High Energy Density of Ball-Milled Fluorinated Carbon Nanofibers as Cathode in Primary Lithium Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Electrochemical Tests
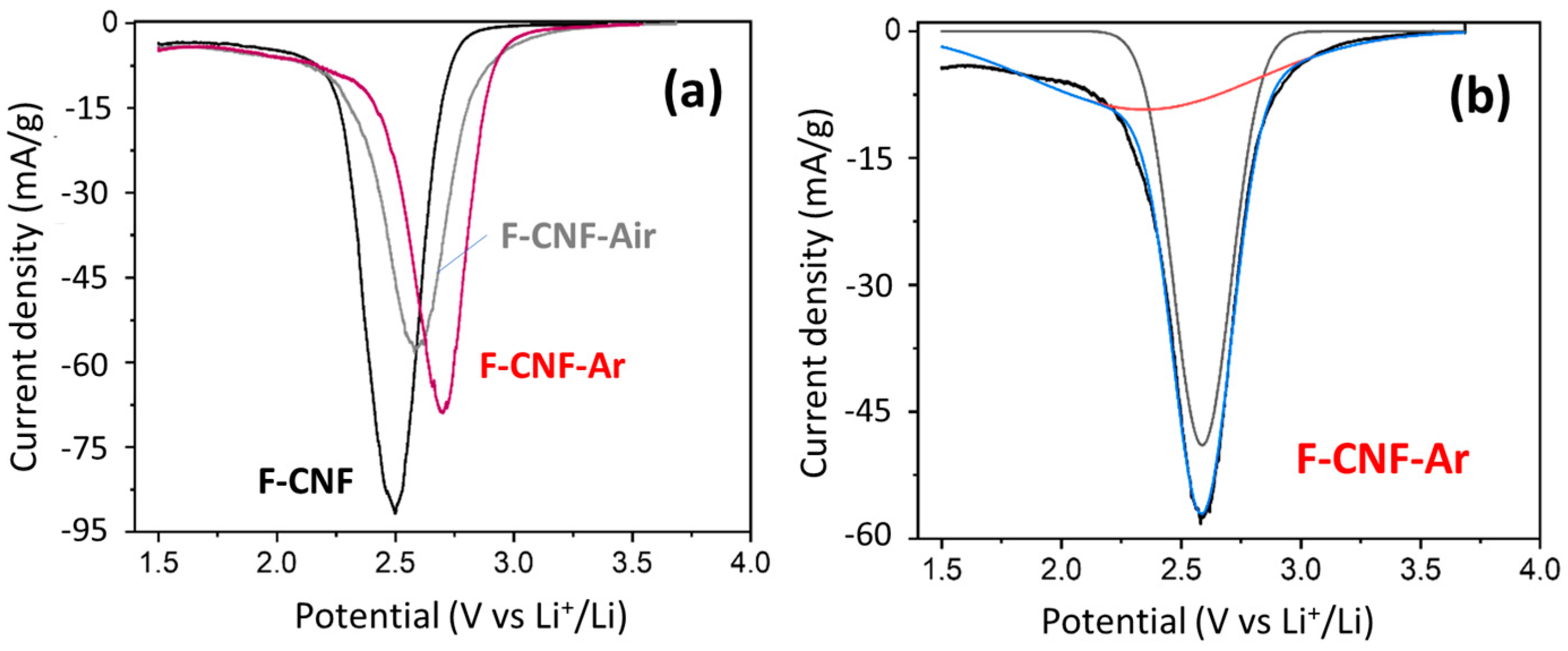
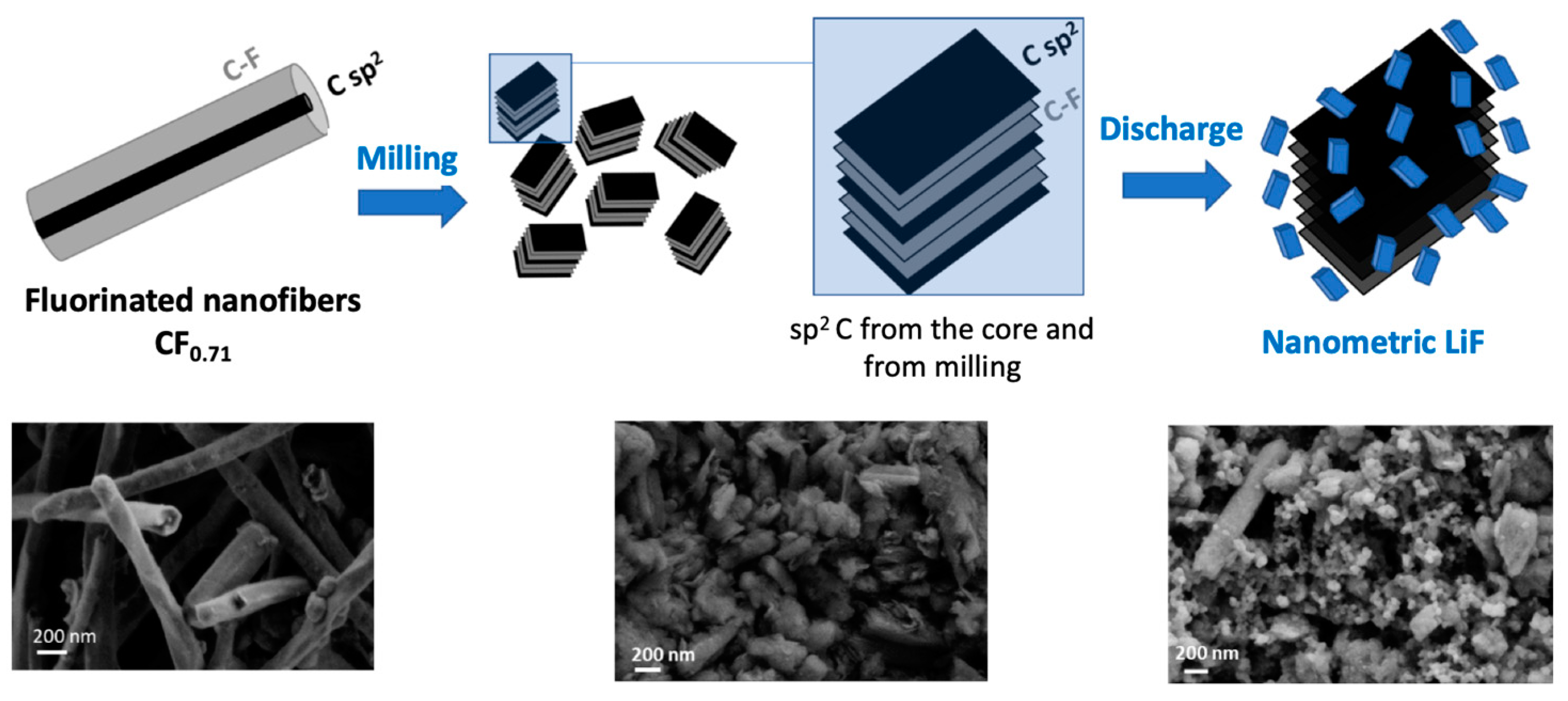
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.X.; Kong, L.C.; Li, Y.; Peng, C.; Feng, W. Fundamentals of Li/CFx Battery Design and Application. Energy Environ. Sci. 2023, 16, 1907–1942. [Google Scholar] [CrossRef]
- Gao, M.T.; Cai, D.M.; Luo, S.F.; Yang, Y.H.; Xie, Y.; Zhu, L.C.; Yuan, Z.Z. Research Progress in Fluorinated Carbon Sources and the Discharge Mechanism for Li/CFx Primary Batteries. J. Mater. Chem. A 2023, 11, 16519–16538. [Google Scholar] [CrossRef]
- Liu, W.; Ma, S.; Li, Y.; Wan, B.X.; Wu, C.; Ma, S.D.; Guo, R.; Pei, H.J.; Xie, J.Y. Electrochemical Impedance Spectroscopy Analysis for Lithium Carbon Fluorides Primary Battery. J. Energy Storage 2023, 68, 107699. [Google Scholar] [CrossRef]
- Liu, W.C.; Deng, N.P.; Wang, G.; Yu, R.R.; Wang, X.X.; Cheng, B.W.; Ju, J.G.; Kang, W.M. Fluoridation Routes, Function Mechanism and Application of Fluorinated/Fluorine-Doped Nanocarbon-Based Materials for Various Batteries: A Review. J. Energy Chem. 2023, 85, 363–393. [Google Scholar] [CrossRef]
- Hu, Y.H.; Kong, L.C.; Li, W.Y.; Sun, L.D.; Peng, C.; Qin, M.M.; Zhao, Z.Y.; Li, Y.; Feng, W. Fluorinated Microporous Carbon Spheres for Li/CFX Batteries with High Volumetric Energy Density. Compos. Commun. 2023, 40, 101607. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, Y.Y.; Li, R.J.; Wang, J.L.; Wu, S.X.; Cai, L.J.; Zhao, Y.; Wang, W.L. Boosting the Rate Performance of Primary Li/CFx Batteries through Interlayer Conductive Network Engineering. J. Mater. Chem. A 2023, 11, 20187–20192. