Immobilization and Characterization of a Processive Endoglucanase EG5C-1 from Bacillus subtilis on Melamine–Glutaraldehyde Dendrimer-Functionalized Magnetic Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Surface Modification of Magnetic Nanoparticles
2.3. Immobilization of EG5C-1 on Carrier MG-DMNPs
2.4. Protein Concentration Measurements and Enzyme Activity Assay
2.5. Characterization
2.6. Biochemical Properties
2.7. Saccharification of Filter Paper by the Immobilized EG5C-1
2.8. Reusability Assay of the Immobilized EG5C-1
3. Results and Discussion
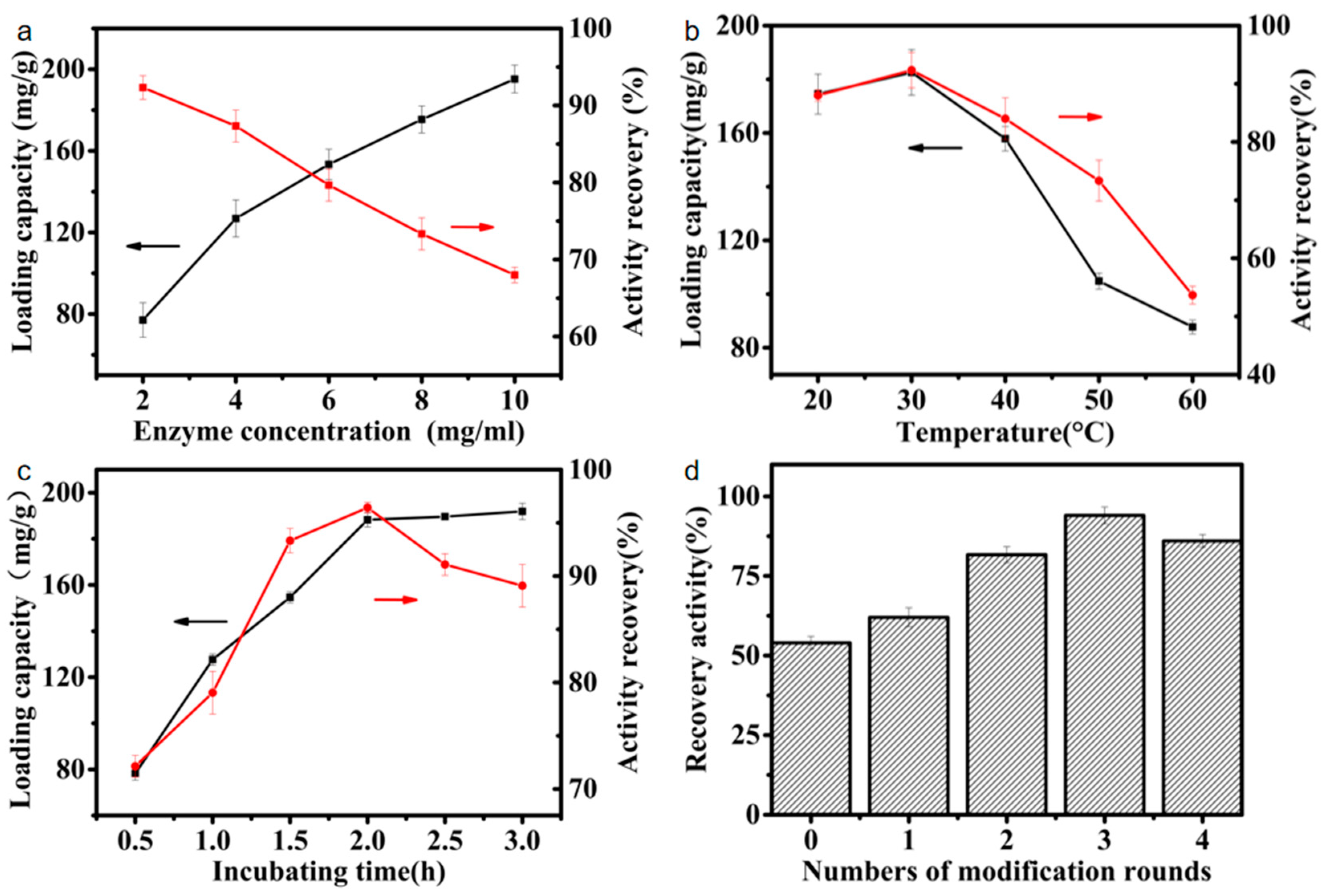
3.1. Immobilization of EG5C-1 onto Melamine–Glutaraldehyde Magnetic Nanoparticles
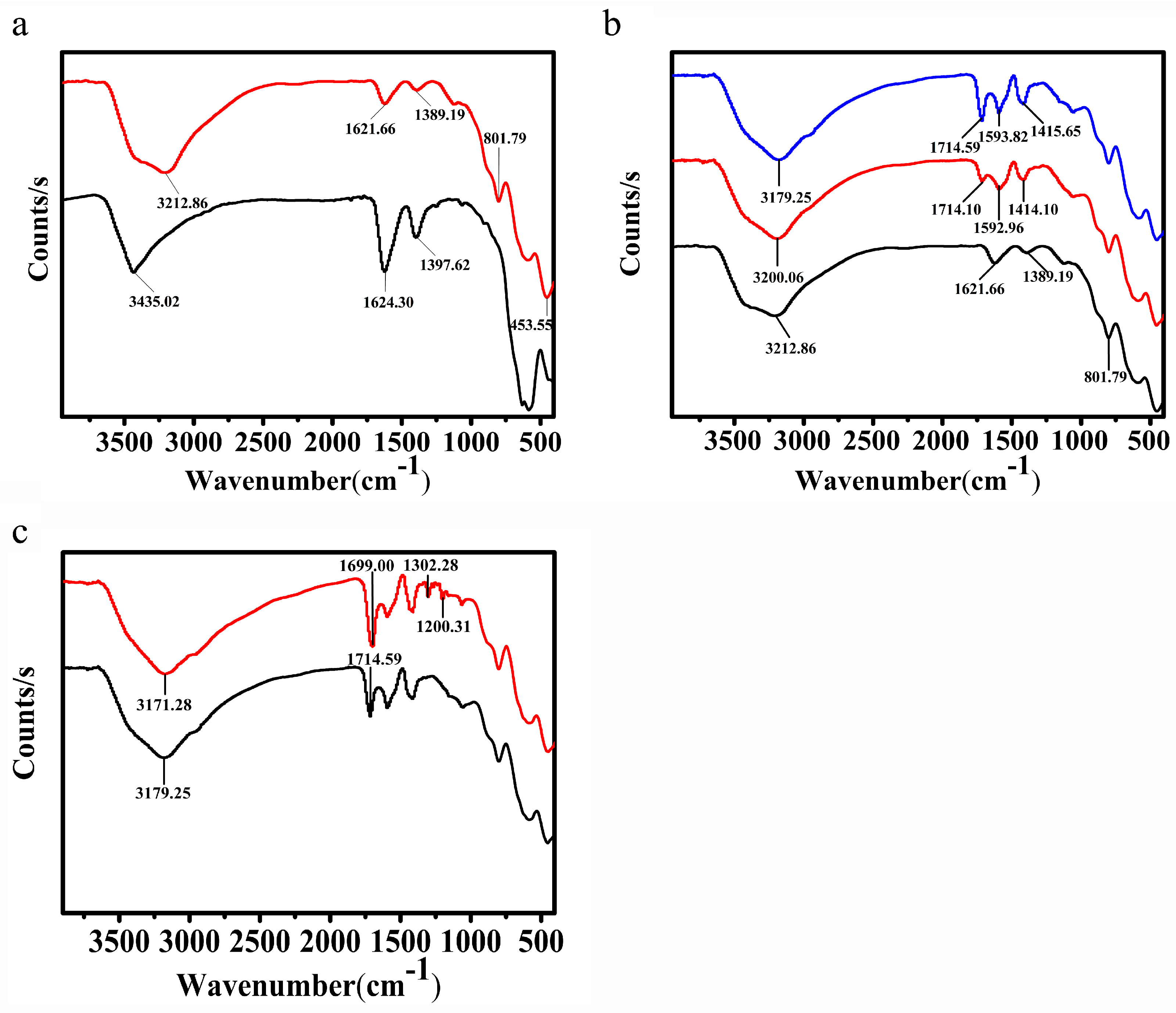
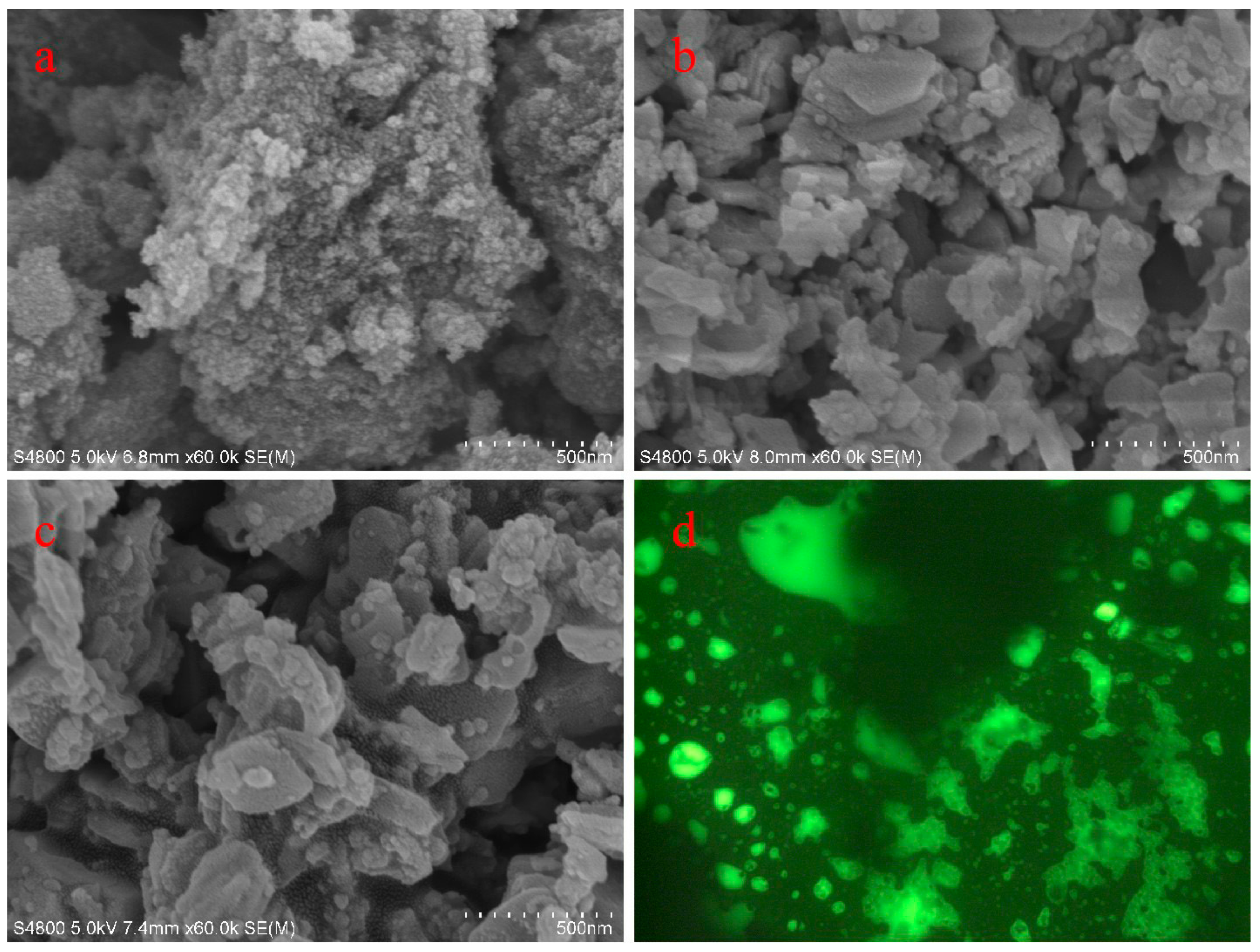
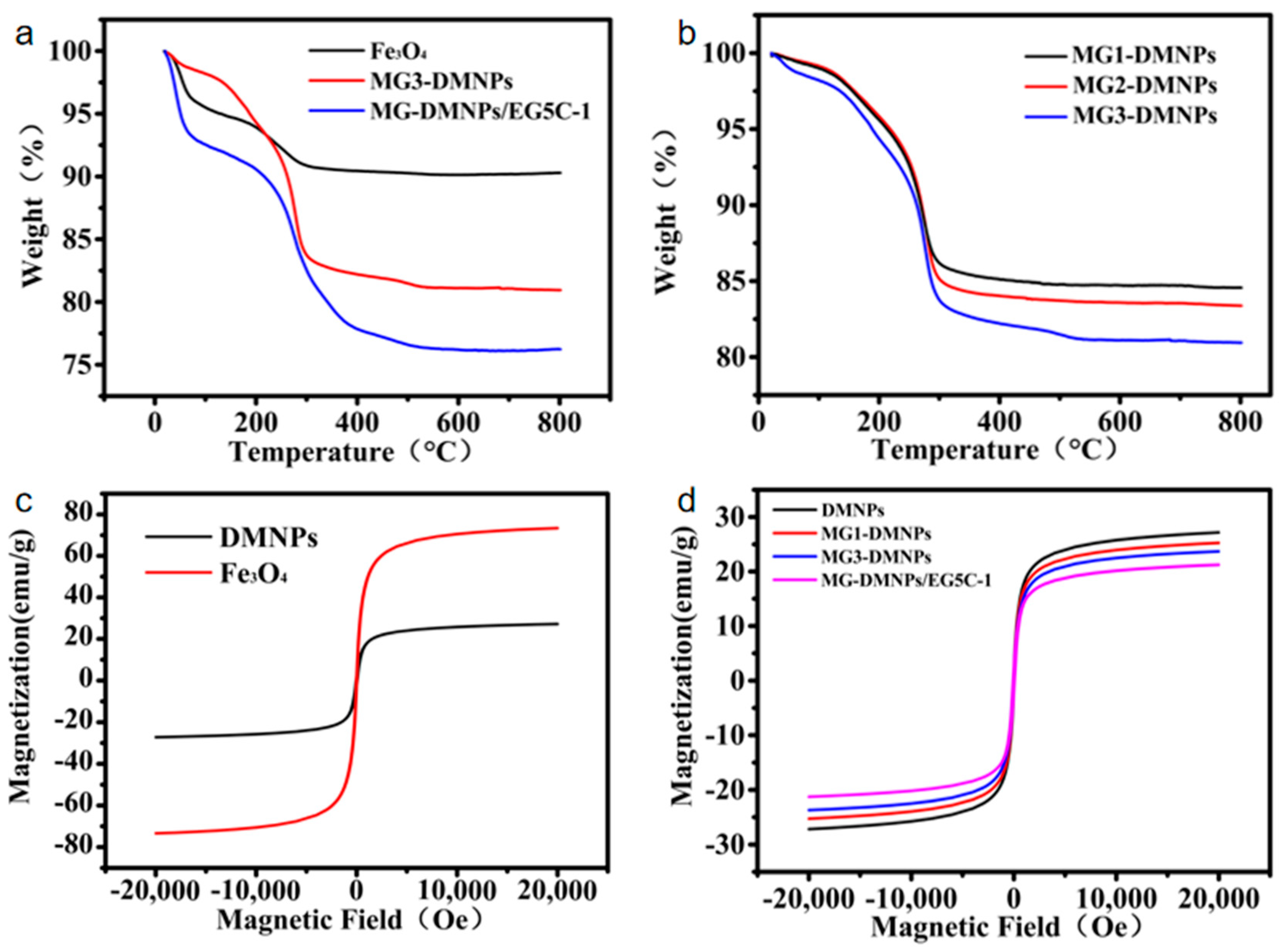
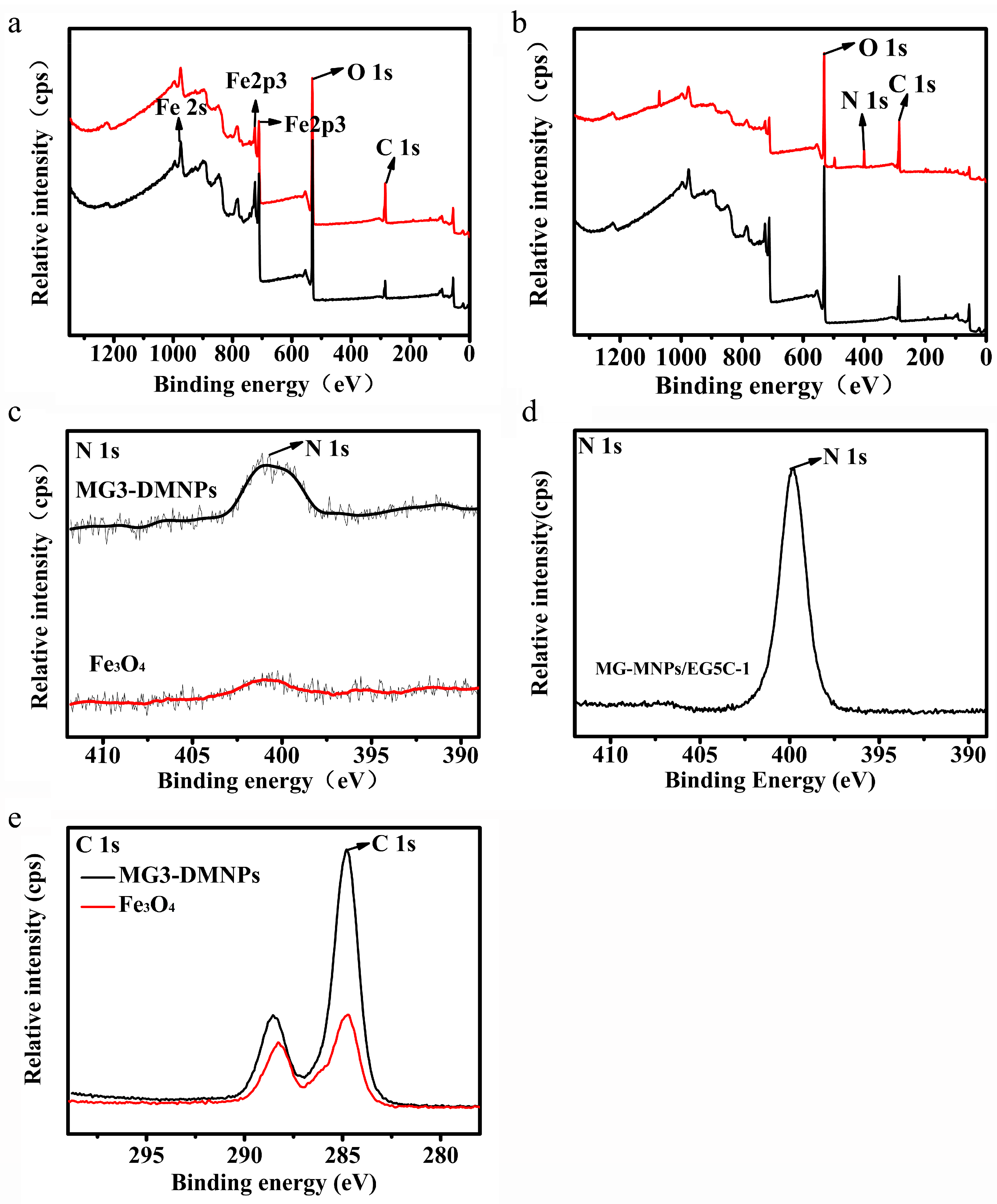
3.2. Characterization
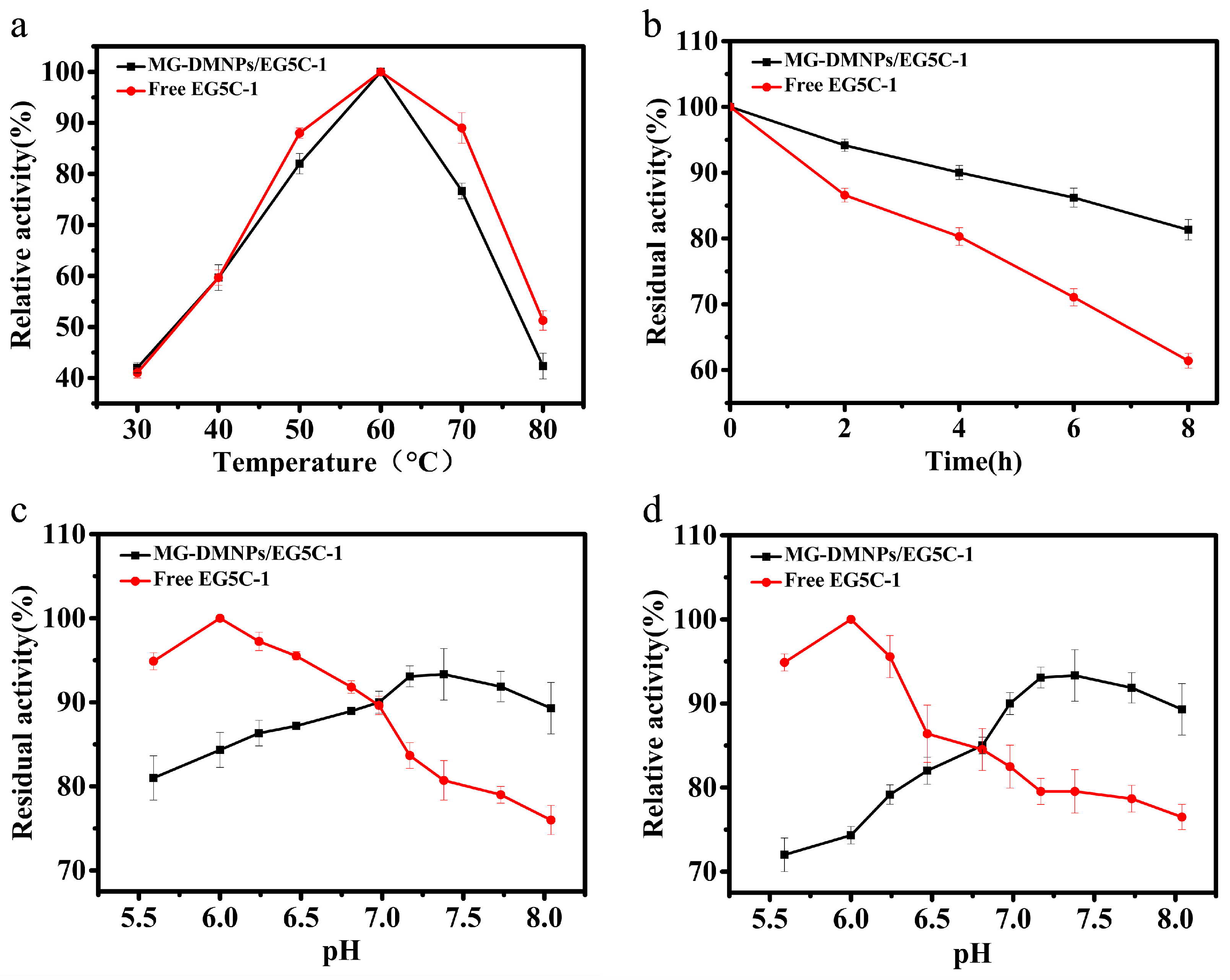
3.3. Biochemical Properties of Immobilized EG5C-1
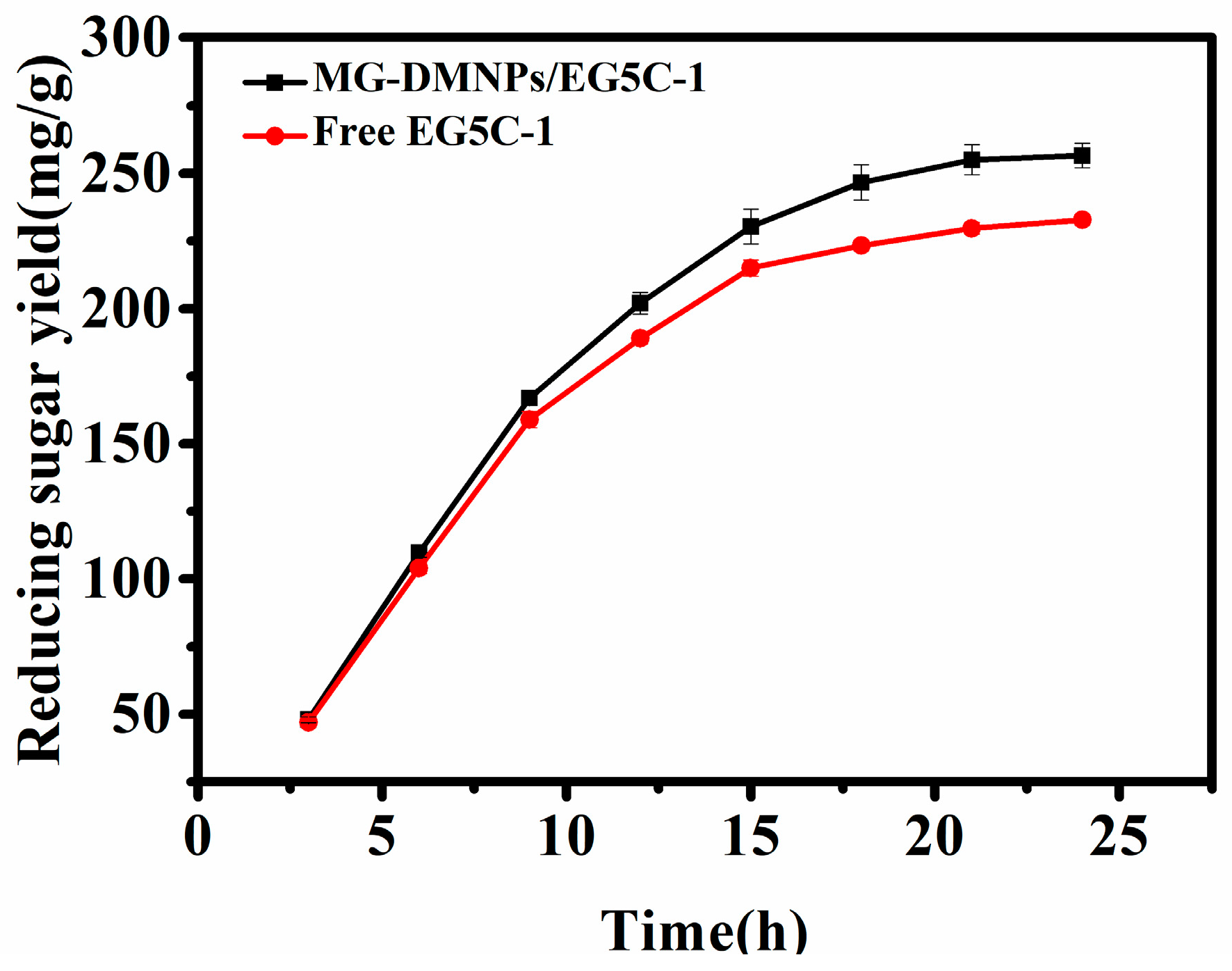
3.4. Hydrolysis of Filter Paper Using Free and Immobilized EG5C-1
