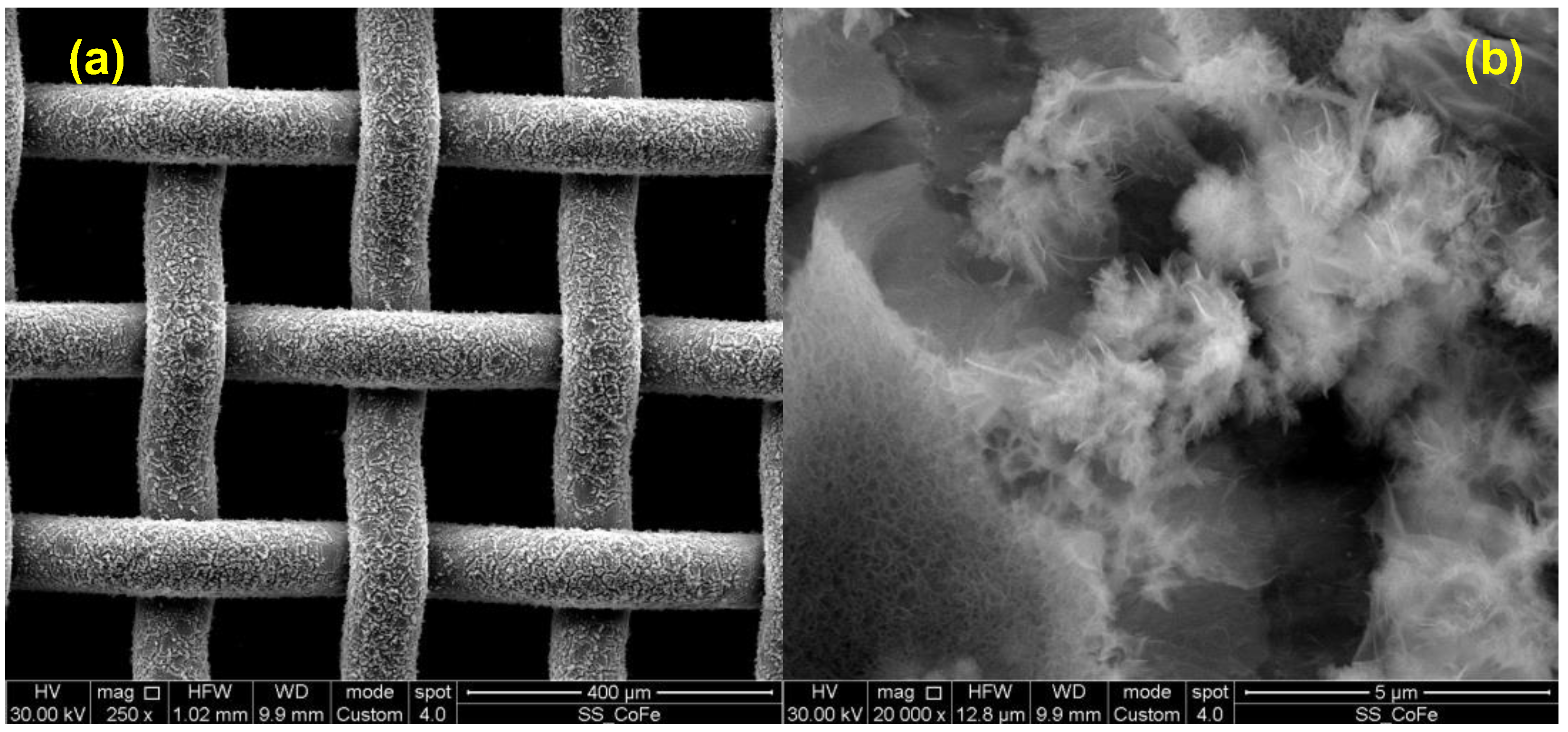
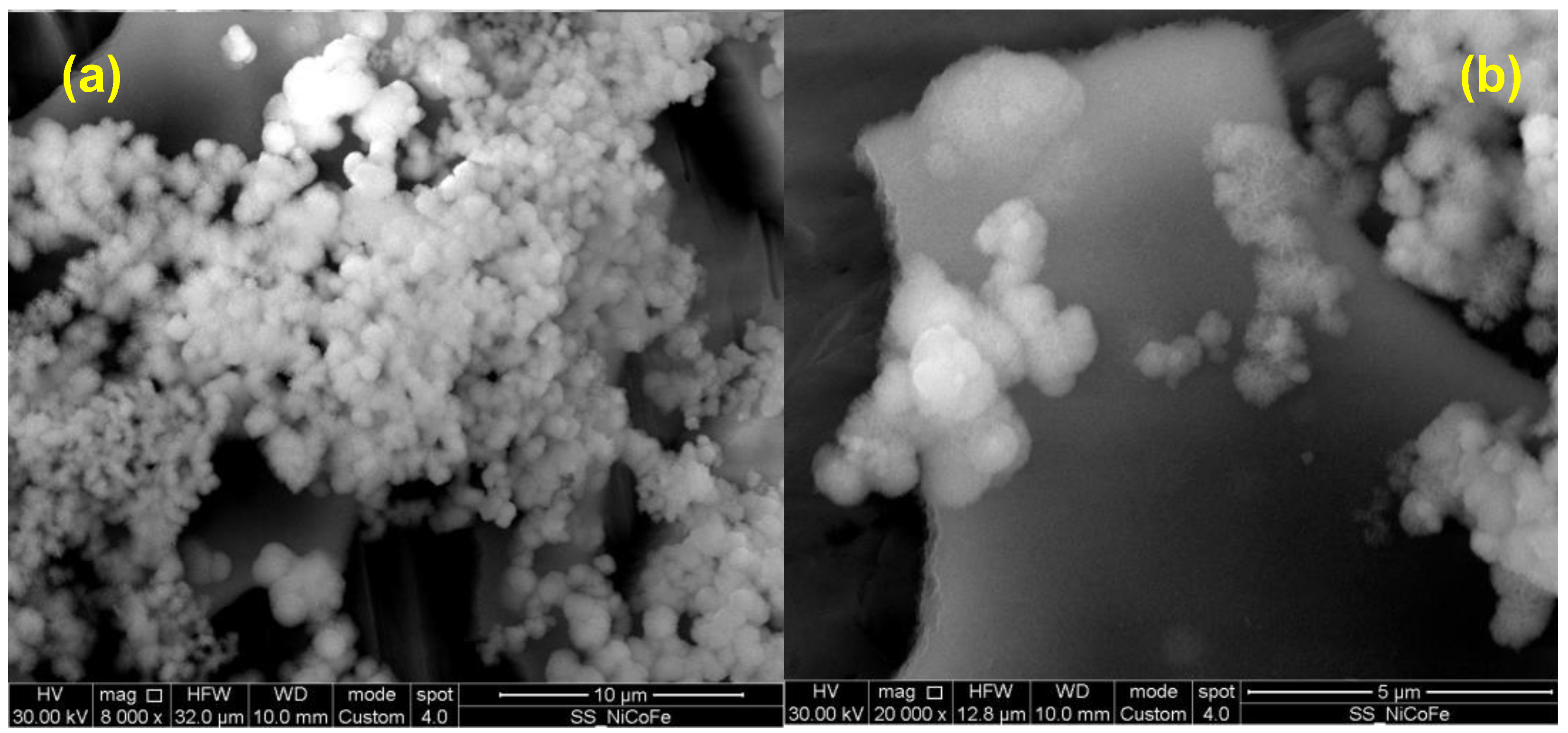
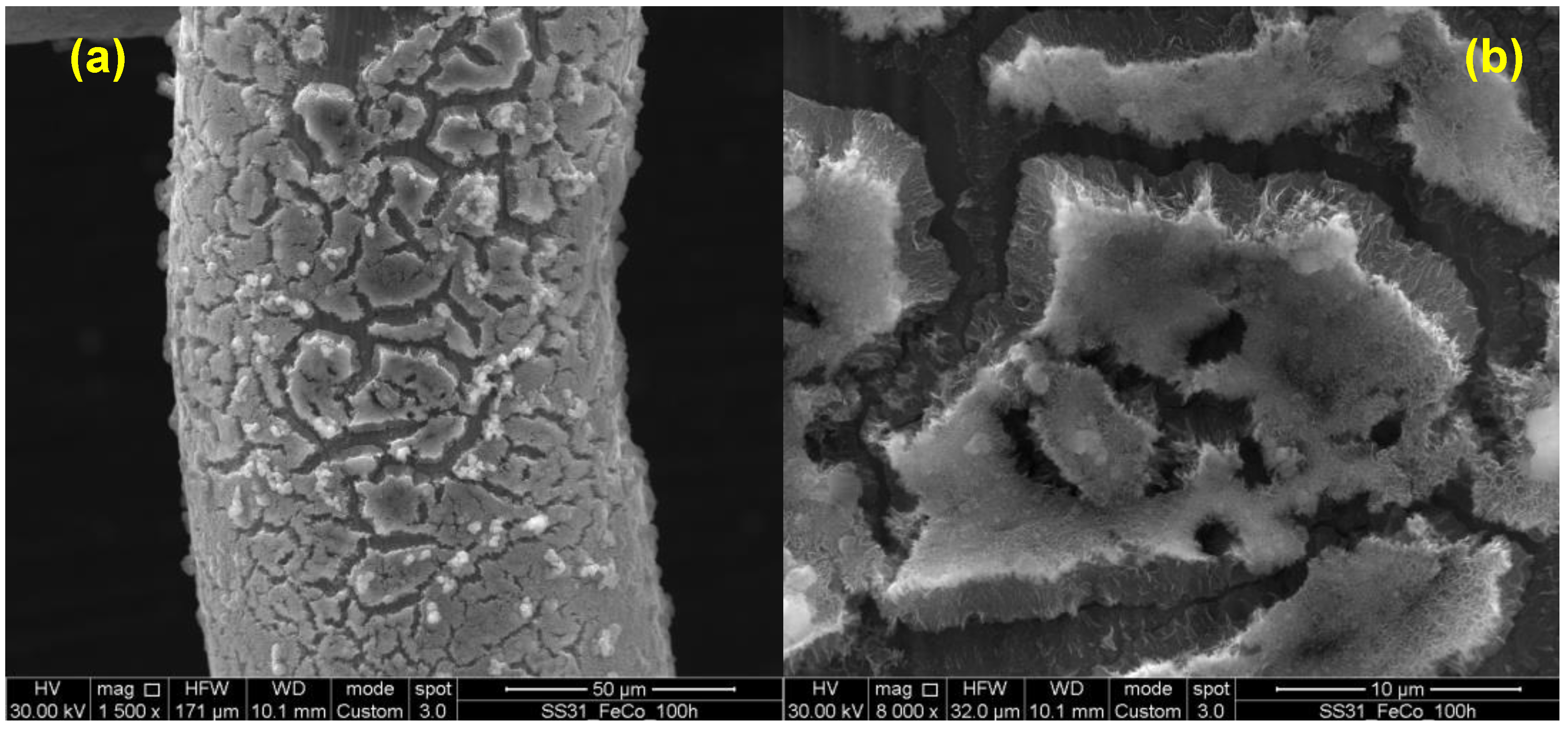
3.2. LDH Morphological Characterization
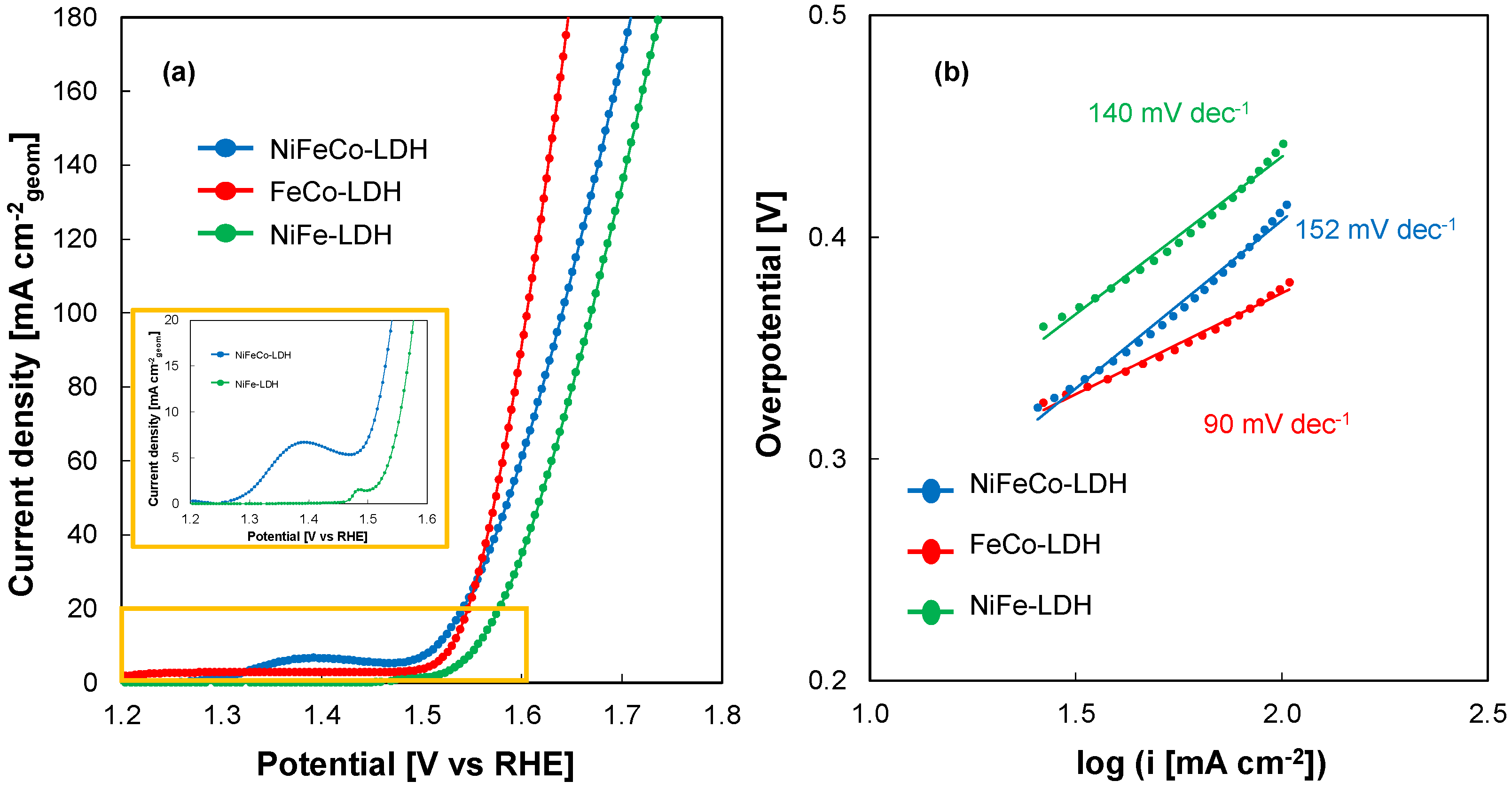
3.3. OER Performance of Electrodeposited PTLs
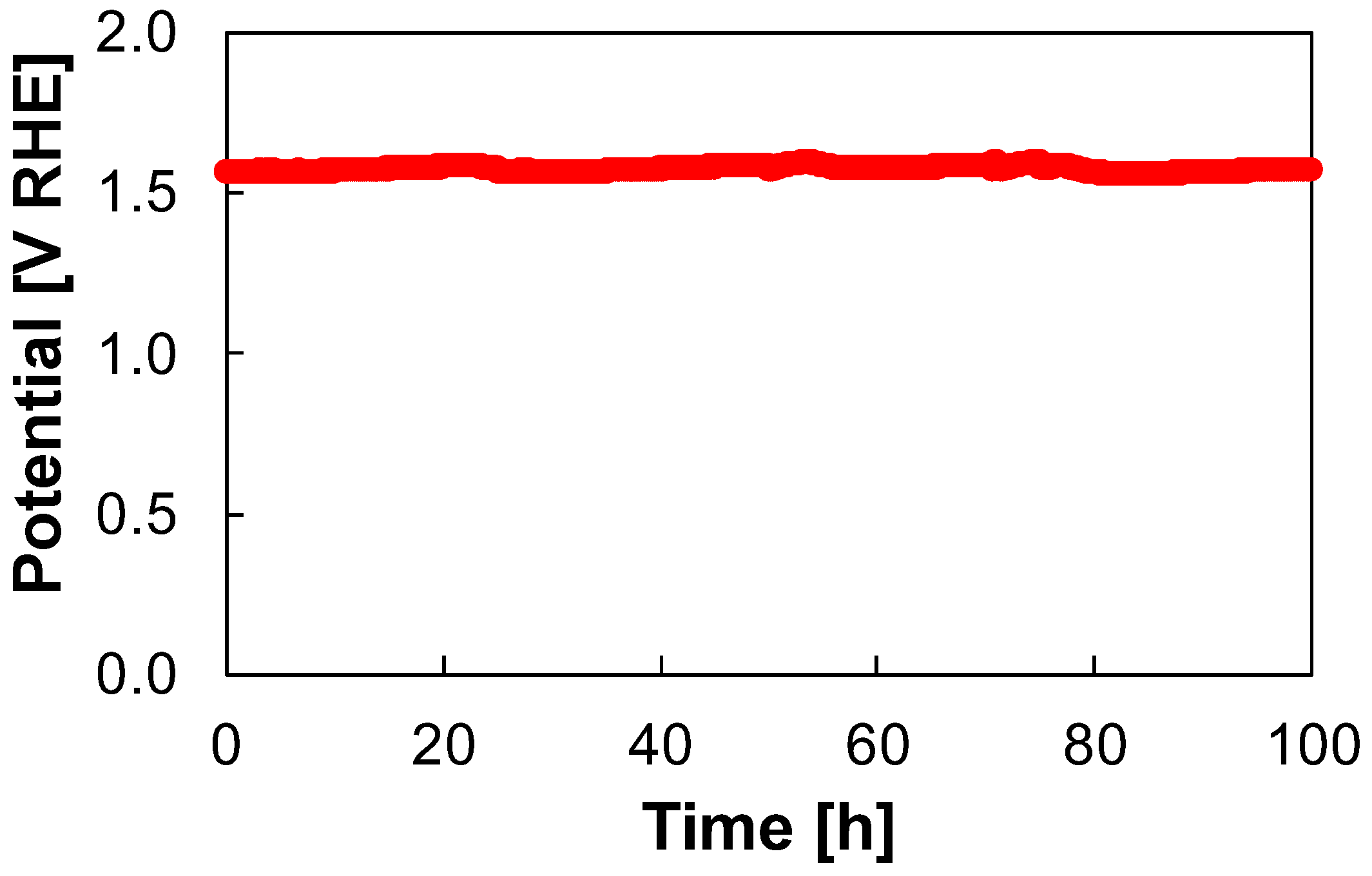
3.4. Stability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 1–29. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W.C. Advanced Electrocatalysts with Unusual Active Sites for Electrochemical Water Splitting. InfoMat 2023, 6, e12494. [Google Scholar] [CrossRef]
- Fei, L.; Sun, H.; Xu, X.; Li, Y.; Ran, R.; Zhou, W.; Shao, Z. Understanding the Bifunctional Catalytic Ability of Electrocatalysts for Oxygen Evolution Reaction and Urea Oxidation Reaction: Recent Advances and Perspectives. Chem. Eng. J. 2023, 471, 144660. [Google Scholar] [CrossRef]
- Santoro, C.; Lavacchi, A.; Mustarelli, P.; Di Noto, V.; Elbaz, L.; Dekel, D.R.; Jaouen, F. What Is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef] [PubMed]
- International Renewable Energy Agency (IRENA). Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Phillips, R.; Dunnill, C.W. Zero Gap Alkaline Electrolysis Cell Design for Renewable Energy Storage as Hydrogen Gas. RSC Adv. 2016, 6, 100643–100651. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, e2201099. [Google Scholar] [CrossRef]
- Zaffora, A.; Di Franco, F.; Pupillo, D.; Seminara, B.; Tranchida, G.; Santamaria, M. Highly Active and Stable NiCuMo Electrocatalyst Supported on 304 Stainless Steel Porous Transport Layer for Hydrogen Evolution in Alkaline Water Electrolyzer. Adv. Sustain. Syst. 2023, 7, 2200486. [Google Scholar] [CrossRef]