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.X.; Kong, L.C.; Xu, H.; Li, Y.; Feng, W. Fluorinated Carbon Nanohorns as Cathode Materials for Ultra-High Power Li/CFx Batteries. Small Methods 2023, 2301090. [Google Scholar] [CrossRef]
- Cheon, S.; Ha, N.; Lim, C.; Myeong, S.; Lee, I.W.; Lee, Y.S. Fabrication and Electrochemical Characterization of Carbon Fluoride-Based Lithium-Ion Primary Batteries with Improved Rate Performance Using Oxygen Plasma. Appl. Chem. Eng. 2023, 34, 534–540. [Google Scholar] [CrossRef]
- Ha, N.; Lim, C.; Ha, S.; Myeong, S.; Lee, Y.S. Electrochemical Characteristics of CFX Based Lithium Primary Batteries Produced by Carbon Fiber Reinforced Plastic-Derived Waste Carbon Fibers. Appl. Chem. Eng. 2023, 34, 515–521. [Google Scholar] [CrossRef]
- Chen, N.E.; Zhang, G.J.; Chen, H.X.; Yue, H.J. Conductive Carbon-Wrapped Fluorinated Hard Carbon Composite as High-Performance Cathode for Primary Lithium Batteries. Coatings 2023, 13, 812. [Google Scholar] [CrossRef]
- Chen, G.B.; Cao, F.; Li, Z.X.; Fu, J.A.; Wu, B.S.; Liu, Y.F.; Jian, X. Helical Fluorinated Carbon Nanotubes/Iron(III) Fluoride Hybrid with Multilevel Transportation Channels and Rich Active Sites for Lithium/Fluorinated Carbon Primary Battery. Nanotechnol. Rev. 2023, 12, 20230108. [Google Scholar] [CrossRef]
- Wang, C.; Teng, J.K.; Chen, X.T.; Wei, J.H.; Shi, B.; Yuan, Z.F.; Li, X.L.; Kang, S.S.; Tang, K.K. Preparation of High-Power Lithium Fluoride Carbon Battery via Microstructural Modulation of Ketjen Black. Energy Technol. 2023, 11, 2300635. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Jiang, J.M.; Zhang, L.; Tang, C.; Tong, Z.K.; Wang, X.M.; Chen, Z.Y.; Li, M.R.; Zhuang, Q.C. BF3-Based Electrolyte Additives Promote Electrochemical Reactions to Boost the Energy Density of Li/CFx Primary Batteries. Electrochim. Acta 2023, 470, 143311. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.Y.; Liu, C.; Guo, F.F.; Wu, X.Z.; Zhou, P.F.; Fang, Z.W.; Zhou, J. Fluorinated Saccharide-Derived Hard Carbon as a Cathode Material of Lithium Primary Batteries: Effect of the Polymerization Degree of the Starting Saccharide. RSC Adv. 2023, 13, 14797–14807. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, J.; Wang, X.; Chen, D.W.; Wu, D.Z.; Pan, J.; Pan, Y.; Ouyang, X.P. Surface Engineering of Fluorinated Graphene Nanosheets Enables Ultrafast Lithium/Sodium/Potassium Primary Batteries. Adv. Mater. 2023, 35, 2303444. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.Y.; Zhang, Q.F.; Pan, J.A.; Yuan, T.; Yang, Y.; Xie, S.H. Succinonitrile Broadening the Temperature Range of Li/CFx Primary Batteries. J. Cent. South Univ. 2023, 30, 443–453. [Google Scholar] [CrossRef]
- Lim, C.; Ha, S.; Ha, N.; Jeong, S.G.; Lee, Y.S. Plasma Treatment of CFX: The Effect of Surface Chemical Modification Coupled with Surface Etching. Carbon Lett. 2023. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, G.; Yao, L.; Zhang, S.; Feng, T.; Xu, Z.; Fang, Z.; Wu, M. Plasma-Enhanced Fluorination of Layered Carbon Precursors for High-Performance CFx Cathode Materials. J. Alloys Compd. 2023, 941, 168998. [Google Scholar] [CrossRef]
- Li, L.Y.; Wu, R.Z.; Ma, H.C.; Cheng, B.B.; Rao, S.Q.; Lin, S.; Xu, C.B.; Li, L.; Ding, Y.; Mai, L.Q. Toward the High-Performance Lithium Primary Batteries by Chemically Modified Fluorinate Carbon with d-MnO2. Small 2023, 19, 2300762. [Google Scholar] [CrossRef]