3.5. Reusability of Immobilized EG5C-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.F.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Palanisamy, G.; Oh, T.H.; Thangarasu, S. Modified Cellulose Proton-Exchange Membranes for Direct Methanol Fuel Cells. Polymers 2023, 15, 659. [Google Scholar] [CrossRef]
- Nayeri, M.D.; Nikkhah, H.; Zilouei, H.; Bazarganipour, M. Immobilization of cellulase on graphene oxide coated with NiFe2O4 and Fe3O4 for hydrolysis of rice straw. Cellulose 2023, 30, 5549–5571. [Google Scholar] [CrossRef]
- Gennari, A.; Führ, A.J.; Volpato, G.; de Souza CF, V. Magnetic cellulose: Versatile support for enzyme immobilization—A review. Carbohydr. Polym. 2020, 246, 116646. [Google Scholar] [CrossRef]
- Domingues, S.Z.; Timmers, L.; Granada, C.E. Cellulase production by bacteria is a strain-specific characteristic with a high biotechnological potential. A review of cellulosome of highly studied strains. Cellulose 2022, 29, 8065–8083. [Google Scholar] [CrossRef]
- Suo, H.B.; Xu, L.L.; Xue, Y.; Qiu, X.; Huang, H.; Hu, Y. Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: Improvement of catalytic performance. Carbohydr. Polym. 2020, 234, 115914. [Google Scholar] [CrossRef]
