Enhanced Electrocatalytic Oxygen Reduction Reaction of TiO2 Nanotubes by Combining Surface Oxygen Vacancy Engineering and Zr Doping
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Fabrication of TNT Arrays
2.3. Fabrication of Zr:TNT Electrodes
2.4. Characterization of Electrodes
3. Results and Discussion
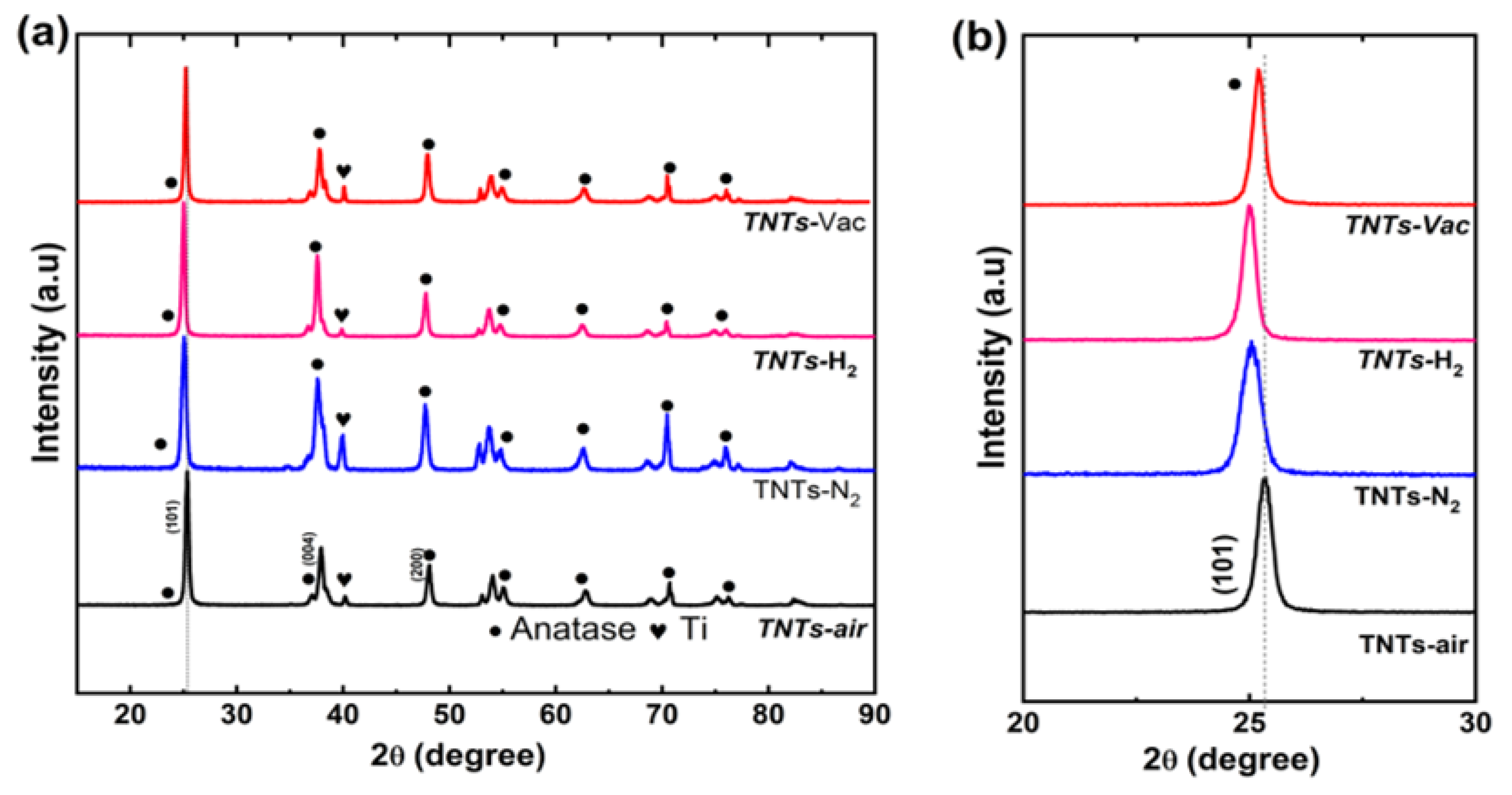
3.1. Crystalline Properties of Zr:TNT Arrays
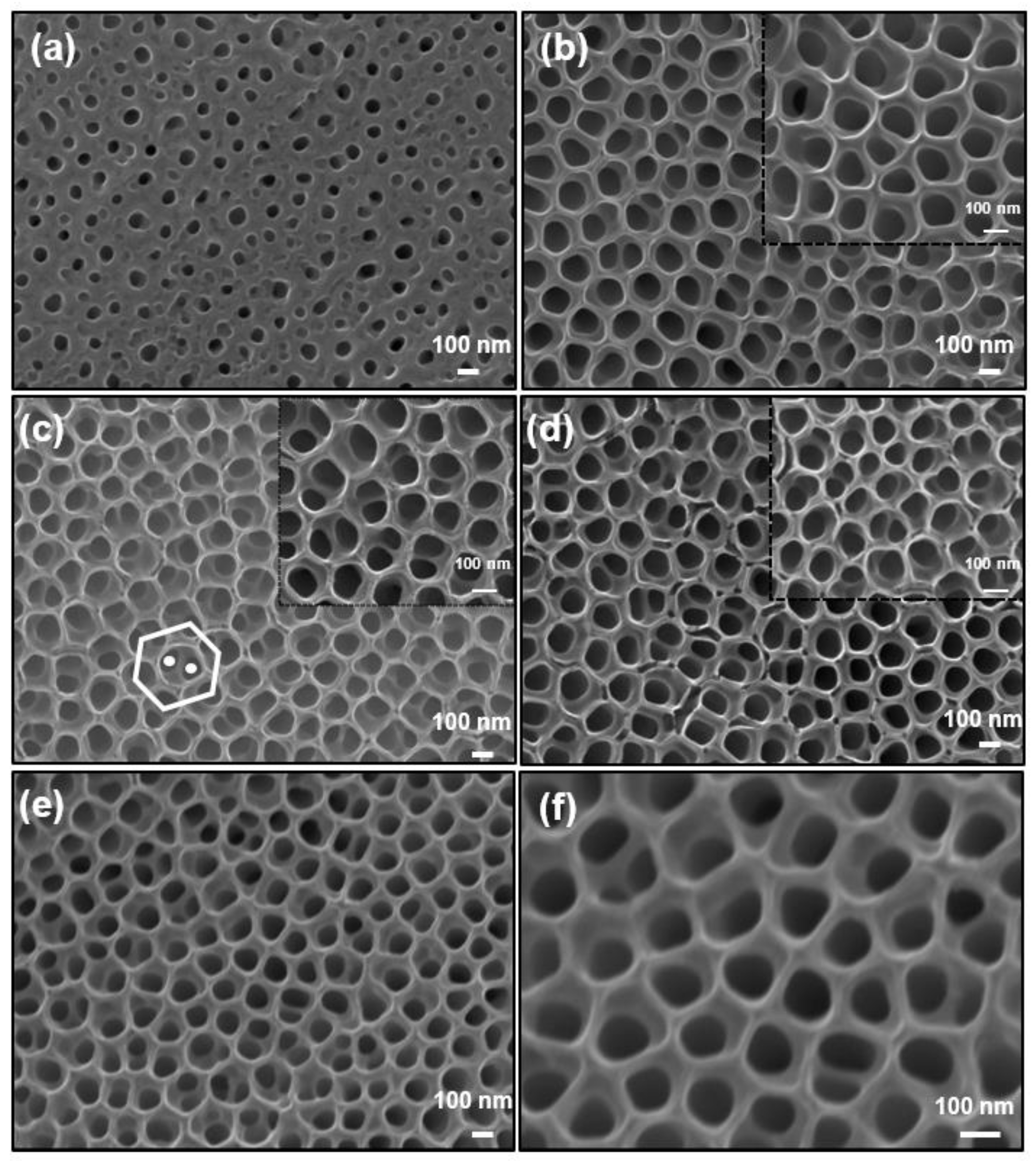
3.2. Morphological Features of Zr:TNT Arrays
3.3. Electrochemical Performance of TNT Arrays
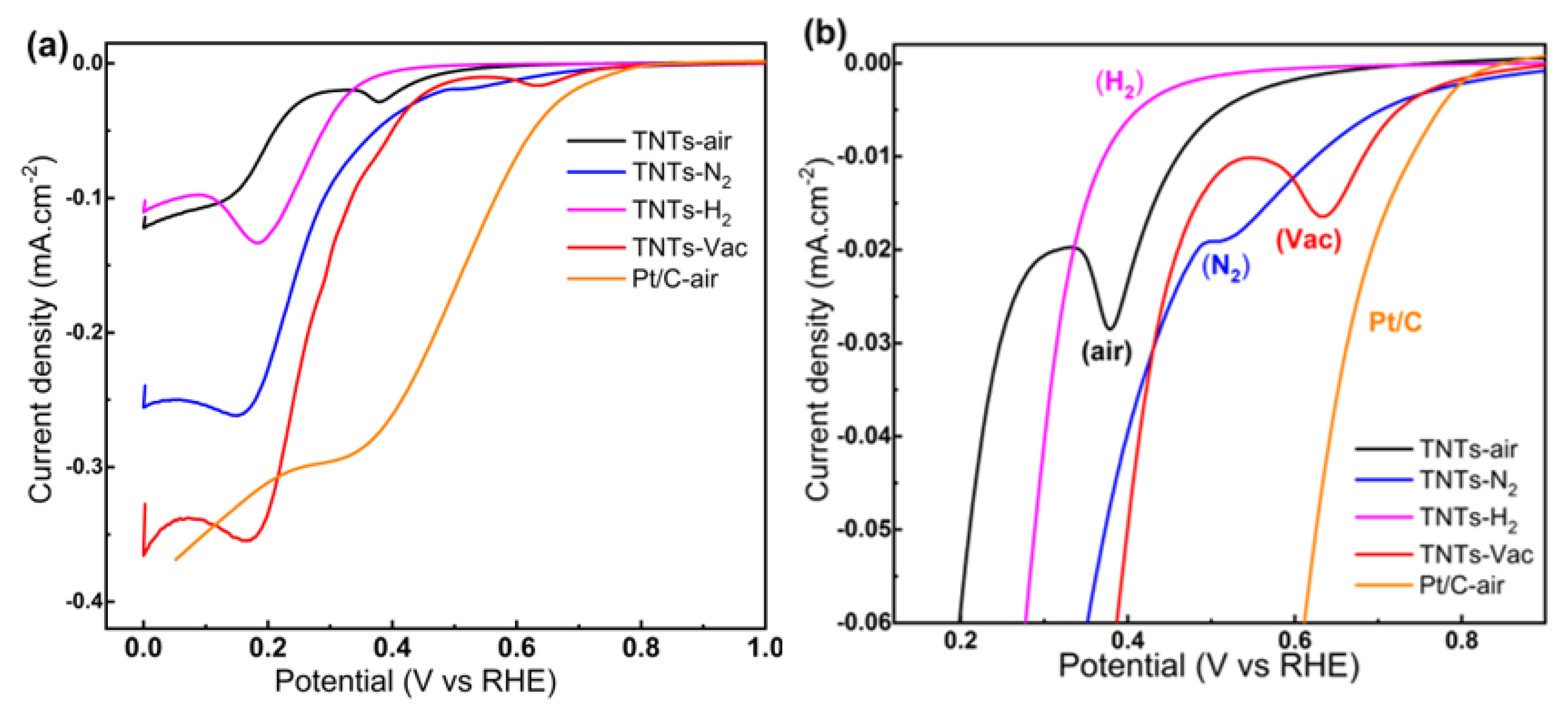
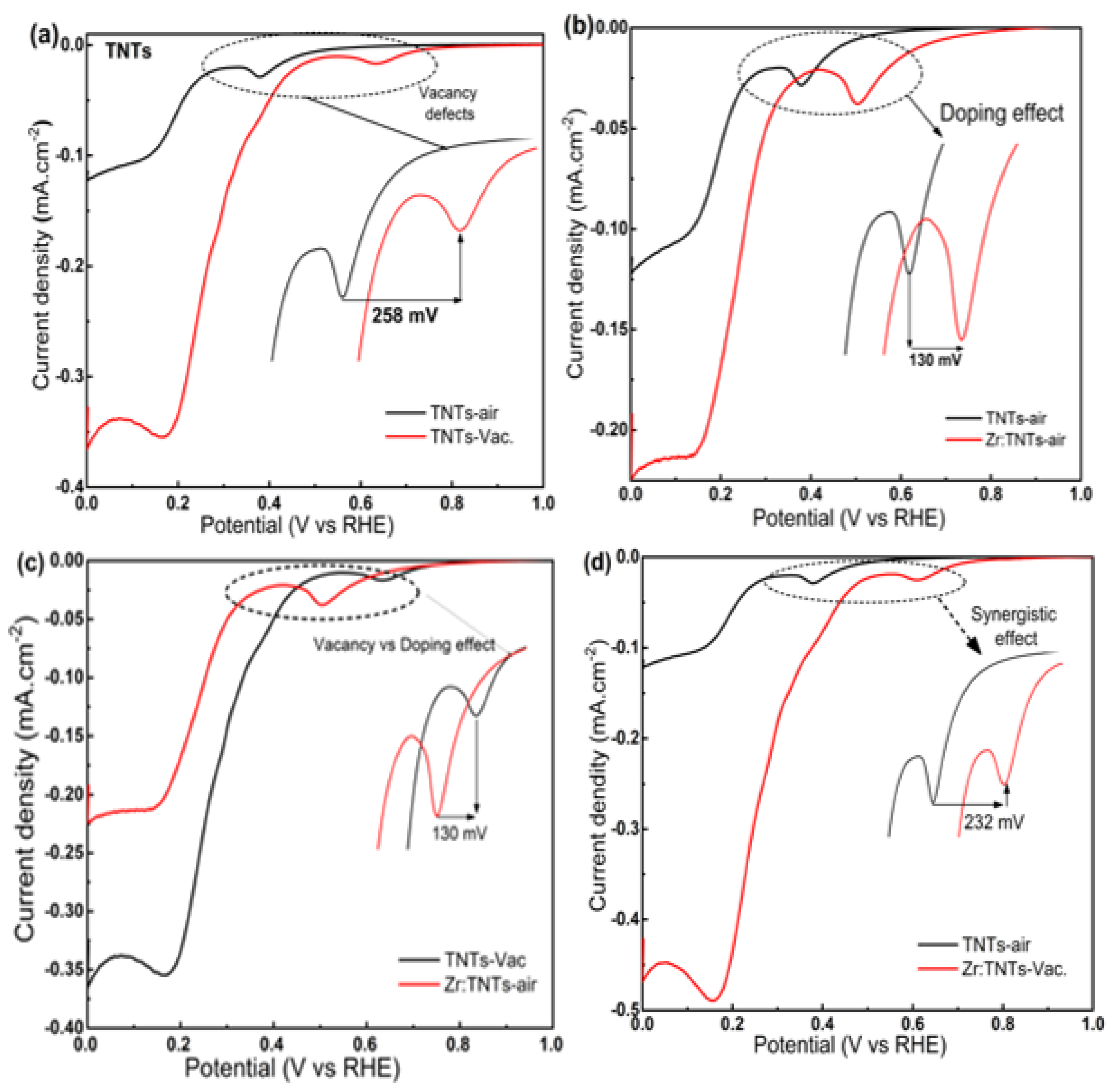
3.3.1. Electrochemical Performance of TNT Arrays without Zr Doping
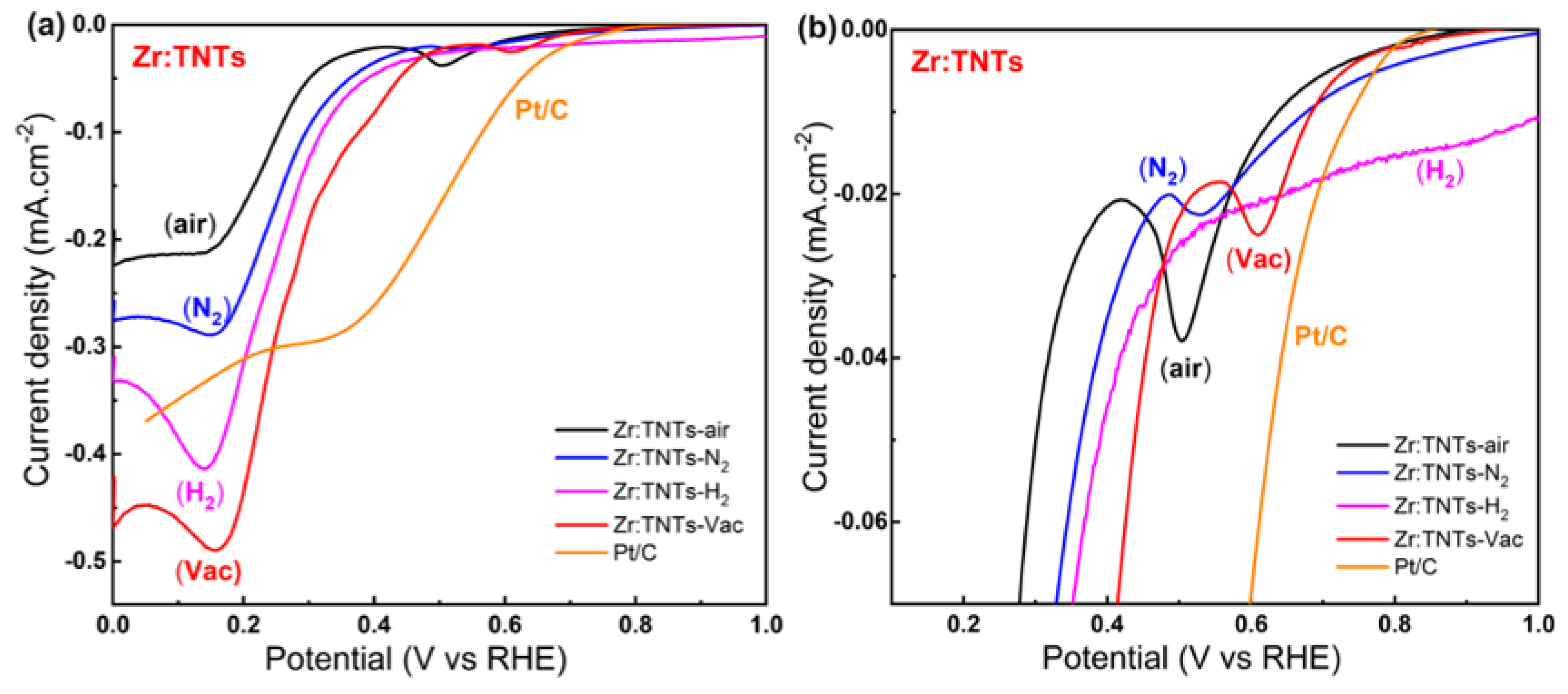
3.3.2. Electrochemical Performances of Zr-Doped TNT Arrays
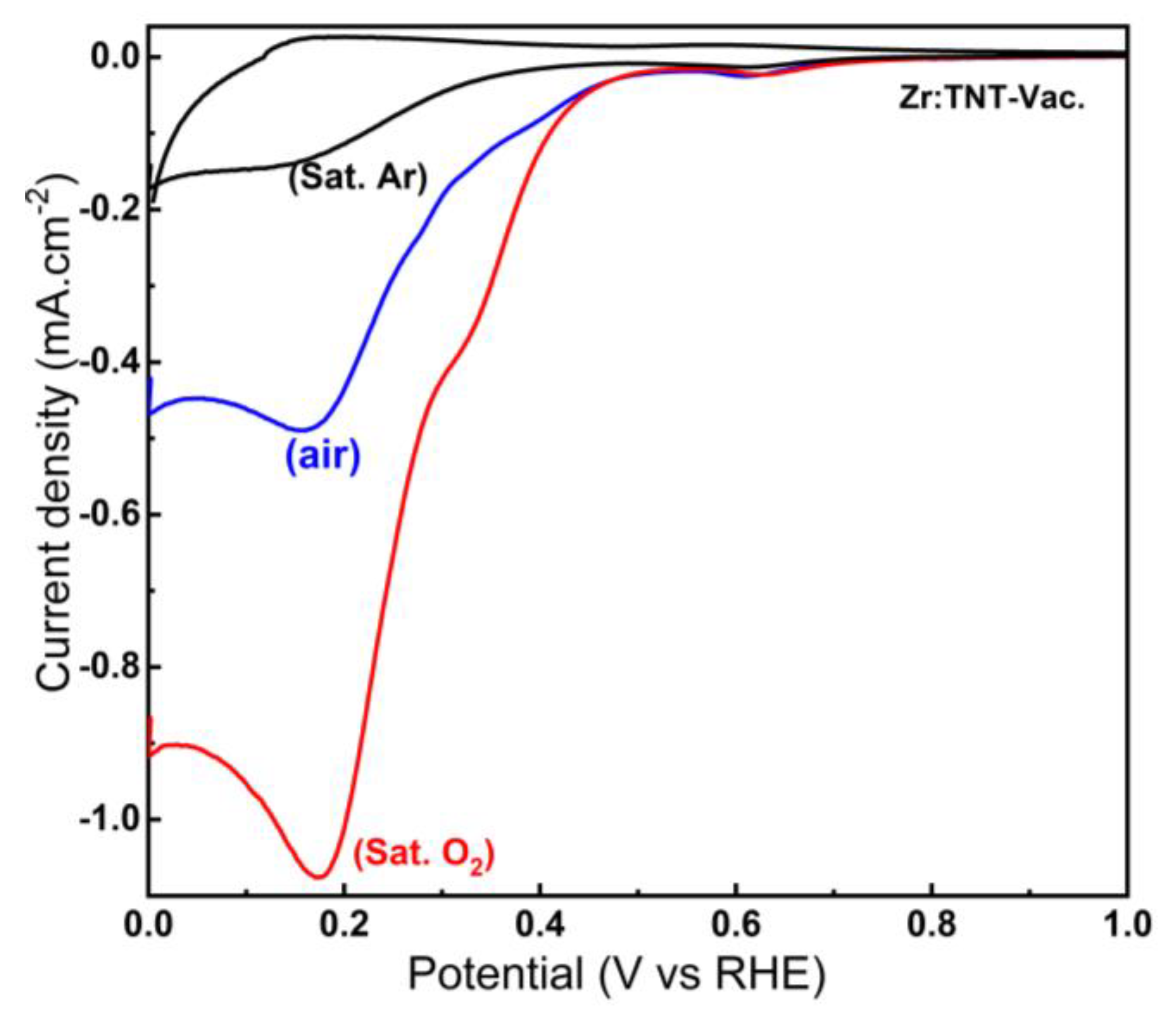
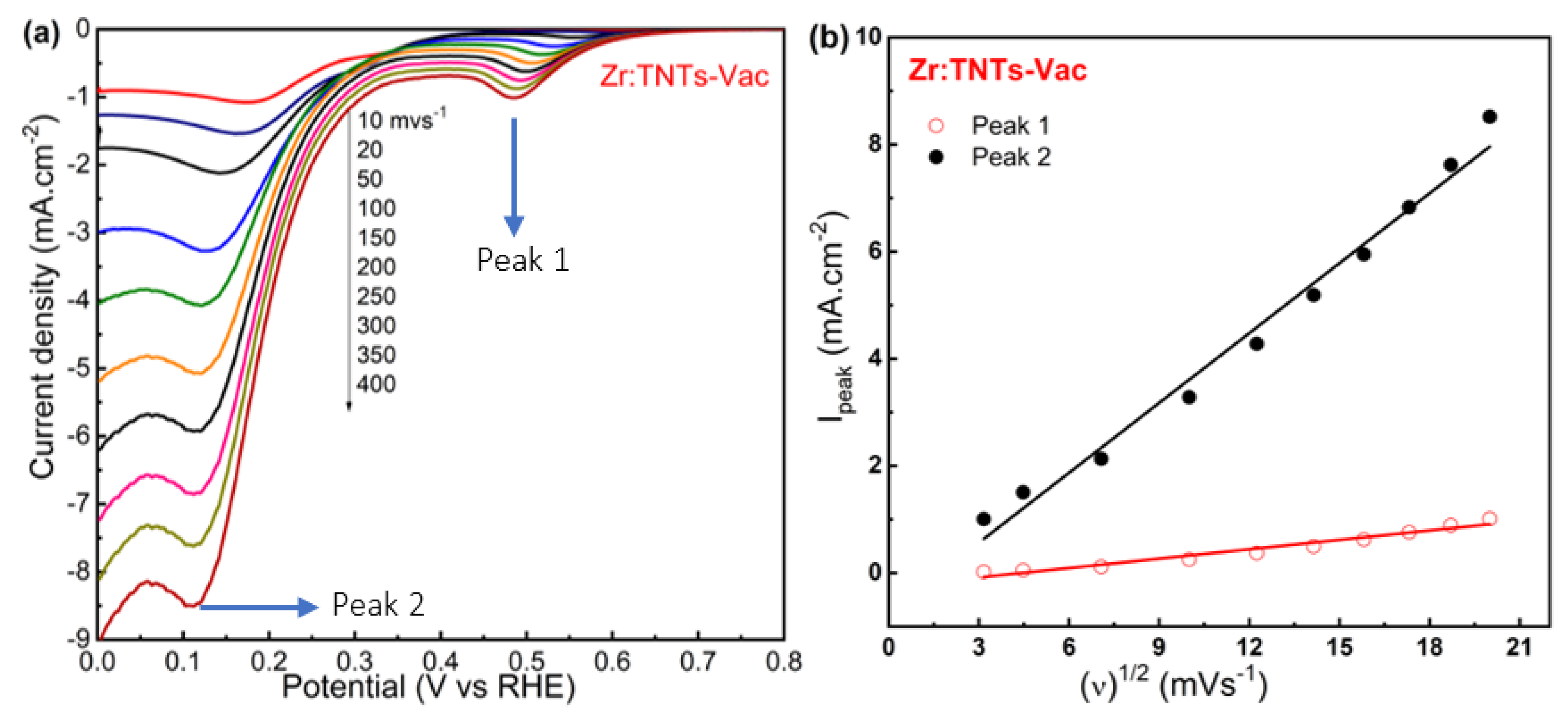
3.3.3. Effect of Oxygen Concentration on the Reduction Peaks in Vacuum-Annealed Zr-Doped TNT Arrays
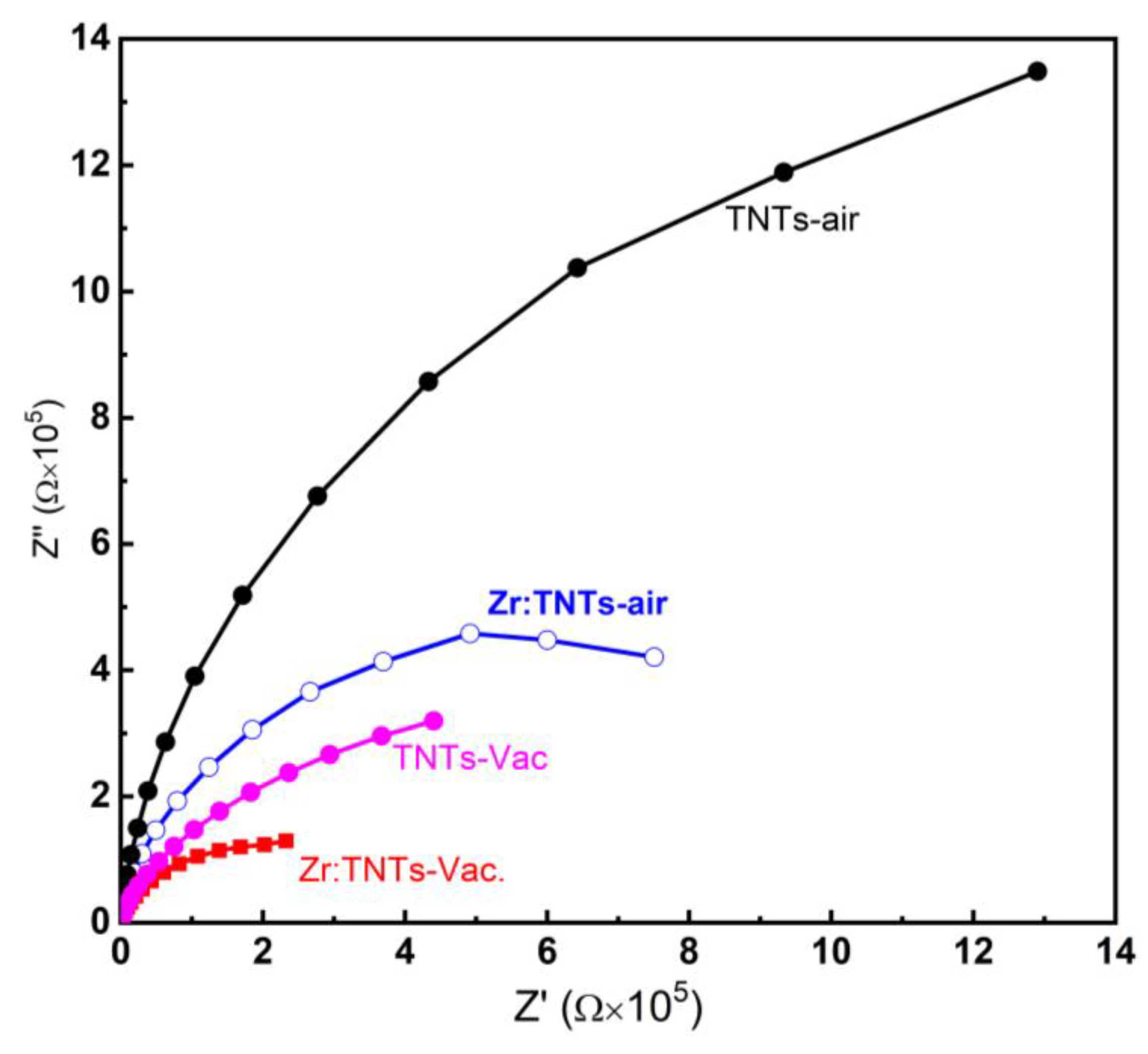
3.3.4. EIS Measurements
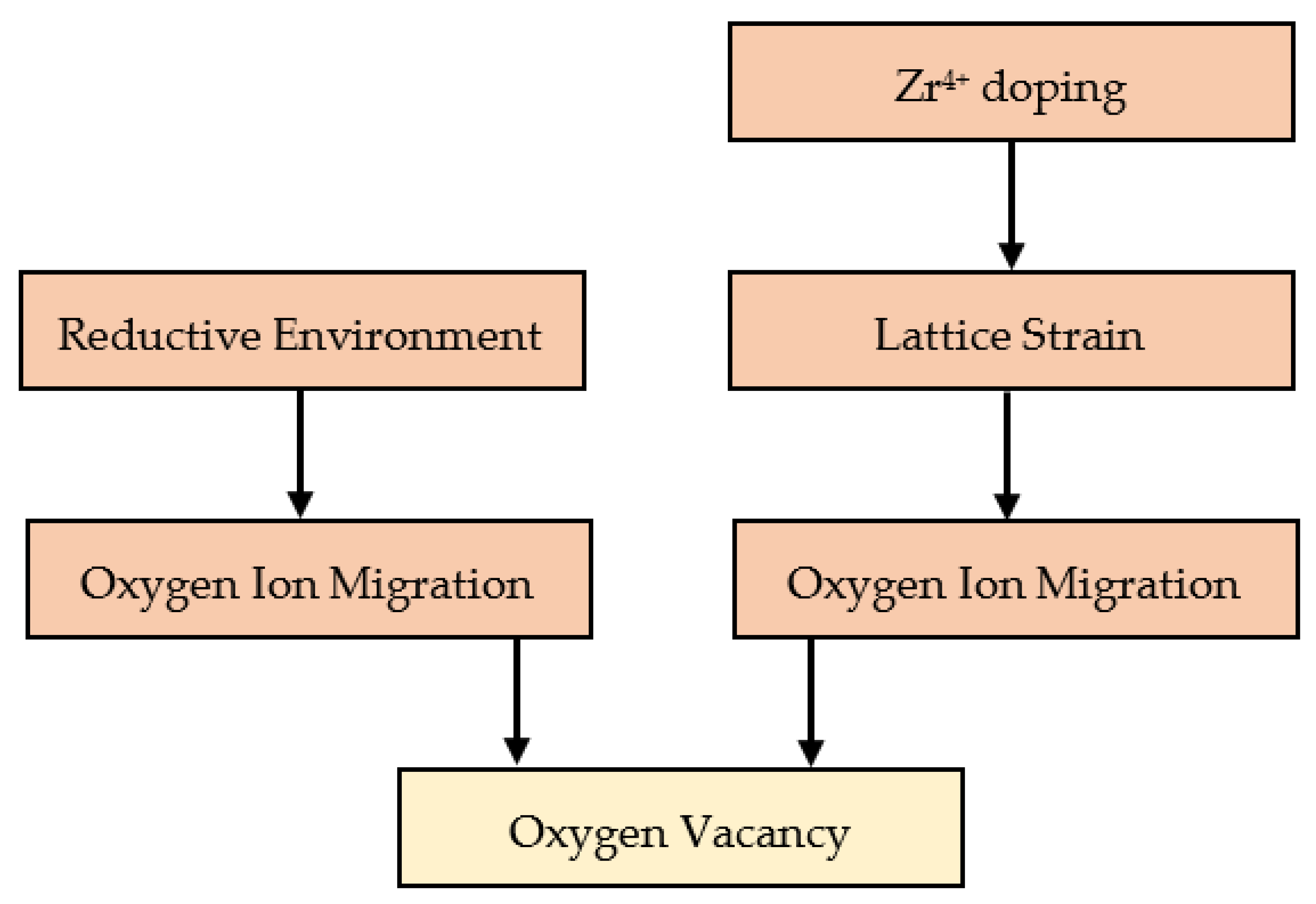
3.3.5. Mechanisms of ORR Enhancement
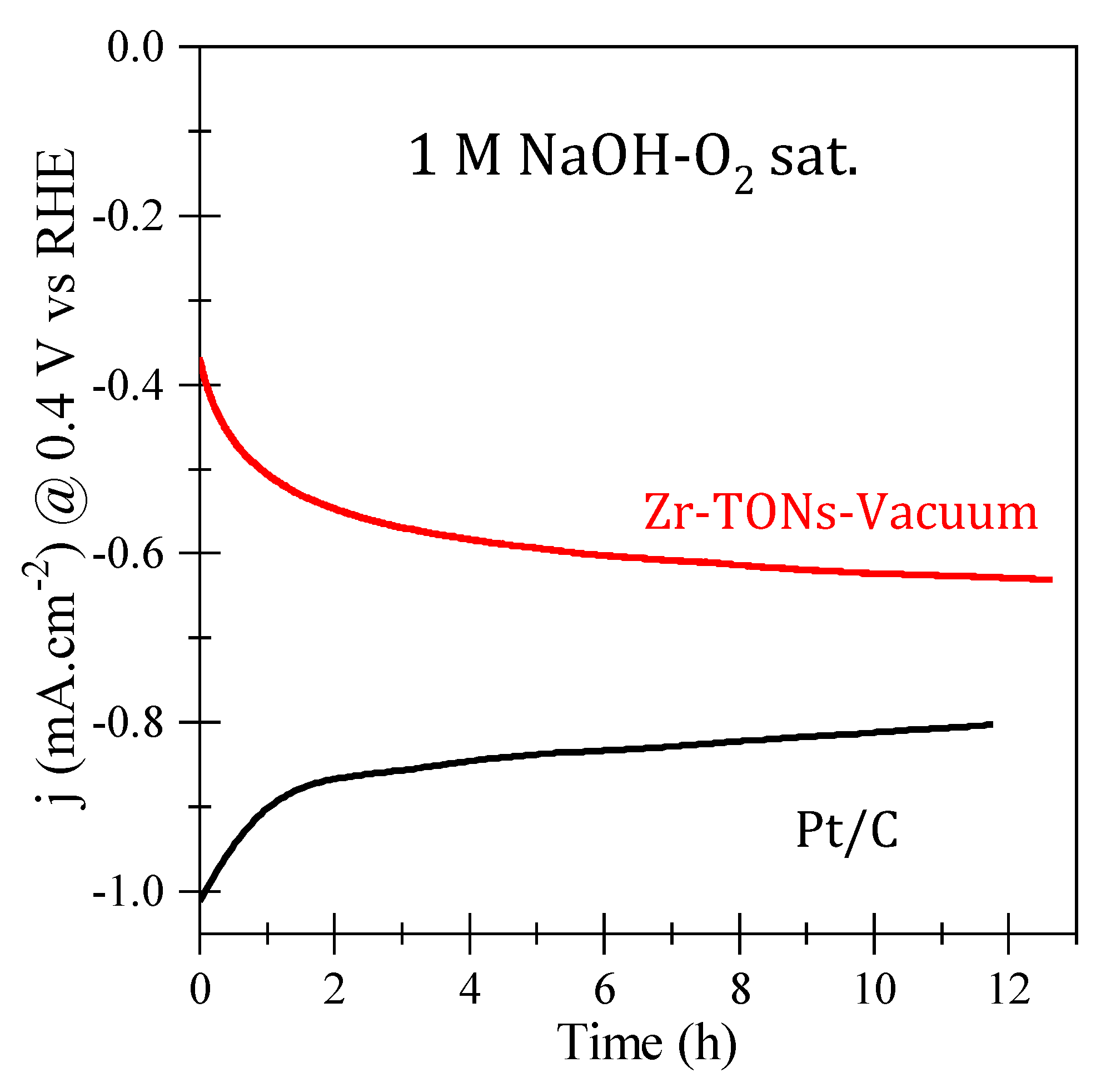
3.3.6. ORR Catalytic Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, V.; Cooper, J.S. Review and Analysis of PEM Fuel Cell Design and Manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent Advancements in Pt and Pt-Free Catalysts for Oxygen Reduction Reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, S.; Chen, S. Pt Utilization in Proton Exchange Membrane Fuel Cells: Structure Impacting Factors and Mechanistic Insights. Chem. Soc. Rev. 2022, 51, 1529–1546. [Google Scholar] [CrossRef]