- Tang, C.; Jiang, J.M.; Wang, X.F.; Liu, G.F.; Cui, Y.H.; Zhuang, Q.C. Modifying CF0.75 Cathode by Ultrathin Carbon Layer to Boosts Electrons Transmission and Discharge Capacity for Lithium/Fluorinated Graphite Primary Batteries. Mater. Lett. 2023, 336, 133901. [Google Scholar] [CrossRef]
- Ma, S.; Liu, W.; Zhang, D.M.; Yang, C.; Luo, Y.; Lou, X.B.; Guo, R.; Wang, Y.; Xie, J.Y. Controllable Solvent Treatment of Fluorinated Graphite for High Power Density and Low Cathode Swelling Lithium Primary Batteries. Chem. Eng. J. 2023, 474, 145819. [Google Scholar] [CrossRef]
- Li, P.; Cheng, Z.; Liu, J.L.; Che, L.K.; Zhou, Y.K.; Xu, E.M.; Tian, X.H.; Yuan, Z.Z. Solvation Structure Tuning Induces LiF/Li3N-Rich CEI and SEI Interfaces for Superior Li/CFx Batteries. Small 2023, 19, 2303149. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, J.; Chen, Y.; Wu, Z.R.; Niu, X.B.; Ouyang, C.Y.; Liu, J.; Wang, L. Lithium-Ion and Solvent Co-Intercalation Enhancing the Energy Density of Fluorinated Graphene Cathode. J. Energy Chem. 2024, 89, 208–215. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Chen, C.; Xu, C.L.; Wang, R.; Wu, M.Q. Potassium Ion Electrolytes Enable High Rate Performance of Li/CFx Primary Batteries. J. Electrochem. Soc. 2023, 170, 040506. [Google Scholar] [CrossRef]
- Huo, H.B.; Radhakrishnan, S.; Shaw, L.L.; Nemeth, K. High-Energy and High-Power Primary Li-CFx Batteries Enabled by the Combined Effects of the Binder and the Electrolyte. Batteries 2023, 9, 268. [Google Scholar] [CrossRef]