- Park, M.; Park, S.; Hyun, J. Use of Magnetic Nanoparticles to Manipulate the Metabolic Environment of Bacteria for Controlled Biopolymer Synthesis. ACS Appl. Mater. Interfaces 2012, 4, 5114–5117. [Google Scholar] [CrossRef]
- Guo, H.; Lei, B.S.; Yu, J.W.; Chen, Y.F.; Qian, J.Q. Immobilization of lipase by dialdehyde cellulose crosslinked magnetic nanoparticles. Int. J. Biol. Macromol. 2021, 185, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Schnell, F.; Kube, M.; Berensmeier, S.; Schwaminger, S.P. Magnetic Recovery of Cellulase from Cellulose Substrates with Bare Iron Oxide Nanoparticles. Chemnanomat 2019, 5, 422–426. [Google Scholar] [CrossRef]
- Cho, E.J.; Jung, S.; Kim, H.J.; Lee, Y.G.; Nam, K.C.; Lee, H.J.; Bae, H.J. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose. Chem. Commun. 2012, 48, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Yee, Y.C.; Hashim, R.; Yahya AR, M.; Bustami, Y. Colorimetric Analysis of Glucose Oxidase-Magnetic Cellulose Nanocrystals (CNCs) for Glucose Detection. Sensors 2019, 19, 2511. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Lee, S.L.; Wei, G.T.; Hsieh, H.C.; Yu, C.Y. Immobilization of cellulase from Trichoderma reesei onto magnetic nanoparticles and effects of ionic liquid on cellulose hydrolysis. J. Biosci. Bioeng. 2009, 108, S112. [Google Scholar] [CrossRef]