- Fang, B.; Chaudhari, N.K.; Kim, M.-S.; Kim, J.H.; Yu, J.-S. Homogeneous Deposition of Platinum Nanoparticles on Carbon Black for Proton Exchange Membrane Fuel Cell. J. Am. Chem. Soc. 2009, 131, 15330–15338. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ou, L.; Liang, W.; Yang, F.; Liu, Y.; Chen, S. Efficient and Superiorly Durable Pt-Lean Electrocatalysts of Pt−W Alloys for the Oxygen Reduction Reaction. J. Phys. Chem. C 2011, 115, 2162–2168. [Google Scholar] [CrossRef]
- Li, Z.; Ge, R.; Su, J.; Chen, L. Recent Progress in Low Pt Content Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2020, 7, 2000396. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Delmondo, L.; Salvador, G.P.; Muñoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Gerosa, M.; Massaglia, G.; Chiodoni, A.; Quaglio, M. Nanostructured MnxOy for Oxygen Reduction Reaction (ORR) Catalysts. Appl. Surf. Sci. 2016, 388, 631–639. [Google Scholar] [CrossRef]
- Garino, N.; Sacco, A.; Castellino, M.; Muñoz-Tabares, J.A.; Armandi, M.; Chiodoni, A.; Pirri, C.F. One-Pot Microwave-Assisted Synthesis of Reduced Graphene Oxide/Iron Oxide Nanocomposite Catalyst for the Oxygen Reduction Reaction. ChemistrySelect 2016, 1, 3640–3646. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An Overview of Metal Oxide Materials as Electrocatalysts and Supports for Polymer Electrolyte Fuel Cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite in Energy Storage and Conversion: Past, Present, and