- Liang, H.J.; Su, M.Y.; Zhao, X.X.; Gu, Z.Y.; Yang, J.L.; Guo, W.; Liu, Z.M.; Zhang, J.; Wu, X.L. Weakly-Solvating Electrolytes Enable Ultralow-Temperature (−80 °C) and High-Power CFx/Li Primary Batteries. Sci. China Chem. 2023, 66, 1982–1988. [Google Scholar] [CrossRef]
- Li, H.; Cabañas-Gac, F.; Hadidi, L.; Bilodetau-Calame, M.; Abid, A.; Mameri, K.; Rigamonti, M.G.; Rousselot, S.; Dollé, M.; Patience, G.S. Ultrasound assisted wet media milling synthesis of nanofiber-cage LiFePO4/C, Ultrason. Sonochemistry 2020, 68, 105177. [Google Scholar] [CrossRef]
- Cabello, M.; Gucciardi, E.; Herrán, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a High-Power Si@graphite Anode for Lithium Ion Batteries through a Wet Ball Milling Process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef]
- Kang, H.-C.; Jun, D.-K.; Jin, B.; Jin, E.M.; Palrk, K.-H.; Gu, H.-B.; Kim, K.-W. Optimized solid-state synthesis of LiFePO4 cathode materials using ball-milling. J. Power Sources 2008, 179, 340–346. [Google Scholar] [CrossRef]
- Kim, S.-B.; Kim, S.-J.; Kim, C.-H.; Kim, W.-S.; Park, K.-W. Nanostructure cathode materials prepared by high-energy ball milling method. Mater. Lett. 2011, 65, 3313–3316. [Google Scholar] [CrossRef]
- Eguchi, T.; Kanamoto, Y.; Tomioka, M.; Tashima, D.; Kumagai, S. Effect of Ball Milling on the Electrochemical Performance of Activated Carbon with a Very High Specific Surface Area. Batteries 2020, 6, 22. [Google Scholar] [CrossRef]
- Zhou, P.; Weng, J.; Liu, X.; Li, Y.; Wang, L.; Wu, X.; Zhou, T.; Zhou, J.; Zhuo, S. Urea-assistant ball-milled CF as electrode material for primary lithium battery with improved energy density and power density. J. Power Sources 2019, 414, 210–217. [Google Scholar] [CrossRef]