- Kuang, G.L.; Wang, Z.C.; Luo, X.Y.; Geng, Z.X.; Cui, J.D.; Bilal, M.; Wang, Z.Y.; Jia, S.R. Immobilization of lipase on hydrophobic MOF synthesized simultaneously with oleic acid and application in hydrolysis of natural oils for improving unsaturated fatty acid production. Int. J. Biol. Macromol. 2023, 242, 124807. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Meng, H.C.; Zhou, L.; Chang, J. Synthesis of Novel Magnetic Cellulose-Chitosan Composite Microspheres and Their Application in Laccase Immobilization. J. Nanosci. Nanotechnol. 2014, 14, 7010–7014. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Munawar, K.; Chee, C.Y.; Pramanik, S.; Halilu, A.; Illias, H.A.; Rizwan, M.; Senthilnithy, R.; Mahanama, K.R.R.; Tripathy, A.; et al. Cellulose supported magnetic nanohybrids: Synthesis, physicomagnetic properties and biomedical applications—A review. Carbohydr. Polym. 2021, 267, 118136. [Google Scholar] [CrossRef] [PubMed]
- Je, H.H.; Noh, S.; Hong, S.G.; Ju, Y.; Kim, J.; Hwang, D.S. Cellulose nanofibers for magnetically-separable and highly loaded enzyme immobilization. Chem. Eng. J. 2017, 323, 425–433. [Google Scholar] [CrossRef]