- Tricker, A.W.; Ertugrul, T.Y.; Lee, J.K.; Shin, J.R.; Choi, W.; Kushner, D.I.; Wang, G.; Lang, J.; Zenyuk, I.V.; Weber, A.Z.; et al. Pathways Toward Efficient and Durable Anion Exchange Membrane Water Electrolyzers Enabled By Electro-Active Porous Transport Layers. Adv. Energy Mater. 2023, 2303629. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.; Duan, X. Layered Double Hydroxide-Based Electrocatalysts for the Oxygen Evolution Reaction: Identification and Tailoring of Active Sites, and Superaerophobic Nanoarray Electrode Assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Evans, D.G.; Duan, X.; Vial, C.; Ghanbaja, J.; Prevot, V.; De Roy, M.; Forano, C. Synthesis of [Zn–Al–CO3] Layered Double Hydroxides by a Coprecipitation Method under Steady-State Conditions. J. Solid. State Chem. 2005, 178, 2766–2777. [Google Scholar] [CrossRef]
- Prevot, V.; Caperaa, N.; Taviot-Guého, C.; Forano, C. Glycine-Assisted Hydrothermal Synthesis of NiAl-Layered Double Hydroxide Nanostructures. Cryst. Growth Des. 2009, 9, 3646–3654. [Google Scholar] [CrossRef]
- Prince, J.; Montoya, A.; Ferrat, G.; Valente, J.S. Proposed General Sol—Gel Method to Prepare Multimetallic Layered Double Hydroxides: Synthesis, Characterization, and Envisaged Application. Chem. Mater. 2009, 21, 5826–5835. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Xiao, H.; Zhang, L.; Qin, Y.; Liu, D.; Hou, W.; Du, N. Synthesis and Thermal Properties of ZnAl Layered Double Hydroxide by Urea Hydrolysis. Powder Technol. 2014, 253, 41–45. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Q.; Li, B.; Xue, H.; Pang, H.; Chen, C. Recent Progress in Layered Double Hydroxide Based Materials for Electrochemical Capacitors: Design, Synthesis and Performance. Nanoscale 2017, 9, 15206–15225. [Google Scholar] [CrossRef]