Future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, H.; Li, Y.; Gao, Y.; Zhao, Y.; Song, W.; Shao, Z.; Yi, B. Supported Noble Metals on Hydrogen-Treated TiO2 Nanotube Arrays as Highly Ordered Electrodes for Fuel Cells. ChemSusChem 2013, 6, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Shaddad, M.N.; Al-Mayouf, A.M.; Ghanem, M.A.; AlHoshan, M.S.; Singh, J.P.; Al-Suhybani, A.A. Chemical Deposition and Electrocatalytic Activity of Platinum Nanoparticles Supported on TiO2 Nanotubes. Int. J. Electrochem. Sci. 2013, 8, 2468–2478. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Al-Mayouf, A.M.; Shaddad, M.N.; Marken, F. Selective Formation of Hydrogen Peroxide by Oxygen Reduction on TiO2 Nanotubes in Alkaline Media. Electrochim. Acta 2015, 174, 557–562. [Google Scholar] [CrossRef]
- Wang, W.; Lv, F.; Lei, B.; Wan, S.; Luo, M.; Guo, S. Tuning Nanowires and Nanotubes for Efficient Fuel-Cell Electrocatalysis. Adv. Mater. 2016, 28, 10117–10141. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A Review on Highly Ordered, Vertically Oriented TiO2 Nanotube Arrays: Fabrication, Material Properties, and Solar Energy Applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Grimes, C.; Mor, G. TiO2 Nanotube Arrays: Synthesis, Properties, and Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Arunachalam, P.; AlOraij, H.A.; Amer, M.S.; Hezam, M.; Shaddad, M.N.; Madhavan, J. Activation Effect of Nickel Phosphate Co-Catalysts on the Photoelectrochemical Water Oxidation Performance of TiO2 Nanotubes. J. Saudi Chem. Soc. 2022, 26, 