- Javid, A.; Amiri, H.; Kafrani, A.T.; Rismani-Yazdi, H. Post-hydrolysis of cellulose oligomers by cellulase immobilized on chitosan-grafted magnetic nanoparticles: A key stage of butanol production from waste textile. Int. J. Biol. Macromol. 2022, 207, 324–332. [Google Scholar] [CrossRef]
- Gennari, A.; Simon, R.; Sperotto, N.D.D.; Bizarro, C.V.; Basso, L.A.; Machado, P.; Benvenutti, E.V.; Viegas, A.D.; Nicolodi, S.; Renard, G.; et al. One-step purification of a recombinant beta-galactosidase using magnetic cellulose as a support: Rapid immobilization and high thermal stability. Bioresour. Technol. 2022, 345, 126497. [Google Scholar] [CrossRef]
- Honda, T.; Tanaka, T.; Yoshino, T. Stoichiometrically Controlled Immobilization of Multiple Enzymes on Magnetic Nanoparticles by the Magnetosome Display System for Efficient Cellulose Hydrolysis. Biomacromolecules 2015, 16, 3863–3868. [Google Scholar] [CrossRef]
- Alftrén, J.; Hobley, T.J. Immobilization of cellulase mixtures on magnetic particles for hydrolysis of lignocellulose and ease of recycling. Biomass Bioenergy 2014, 65, 72–78. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Tiwari, R.; Goel, R.; Nain, L. Immobilization of indigenous holocellulase on iron oxide (Fe2O3) nanoparticles enhanced hydrolysis of alkali pretreated paddy straw. Int. J. Biol. Macromol. 2017, 96, 538–549. [Google Scholar] [CrossRef]
- Arevalo-Cid, P.; Isasi, J.; Caballero, A.C.; Martin-Hernandez, F.; Gonzalez-Rubio, R. Effects of shell-thickness on the powder morphology, magnetic behavior and stability of the chitosan-coated Fe3O4 nanoparticles. Bol. Soc. Esp. Ceram. Vidr. 2022, 61, 300–312. [Google Scholar] [CrossRef]
- Hou, C.; Zhu, H.; Wu, D.M.; Li, Y.J.; Hou, K.; Jiang, Y.; Li, Y.F. Immobilized lipase on macroporous polystyrene modified by PAMAM-dendrimer and their enzymatic hydrolysis. Process Biochem. 2014, 49, 244–249. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, Y.; Dong, J.; Tang, X.; Ni, L.; Wang, L. Preparation and characterization of Fe3O4-NH2@4-arm-PEG-NH2, a novel magnetic four-arm polymer-nanoparticle composite for cellulase immobilization. Biochem. Eng. J. 2018, 130, 90–98. [Google Scholar] [CrossRef]