- Reddy, M.A.; Breitung, B.; Fichtner, M. Improving the Energy Density and Power Density of CFx by Mechanical Milling: A Primary Lithium Battery Electrode. ACS Appl. Mater. Interfaces 2013, 5, 11207–11211. [Google Scholar] [CrossRef]
- Ahmad, Y.; Dubois, M.; Guérin, K.; Hamwi, A.; Zhang, W. Pushing the theoretical limit of Li–CFx batteries using fluorinated nanostructured carbon nanodiscs. Carbon 2015, 94, 1061–1070. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Liu, C.; Wang, S.; Zhou, P.; Zhou, T.; Mialo, Z.; Xing, W.; Zhuo, S.; Zhou, J. Fluorinated multi-walled carbon nanotubes as cathode materials of lithium and sodium primary batteries: Effect of graphitization of carbon nanotubes. J. Mater. Chem. A 2019, 7, 7128–7137. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Wu, B.; Ma, J.; Zhou, G.; Mahmood, N.; Jian, X.; Liu, H. Pushing capacities and energy densities beyond theoretical limits of lithium primary batteries using active CFx nanocapsules with x > 1. Inorg. Chem. Front. 2023, 10, 127–136. [Google Scholar] [CrossRef]
- Guérin, K.; Dubois, M.; Hamwi, A. Electrochemical discharge mechanism of fluorinated graphite used as electrode in primary lithium batteries. J. Phys. Chem. Solids 2006, 67, 1173–1177. [Google Scholar] [CrossRef]
- Sayahpour, B.; Hirsh, H.; Bai, S.; Schorr, N.B.; Lambert, T.N.; Mayer, M.; Bao, W.; Cheng, D.; Zhang, M.; Leung, K.; et al. Revisiting Discharge Mechanism of CFx as a High Energy Density Cathode Material for Lithium Primary Battery. Adv. Energy Mater. 2022, 12, 2103196. [Google Scholar] [CrossRef]
- Read, J.; Collins, E.; Piekarski, B.; Zhang, S. LiF Formation and Cathode Swelling in the Li/CF x Battery. J. Electrochem. Soc. 2011, 158, A504–A510. [Google Scholar] [CrossRef]