- Rohit, R.C.; Jagadale, A.D.; Shinde, S.K.; Kim, D.Y. A Review on Electrodeposited Layered Double Hydroxides for Energy and Environmental Applications. Mater. Today Commun. 2021, 27, 102275. [Google Scholar] [CrossRef]
- Etesami, M.; Mohamad, A.A.; Nguyen, M.T.; Yonezawa, T.; Pornprasertsuk, R.; Somwangthanaroj, A.; Kheawhom, S. Benchmarking Superfast Electrodeposited Bimetallic (Ni, Fe, Co, and Cu) Hydroxides for Oxygen Evolution Reaction. J. Alloys Compd. 2022, 889, 161738. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-Situ Structure and Catalytic Mechanism of NiFe and CoFe Layered Double Hydroxides during Oxygen Evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Yan, Z.; Sun, H.; Chen, X.; Liu, H.; Zhao, Y.; Li, H.; Xie, W.; Cheng, F.; Chen, J. Anion Insertion Enhanced Electrodeposition of Robust Metal Hydroxide/Oxide Electrodes for Oxygen Evolution. Nat. Commun. 2018, 9, 2373. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Shin, H.H.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Cobalt Iron Hydroxide as a Precious Metal-Free Bifunctional Electrocatalyst for Efficient Overall Water Splitting. Small 2018, 14, 1702568. [Google Scholar] [CrossRef]
- Zhao, N.; Feng, Y.; Zhao, H.; Fan, H.; Tian, S.; Hu, B. Simple Electrodeposition of 3D NiCoFe-Layered Double Hydroxide Nanosheet Assembled Nanospheres/Nanoflowers on Carbon Cloth for High Performance Hybrid Supercapacitors. J. Alloys Compd. 2022, 901, 163566. [Google Scholar] [CrossRef]
- Gao, X.; Pan, X.; Long, X.; Yi, Z. Room-Temperature Synthesis FeNiCo Layered Double Hydroxide as an Excellent Electrochemical Water Oxidation Catalyst. J. Electrochem. Soc. 2017, 164, H755–H759. [Google Scholar] [CrossRef]