- Lv, K.M.; Shao, W.Y.; Pedroso, M.M.; Peng, J.Y.; Wu, B.; Li, J.H.; He, B.F.; Schenk, G. Enhancing the catalytic activity of a GH5 processive endoglucanase from Bacillus subtilis BS-5 by site-directed mutagenesis. Int. J. Biol. Macromol. 2021, 168, 442–452. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.J.; Gu, C.R.; Zhang, Y.; Xu, J.D.; Bian, Z.P.; Yang, D.; Gu, N. The Effect of Iron Oxide Magnetic Nanoparticles on Smooth Muscle Cells. Nanoscale Res. Lett. 2009, 4, 70–77. [Google Scholar] [CrossRef]
- Yang, R.B.; Reddy, P.M.; Chang, C.J.; Chen, P.A.; Chen, J.K.; Chang, C.C. Synthesis and characterization of Fe3O4/polypyrrole/carbon nanotube composites with tunable microwave absorption properties: Role of carbon nanotube and polypyrrole content. Chem. Eng. J. 2016, 285, 497–507. [Google Scholar] [CrossRef]
- Lv, K.M.; Yu, Z.M.; Pedroso, M.M.; Wu, B.; Gao, Z.; He, B.F.; Schenk, G. Metal Affinity Immobilization of the Processive Endoglucanase EG5C-1 from Bacillus subtilis on a Recyclable pH-Responsive Polymer. ACS Sustain. Chem. Eng. 2021, 9, 7948–7959. [Google Scholar] [CrossRef]