101484. [Google Scholar] [CrossRef]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Xu, W.; Kulkarni, S.A.; Batabyal, S.K.; Zhang, S.; Cao, A.; Wong, L.H. TiO2 Nanotube Arrays Based Flexible Perovskite Solar Cells with Transparent Carbon Nanotube Electrode. Nano Energy 2015, 11, 728–735. [Google Scholar] [CrossRef]
- Arunachalam, P.; Amer, M.S.; AlOraij, H.A.; Al-Mayouf, A.M.; Hezam, M.; Al-Shalwi, M. Boosting the Photoelectrochemical Water Oxidation Performance of TiO2 Nanotubes by Surface Modification Using Silver Phosphate. Catalysts 2022, 12, 1440. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 Nanotubes: Self-Organized Electrochemical Formation, Properties and Applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Xing, L.; Jia, J.; Wang, Y.; Zhang, B.; Dong, S. Pt Modified TiO2 Nanotubes Electrode: Preparation and Electrocatalytic Application for Methanol Oxidation. Int. J. Hydrog. Energy 2010, 35, 12169–12173. [Google Scholar] [CrossRef]
- Baez, V.B.; Graves, J.E.; Pletcher, D. The Reduction of Oxygen on Titanium Oxide Electrodes. J. Electroanal. Chem. 1992, 340, 273–286. [Google Scholar] [CrossRef]
- Parkinson, B.; Decker, F.; Julião, J.F.; Abramovich, M.; Chagas, H.C. The Reduction of Molecular Oxygen at Single Crystal Rutile Electrodes. Electrochim. Acta 1980, 25, 521–525. [Google Scholar] [CrossRef]
- Mentus, S.V. Oxygen Reduction on Anodically Formed Titanium Dioxide. Electrochim. Acta 2004, 50, 27–32. [Google Scholar] [CrossRef]