- Riaz, A.; Fusco, Z.; Kremer, F.; Gupta, B.; Zhang, D.; Jagadish, C.; Tan, H.H.; Karuturi, S. Hierarchically Multiscale Vertically Oriented NiFeCo Nanoflakes for Efficient Electrochemical Oxygen Evolution at High Current Densities. Adv. Energy Mater. 2023, 14, 2303001. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Shi, Y.; Sun, Z.; Liu, R. Role of CO in the Electrocatalytic Activity of Monolayer Ternary Nifeco-Double Hydroxide Nanosheets for Oxygen Evolution Reaction. Materials 2021, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Jiang, H.; Xian, J.; Mi, S.; Wei, L.; Fang, G.; Guo, J.; Xu, S.; Liu, Z.; Jin, H.; et al. Pearson’s Principle-Inspired Robust 2D Amorphous Ni-Fe-Co Ternary Hydroxides on Carbon Textile for High-Performance Electrocatalytic Water Splitting. Nanomaterials 2022, 12, 2416. [Google Scholar] [CrossRef] [PubMed]
- Rohit, R.C.; Jagadale, A.D.; Shinde, S.K.; Kim, D.Y.; Kumbhar, V.S.; Nakayama, M. Hierarchical Nanosheets of Ternary CoNiFe Layered Double Hydroxide for Supercapacitors and Oxygen Evolution Reaction. J. Alloys Compd. 2021, 863, 158081. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.; Vazquez-Samperio, J.; Tufiño-Velázquez, M.; Flores-Moreno, J.; Lartundo-Rojas, L.; Gonzalez-Huerta, R.d.G. Bifunctional Electrocatalysts for Oxygen Reduction/Evolution Reactions Derived from NiCoFe LDH Materials. J. Appl. Electrochem. 2018, 48, 947–957. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-Film Ni-Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Liu, B.; Chen, Z.; Xu, H.; Yan, K. Trimetallic NiCoFe-Layered Double Hydroxides Nanosheets Efficient for Oxygen Evolution and Highly Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural. ACS Catal. 2020, 10, 5179–5189. [Google Scholar] [CrossRef]