- Mar, M.; Ahmad, Y.; Guérin, K.; Dubois, M.; Batisse, N. Fluorinated exfoliated graphite as cathode materials for enhanced performances in primary lithium battery. Electrochim. Acta 2017, 227, 18–23. [Google Scholar] [CrossRef]
- Yazami, R.; Hamwi, A.; Guérin, K.; Ozawa, Y.; Dubois, M.; Giraudet, J.; Malsin, F. Fluorinated carbon nanofibres for high energy and high power densities primary lithium batteries. Electrochem. Commun. 2007, 9, 1850–1855. [Google Scholar] [CrossRef]
- Lam, P.; Yazami, R. Physical characteristics and rate performance of (CFx)n (0.33 < x < 0.66) in lithium batteries. J. Power Sources 2006, 153, 354–359. [Google Scholar] [CrossRef]
- Ahmad, Y.; Guérin, K.; Dubois, M.; Zhang, W.; Hamwi, A. Enhanced performances in primary lithium batteries of fluorinated carbon nanofibers through static fluorination. Electrochim. Acta 2013, 114, 142–151. [Google Scholar] [CrossRef]
- Nomède-Martyr, N.; Disa, E.; Thomas, P.; Romana, L.; Mansot, J.-L.; Dubois, M.; Guérin, K.; Zhang, W.; Hamwi, A. Tribological properties of fluorinated nanocarbons with different shape factors. J. Fluorine Chem. 2012, 144, 10–16. [Google Scholar] [CrossRef]
- Colin, M.; Chen, S.; Farhat, H.; Guérin, K.; Dubois, M. Transparent wafer-scale self-standing fluorinated graphene films. Carbon 2023, 202, 137–149. [Google Scholar] [CrossRef]
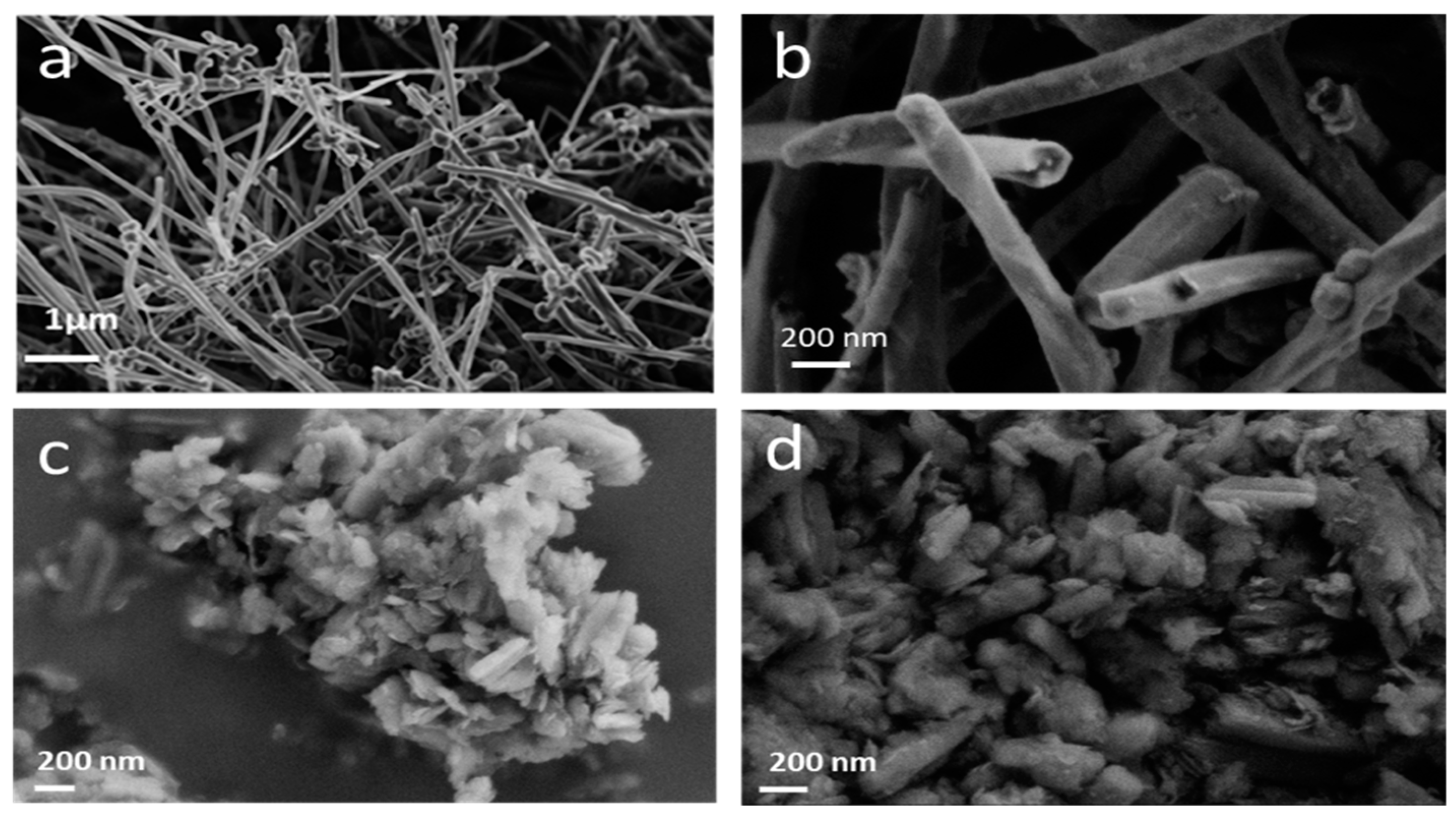
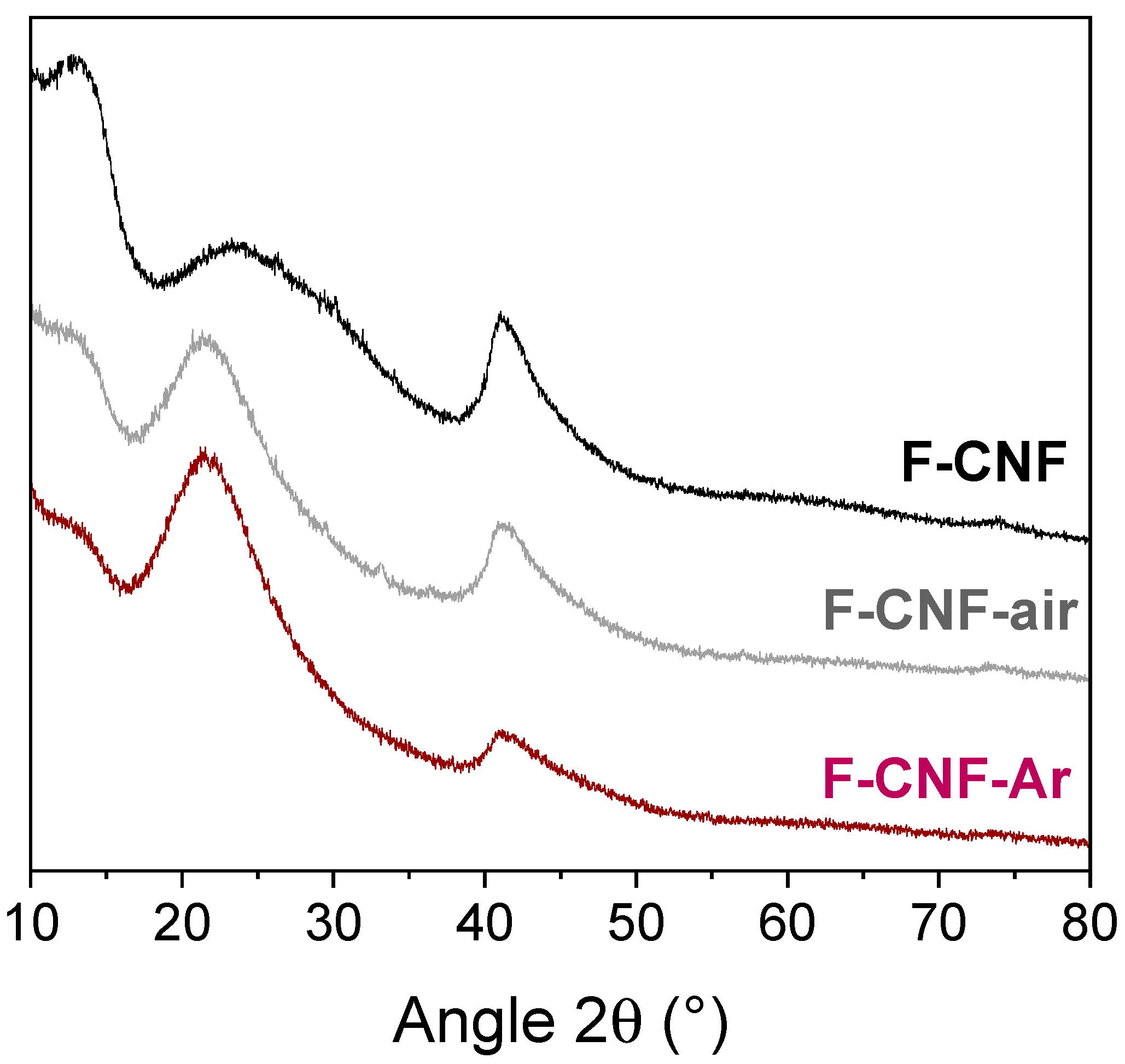
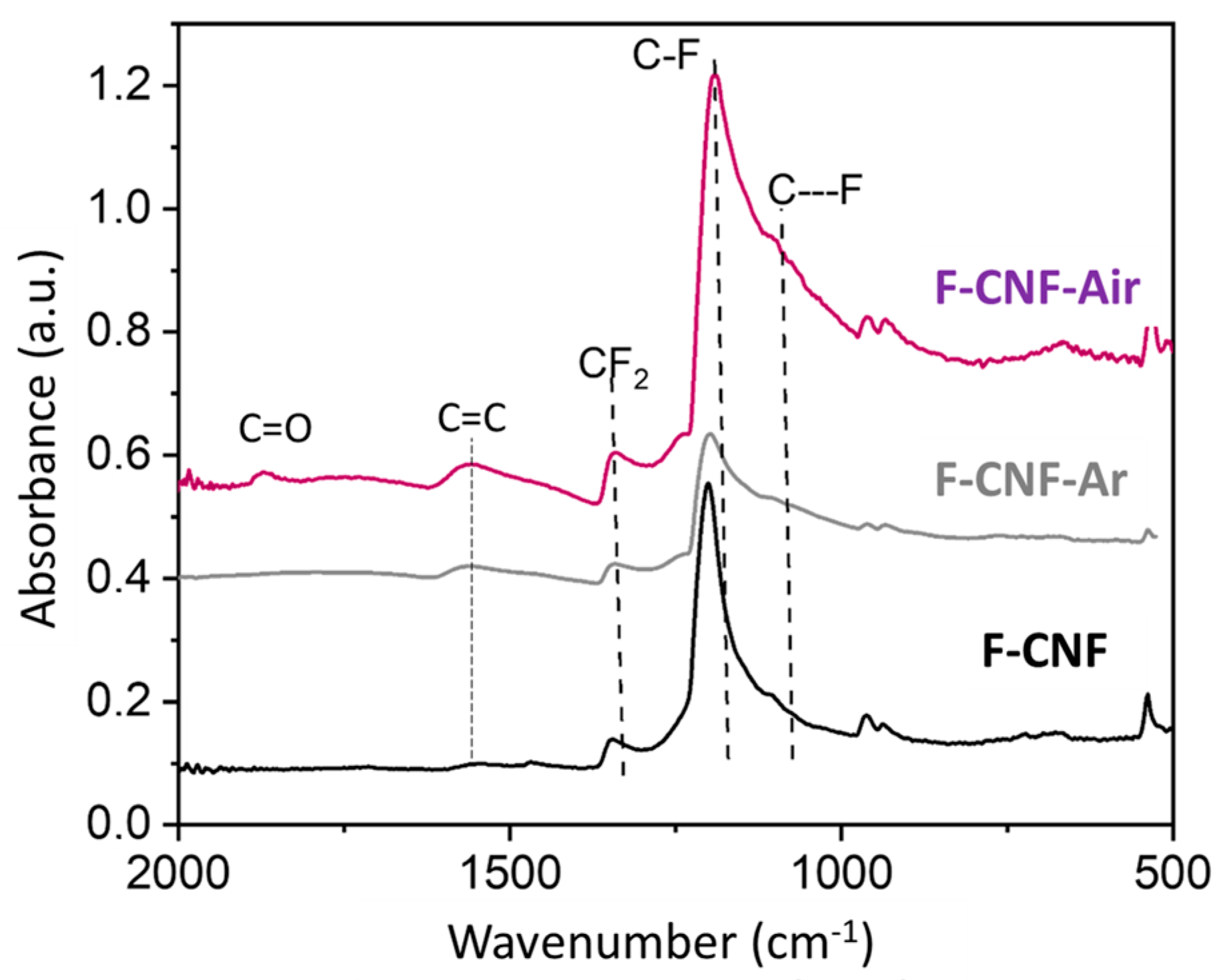
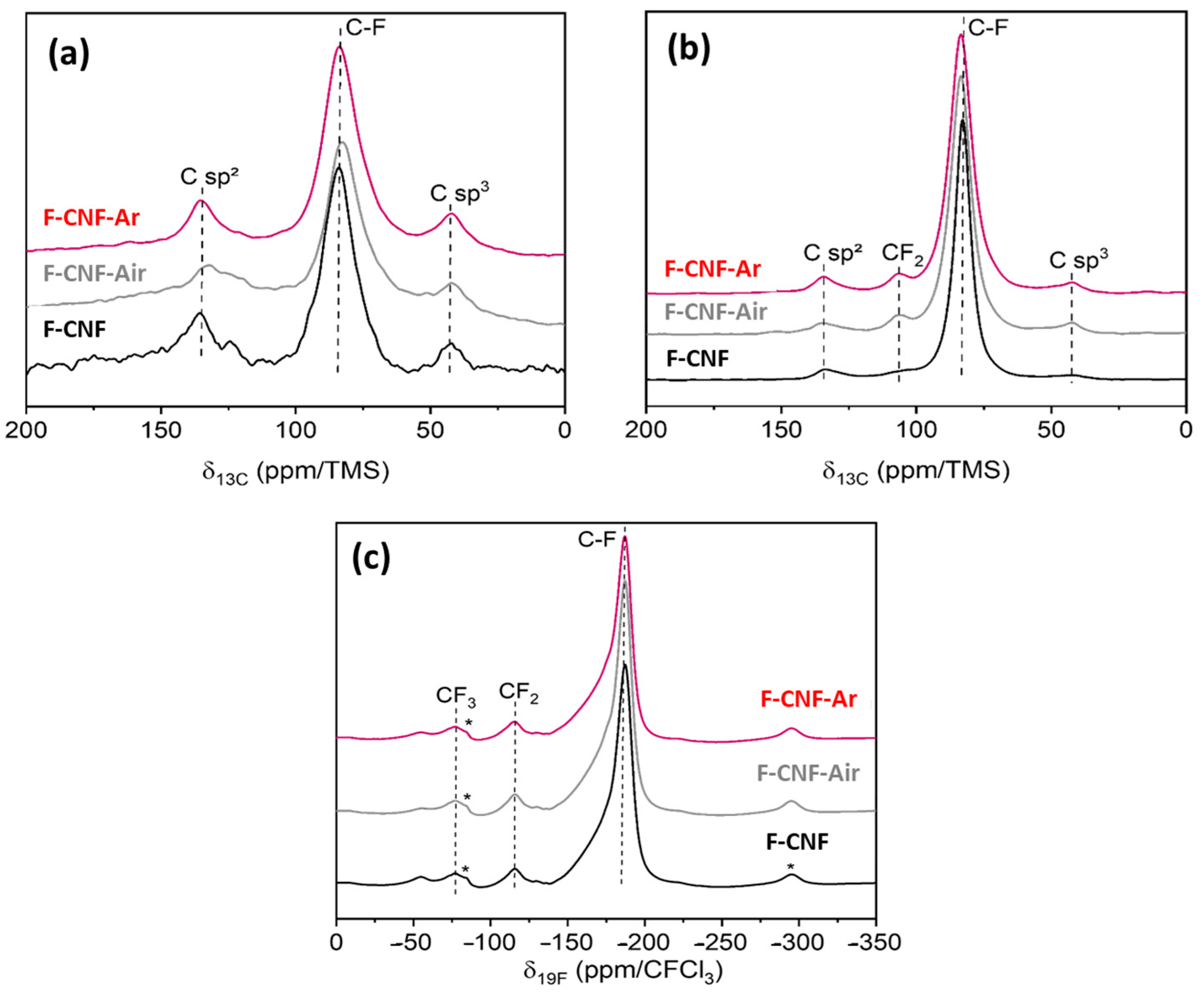
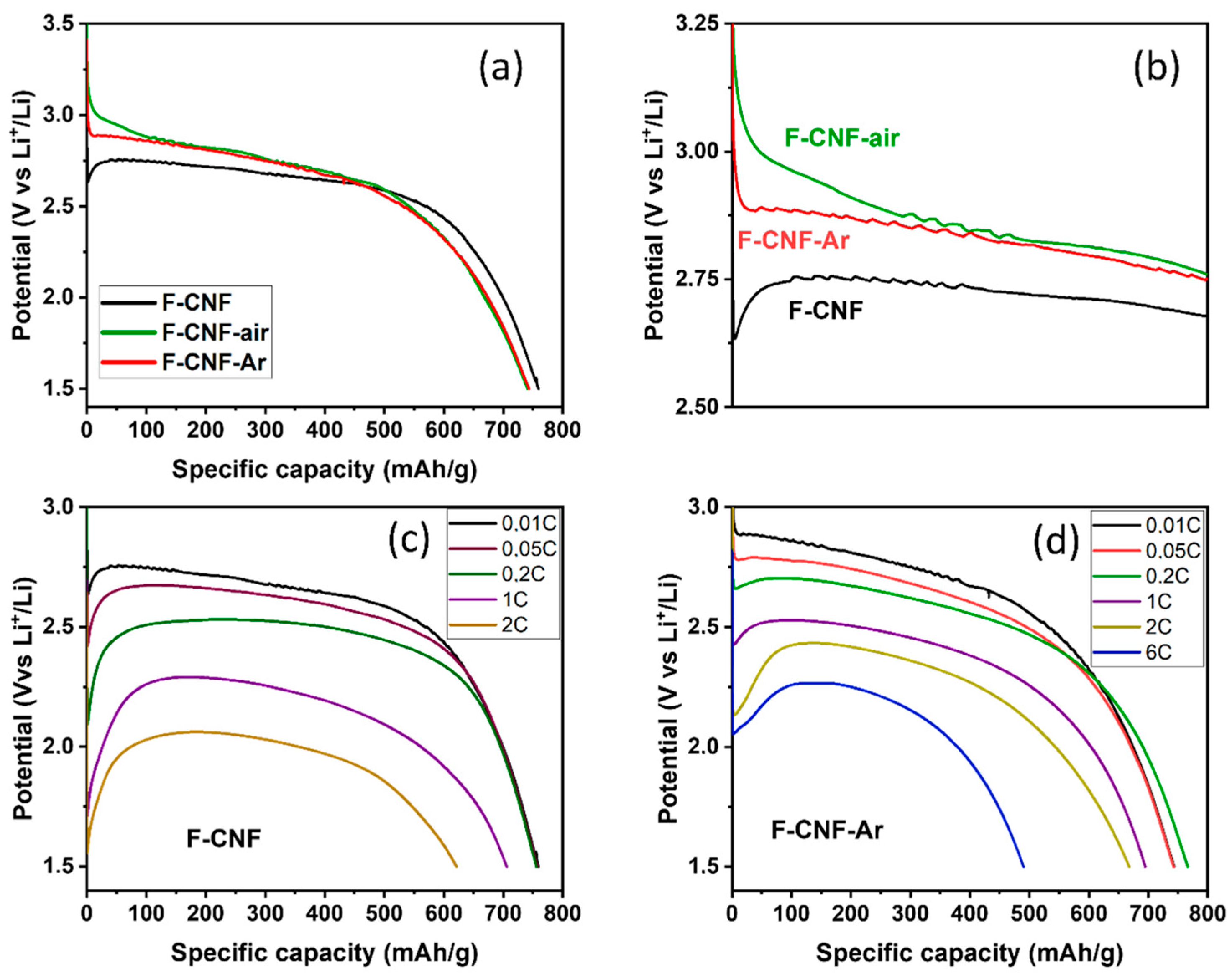
| Current Rate | Current Density (mA/g) | Cexp ± 10% (mAh/g) | E1/2 (V) | Specific Energy (Wh/kg) | Power Density (W/kg) | Faradic Yield (%) ± 10% |