- Clechet, P.; Martelet, C.; Martin, J.R.; Olier, R. Photoelectrochemical Behaviour of TiO2 and Formation of Hydrogen Peroxide. Electrochim. Acta 1979, 24, 457–461. [Google Scholar] [CrossRef]
- Macak, J.; Schmidt-Stein, F.; Communications, P.S.-E. Efficient Oxygen Reduction on Layers of Ordered TiO2 Nanotubes Loaded with Au Nanoparticles. Electrochem. Commun. 2007, 9, 1783–1787. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Cardenas-Morcoso, D.; García-Tecedor, M.; Fabregat-Santiago, F.; Bisquert, J.; Al-Mayouf, A.M.; Gimenez, S. TiO2 Nanotubes for Solar Water Splitting: Vacuum Annealing and Zr Doping Enhance Water Oxidation Kinetics. ACS Omega 2019, 4, 16095–16102. [Google Scholar] [CrossRef]
- Noh, K.J.; Nam, I.; Han, J.W. Nb-TiO2 Nanotubes as Catalyst Supports with High Activity and Durability for Oxygen Reduction. Appl. Surf. Sci. 2020, 521, 146330. [Google Scholar] [CrossRef]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Nagaoka, H.; Ma, F.; Dequilettes, D.W.; Vorpahl, S.M.; Glaz, M.S.; Colbert, A.E.; Ziffer, M.E.; Ginger, D.S. Zr Incorporation into TiO2 Electrodes Reduces Hysteresis and Improves Performance in Hybrid Perovskite Solar Cells While Increasing Carrier Lifetimes. J. Phys. Chem. Lett. 2015, 6, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Tsuchiya, H.; Fujimoto, S.; Schmuki, P. TiO2 Nanotubes–Annealing Effects on Detailed Morphology and Structure. Eur. J. Inorg. Chem. 2010, 2010, 4351–4356. [Google Scholar] [CrossRef]