- Liu, Y.C.; Koza, J.A.; Switzer, J.A. Conversion of Electrodeposited Co(OH)2 to CoOOH and Co3O4, and Comparison of Their Catalytic Activity for the Oxygen Evolution Reaction. Electrochim. Acta 2014, 140, 359–365. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and Correctness in the Evaluation of Electrocatalytic Water Splitting: Revisiting Activity Parameters with a Critical Assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Doyle, R.L.; Lyons, M.E.G. An Electrochemical Impedance Study of the Oxygen Evolution Reaction at Hydrous Iron Oxide in Base. Phys. Chem. Chem. Phys. 2013, 15, 5224–5237. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.P.; Betty, C.A.; Tyagi, D.; Banerjee, A.M.; Pai, M.R.; Tripathi, A.K. Tracking the Role of Fe in NiFe-Layered Double Hydroxide for Solar Water Oxidation and Prototype Demonstration towards PV Assisted Solar Water-Splitting. Int. J. Hydrogen Energy 2021, 46, 2143–2155. [Google Scholar] [CrossRef]
- Zaffora, A.; Santamaria, M.; Di Franco, F.; Habazaki, H.; Di Quarto, F. Photoelectrochemical Evidence of Nitrogen Incorporation during Anodizing Sputtering-Deposited Al-Ta Alloys. Phys. Chem. Chem. Phys. 2016, 18, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780470041406. [Google Scholar]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and Kinetics of HER and OER on NiFe LDH Films in an Alkaline Electrolyte. J. Electrochem. Soc. 2018, 165, J3395–J3404. [Google Scholar] [CrossRef]
- Yin, X.; Sun, G.; Song, A.; Wang, L.; Wang, Y.; Dong, H.; Shao, G. A Novel Structure of Ni-(MoS2/GO) Composite Coatings Deposited on Ni Foam under Supergravity Field as Efficient Hydrogen Evolution Reaction Catalysts in Alkaline Solution. Electrochim. Acta 2017, 249, 52–63. [Google Scholar] [CrossRef]