|---|---|---|---|---|---|---|
| 0.01C | 10 | 759 | 2.65 | 2011 | 26 | 96 |
| 0.05C | 40 | 757 | 2.60 | 1968 | 104 | 96 |
| 0.2C | 158 | 756 | 2.51 | 1898 | 396 | 96 |
| 1C | 790 | 706 | 2.23 | 1574 | 1762 | 89 |
| 2C | 1580 | 621 | 2.02 | 1254 | 3192 | 79 |
| Current Rate | Current Density (mA/g) | Cexp ± 10% (mAh/g) | E1/2 (V) | Specific Energy (Wh/kg) | Power Density (W/kg) | Faradic Yield (%) ± 10% |
|---|---|---|---|---|---|---|
| 0.01C | 10 | 743 | 2.70 | 2006 | 27 | 102 |
| 0.05C | 36 | 742 | 2.63 | 1951 | 95 | 101 |
| 0.2C | 146 | 766 | 2.57 | 1969 | 375 | 105 |
| 1C | 731 | 695 | 2.43 | 1689 | 1776 | 95 |
| 2C | 1462 | 668 | 2.33 | 1556 | 3406 | 91 |
| 6C | 4386 | 490 | 2.21 | 1083 | 9693 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colin, M.; Petit, E.; Guérin, K.; Dubois, M. High Energy Density of Ball-Milled Fluorinated Carbon Nanofibers as Cathode in Primary Lithium Batteries. Nanomaterials 2024, 14, 404. https://doi.org/10.3390/nano14050404
Colin M, Petit E, Guérin K, Dubois M. High Energy Density of Ball-Milled Fluorinated Carbon Nanofibers as Cathode in Primary Lithium Batteries. Nanomaterials. 2024; 14(5):404. https://doi.org/10.3390/nano14050404
Chicago/Turabian StyleColin, Marie, Elodie Petit, Katia Guérin, and Marc Dubois. 2024. "High Energy Density of Ball-Milled Fluorinated Carbon Nanofibers as Cathode in Primary Lithium Batteries" Nanomaterials 14, no. 5: 404. https://doi.org/10.3390/nano14050404
APA StyleColin, M., Petit, E., Guérin, K., & Dubois, M. (2024). High Energy Density of Ball-Milled Fluorinated Carbon Nanofibers as Cathode in Primary Lithium Batteries. Nanomaterials, 14(5), 404. https://doi.org/10.3390/nano14050404