- Macak, J.M.; Aldabergerova, S.; Ghicov, A.; Schmuki, P. Smooth Anodic TiO2 Nanotubes: Annealing and Structure. Phys. Status Solidi (a) 2006, 203, R67–R69. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; LeClere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of Double-Walled TiO2 Nanotubes and Robust Anatase Membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, D.; Garcia, B.B.; Sepehri, S.; Zhang, Y.; Cao, G. Electrochemical and Photoelectrical Properties of Titania Nanotube Arrays Annealed in Different Gases. Sens. Actuators B Chem. 2008, 134, 367–372. [Google Scholar] [CrossRef]
- Hyam, R.S.; Lee, J.; Cho, E.; Khim, J.; Lee, H. Effect of Annealing Environments on Self-Organized TiO2 Nanotubes for Efficient Photocatalytic Applications. J. Nanosci. Nanotechnol. 2012, 12, 8908–8912. [Google Scholar] [CrossRef] [PubMed]
- Talla, A.; Suliali, N.J.; Goosen, W.E.; Urgessa, Z.N.; Motloung, S.V.; Botha, J.R. Effect of Annealing Temperature and Atmosphere on the Structural, Morphological and Luminescent Properties of TiO2 Nanotubes. Phys. B Condens. Matter 2022, 640, 414026. [Google Scholar] [CrossRef]
- Regonini, D.; Satka, A.; Jaroenworaluck, A.; Allsopp, D.W.E.; Bowen, C.R.; Stevens, R. Factors Influencing Surface Morphology of Anodized TiO2 Nanotubes. Electrochim. Acta 2012, 74, 244–253. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization Parameters Influencing the Morphology and Electrical Properties of TiO2 Nanotubes for Living Cell Interfacing and Investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Pishkar, N.; Ghoranneviss, M.; Ghorannevis, Z.; Akbari, H. Study of the Highly Ordered TiO2 Nanotubes Physical Properties Prepared with Two-Step Anodization. Results Phys. 2018, 9, 1246–1249. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Guo, D.; Yu, L.; Zhang, W. Anodization Fabrication of Highly Ordered TiO2 Nanotubes. J. Phys. Chem. C 2009, 113, 12759–12765. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Arunachalam, P.; Amer, M.S.; Al-Mayouf, A.M.; Hezam, M.; AlOraij, H.A.; Gimenez, S. Exploiting the Synergistic Catalytic Effects of CoPi Nanostructures on Zr-Doped Highly Ordered TiO2 Nanotubes for Efficient Solar Water Oxidation. Int. J. Energy Res. 2022, 46, 12608–12622. [Google Scholar] [CrossRef]
- Shaddad, M.; Alharthi, A.; Alotaibi, M.; Alanazi, A.A.; Arunachalam, P.; Al-Mayouf, A.M. Combining Heterointerface/Surface Oxygen Vacancies Engineering of Bismuth Oxide to Synergistically Improve Its Solar Water Splitting Performance. Electrochim. Acta 2023, 469, 143217. [Google Scholar] [CrossRef]
- Schaub, R.; Wahlstrom, E.; Rønnau, A.; Lægsgaard, E.; Stensgaard, I.; Besenbacher, F. Oxygen-Mediated Diffusion of Oxygen Vacancies on the TiO2(110) Surface. Science 2003, 299, 377–379. [Google Scholar] [CrossRef]
- Simka, W.; Majewski, D.; Nawrat, G.; Krząkała, A.; Nieużyła, Ł.; Michalska, J. Electrodeposition of Zirconium from DMSO Solution. Arch. Metall. Mater. 2014, 59, 565–568. [Google Scholar] [CrossRef]
- Bakulin, A.; Elfimov, B.; Matiskina, E.V.; Kulkova, S.E. Impurity Influence on Oxygen Diffusion in TiO2. AIP Conf. Proc. 2020, 2310, 020026. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Baloch, A.A.B.; Alqahtani, S.M.; Mumtaz, F.; Muqaibel, A.H.; Rashkeev, S.N.; Alharbi, F.H. Extending Shannon’s Ionic Radii Database Using Machine Learning. Phys. Rev. Mater. 