- Corrigan, D.A. The Catalysis of the Oxygen Evolution Reaction by Iron Impurities in Thin Film Nickel Oxide Electrodes. J. Electrochem. Soc. 1987, 134, 377–384. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-Iron (Oxy)Hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, F.; Zhu, J.; Zeng, Z.; Merzdorf, T.; Sarodnik, H.; Gliech, M.; Pan, L.; Li, W.X.; Greeley, J.; Strasser, P. Intrinsic Electrocatalytic Activity for Oxygen Evolution of Crystalline 3d-Transition Metal Layered Double Hydroxides. Angew. Chem.-Int. Ed. 2021, 60, 14446–14457. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, M.; Yuan, W.; Shi, G. A High-Performance Three-Dimensional Ni–Fe Layered Double Hydroxide/Graphene Electrode for Water Oxidation. J. Mater. Chem. A Mater. 2015, 3, 6921–6928. [Google Scholar] [CrossRef]
- Huang, L.; Yang, L.; Guo, S.; Li, Y.; Zhao, L.; Jiao, L. Influence of Interlayer Water Molecules in Ni-Based Catalysts for Oxygen Evolution Reaction. J. Energy Chem. 2020, 53, 316–322. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Lim, A.C.; Roy, A.; Seo, J.G. Room-Temperature Ultrafast Synthesis of NiCo-Layered Double Hydroxide as an Excellent Electrocatalyst for Water Oxidation. ChemistrySelect 2019, 4, 2409–2415. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.-J.; Yang, Y.-B.; Guo, P.-F.; Zhu, B.; Wang, K.; Wang, W.-T.; He, Z.-H.; Liu, Z.-T. Ru-Doped NiFe Layered Double Hydroxide as a Highly Active Electrocatalyst for Oxygen Evolution Reaction. J. Electrochem. Soc. 2022, 169, 024503. [Google Scholar] [CrossRef]
- Guo, P.F.; Yang, Y.; Wang, W.J.; Zhu, B.; Wang, W.T.; Wang, Z.Y.; Wang, J.L.; Wang, K.; He, Z.H.; Liu, Z.T. Stable and Active NiFeW Layered Double Hydroxide for Enhanced Electrocatalytic Oxygen Evolution Reaction. Chem. Eng. J. 2021, 426, 130768. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Li, J.G.; Li, Z.; Ao, X.; Xue, K.H.; Ostrikov, K.K.; Tang, J.; Wang, C. Rh-Engineered Ultrathin NiFe-LDH Nanosheets Enable Highly-Efficient Overall Water Splitting and Urea Electrolysis. Appl. Catal. B 2021, 284, 119740. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Ma, W.; Ma, R.; Wang, C.; Liang, J.; Liu, X.; Zhou, K.; Sasaki, T. A Superlattice of Alternately Stacked Ni-Fe Hydroxide Nanosheets and Graphene for Efficient Splitting of Water. ACS Nano 2015, 9, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Lopes, P.P.; Farinazzo Bergamo Dias Martins, P.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic Stability of Active Sites in Hydr(Oxy)Oxides for the Oxygen Evolution Reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]

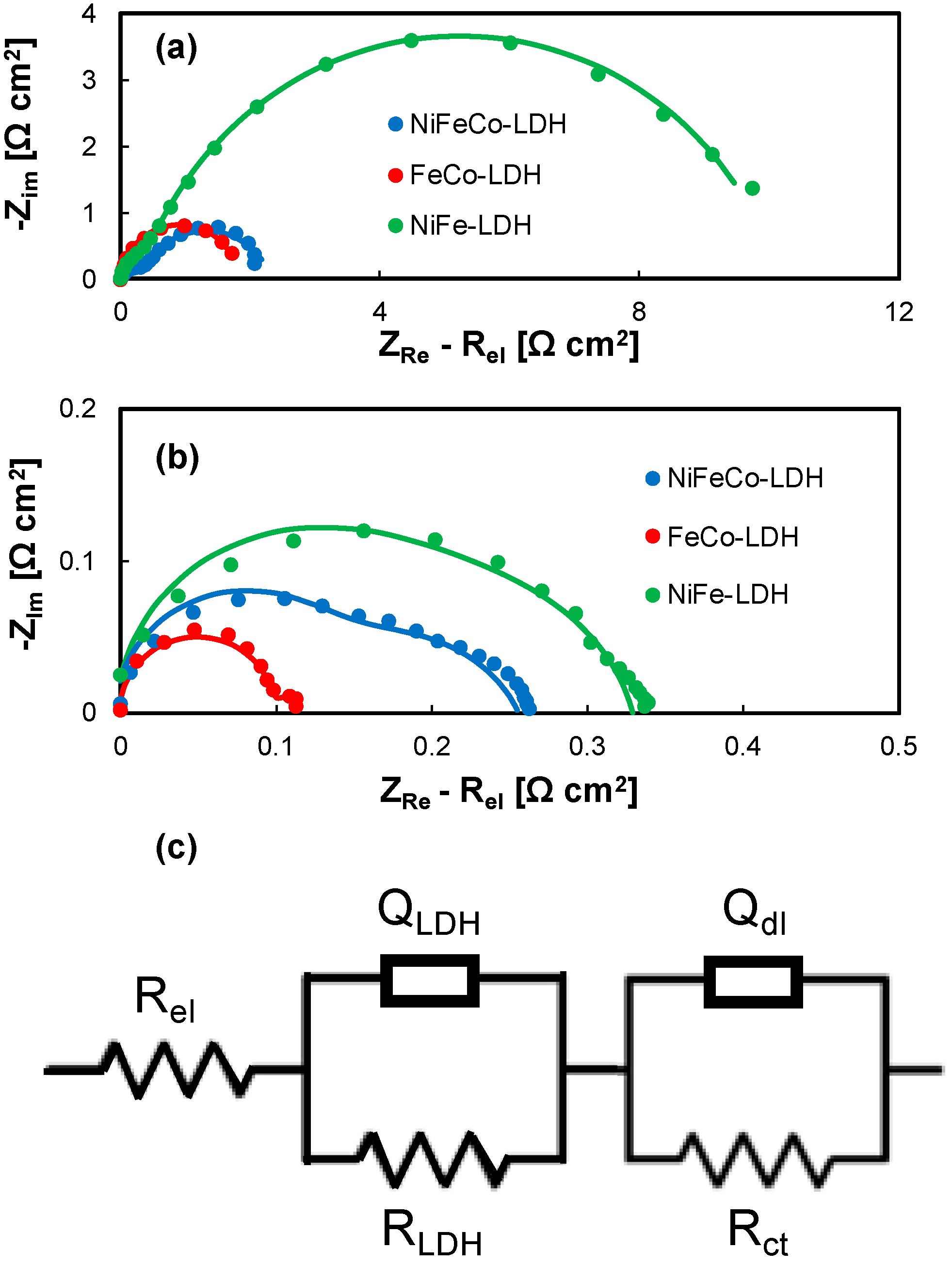
| Sample | O [at.%] | S [at.%] | Cr [at.%] | Fe [at.%] | Ni [at.%] | Co [at.%] |
|---|---|---|---|---|---|---|
| NiFe-LDH | 47.6 | 2.1 | 8.4 | 34.4 | 7.6 | - |
| FeCo-LDH | 53.4 | 3.0 | 6.5 | 27.2 | 2.4 | 7.5 |
| NiFeCo-LDH | 50.0 | 2.5 | 7.4 | 28.7 | 6.0 | 5.6 |
| Sample | Rel [Ω cm2] | RLDH [Ω cm2] | QLDH [S sn cm−2] | n | Rct [Ω cm2] | Qdl [S sn cm−2] | n |
|---|---|---|---|---|---|---|---|
| NiFe-LDH | 0.26 | 0.33 | 2.1 × 10−3 | 1 | 9.86 | 0.012 | 0.81 |
| FeCo-LDH | 0.24 | 0.01 | 4.5 × 10−3 | 0.9 | 1.82 | 0.21 | 0.93 |
| NiFeCo-LDH | 0.32 | 0.34 | 8.5 × 10−3 | 0.87 | 2 | 0.13 | 0.80 |
| Sample | Rel [Ω cm2] | RLDH [Ω cm2] | QLDH [S sn cm−2] | n | Rct [Ω cm2] | Qdl [S sn cm−2] | n |
|---|---|---|---|---|---|---|---|
| NiFe-LDH | 0.26 | 0.18 | 2.7 × 10−3 | 1 | 0.16 | 0.04 | 0.83 |
| FeCo-LDH | 0.24 | 0.02 | 5.0 × 10−3 | 0.93 | 0.10 | 0.23 | 1 |
| NiFeCo-LDH | 0.32 | 0.13 | 4.1 × 10−3 | 1 | 0.13 | 0.21 | 0.73 |
| LDH | Synthesis Method | Test Duration [h] | Current Density [mA cm−2] | Electrolyte | Overpotential [mV] | Reference |