2021, 5, 043804. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C. Inorganic-Modified Semiconductor TiO2 Nanotube Arrays for Photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaddad, M.N.; Arunachalam, P.; Hezam, M.S.; Aladeemy, S.A.; Aljaafreh, M.J.; Abu Alrub, S.; Al-Mayouf, A.M. Enhanced Electrocatalytic Oxygen Reduction Reaction of TiO2 Nanotubes by Combining Surface Oxygen Vacancy Engineering and Zr Doping. Nanomaterials 2024, 14, 366. https://doi.org/10.3390/nano14040366
Shaddad MN, Arunachalam P, Hezam MS, Aladeemy SA, Aljaafreh MJ, Abu Alrub S, Al-Mayouf AM. Enhanced Electrocatalytic Oxygen Reduction Reaction of TiO2 Nanotubes by Combining Surface Oxygen Vacancy Engineering and Zr Doping. Nanomaterials. 2024; 14(4):366. https://doi.org/10.3390/nano14040366
Chicago/Turabian StyleShaddad, Maged N., Prabhakarn Arunachalam, Mahmoud S. Hezam, Saba A. Aladeemy, Mamduh J. Aljaafreh, Sharif Abu Alrub, and Abdullah M. Al-Mayouf. 2024. "Enhanced Electrocatalytic Oxygen Reduction Reaction of TiO2 Nanotubes by Combining Surface Oxygen Vacancy Engineering and Zr Doping" Nanomaterials 14, no. 4: 366. https://doi.org/10.3390/nano14040366
APA StyleShaddad, M. N., Arunachalam, P., Hezam, M. S., Aladeemy, S. A., Aljaafreh, M. J., Abu Alrub, S., & Al-Mayouf, A. M. (2024). Enhanced Electrocatalytic Oxygen Reduction Reaction of TiO2 Nanotubes by Combining Surface Oxygen Vacancy Engineering and Zr Doping. Nanomaterials, 14(4), 366. https://doi.org/10.3390/nano14040366