|---|---|---|---|---|---|---|
| FeCo | Electrodeposition | 50 | 10 | 1 M KOH | 260 | [26] |
| 3D NiFe | Electrodeposition | 2 | 10 | 1 M KOH | 260 | [47] |
| NiFe, NiCo NiCu, CuCo FeCo, FeCu | Electrodeposition | 3 0.3 | 10 100 | 0.1 M KOH | n.s. | [22] |
| NiFe | Hydrothermal | 95 | 100 | 1 M KOH | 240 | [48] |
| NiCo | Electrodeposition | 36 | 13 | 1 M KOH | 270 | [49] |
| Ru-doped NiFe | Hydrothermal | 6 | 10 | 1 M KOH | 240 | [50] |
| NiFeW | Solvothermal | 6 | 10 | 1 M KOH | 270 | [51] |
| Rh-NiFe | Hydrothermal | 7 | 10 | 1 M KOH | 210 | [52] |
| NiFe LDH@NiCoP | Hydrothermal | 100 | 10 | 1 M KOH | 230 | [53] |
| NiFe-rGO | Precipitation | 10 | 10 | 1 M KOH | 240 | [54] |
| FeCo | Electrodeposition | 100 | 50 | 1 M KOH | 346 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaffora, A.; Megna, B.; Seminara, B.; Di Franco, F.; Santamaria, M. Ni,Fe,Co-LDH Coated Porous Transport Layers for Zero-Gap Alkaline Water Electrolyzers. Nanomaterials 2024, 14, 407. https://doi.org/10.3390/nano14050407
Zaffora A, Megna B, Seminara B, Di Franco F, Santamaria M. Ni,Fe,Co-LDH Coated Porous Transport Layers for Zero-Gap Alkaline Water Electrolyzers. Nanomaterials. 2024; 14(5):407. https://doi.org/10.3390/nano14050407
Chicago/Turabian StyleZaffora, Andrea, Bartolomeo Megna, Barbara Seminara, Francesco Di Franco, and Monica Santamaria. 2024. "Ni,Fe,Co-LDH Coated Porous Transport Layers for Zero-Gap Alkaline Water Electrolyzers" Nanomaterials 14, no. 5: 407. https://doi.org/10.3390/nano14050407
APA StyleZaffora, A., Megna, B., Seminara, B., Di Franco, F., & Santamaria, M. (2024). Ni,Fe,Co-LDH Coated Porous Transport Layers for Zero-Gap Alkaline Water Electrolyzers. Nanomaterials, 14(5), 407. https://doi.org/10.3390/nano14050407