- Asha, P.; Jose, D.; Singh, I.S.B. Purification and characterisation of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresour. Technol. 2016, 213, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, T.R.; Long, L.K.; Ding, S.J. Altering the linker in processive GH5 endoglucanase 1 modulates lignin binding and catalytic properties. Biotechnol. Biofuels 2018, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yang, Y.L.; Tang, X.Z.; Chen, Y.J.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Lin, X.M.; Li, P.P.; Zhang, J.; Wang, S.Q.; Ma, H.L. Effects of low intensity ultrasound on cellulase pretreatment. Bioresour. Technol. 2012, 117, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dhillon, A.; Goyal, A. Enhanced catalytic efficiency of Bacillus amyloliquefaciens SS35 endoglucanase by ultraviolet directed evolution and mutation analysis. Renew. Energy 2020, 151, 1124–1133. [Google Scholar] [CrossRef]
- Shyaula, M.; Regmi, S.; Khadka, D.; Poudel, R.C.; Dhakal, A.; Koirala, D.; Sijapati, J.; Singh, A.; Maharjan, J. Characterization of Thermostable Cellulase from Bacillus licheniformis PANG L Isolated from the Himalayan Soil. Int. J. Microbiol. 2023, 2023, 3615757. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.M.; Qiu, J.H.; Wu, X.L.; Zhang, W.J.; Sakai, E.; Wei, Y. Preparation of Magnetic Chitosan Nanoparticles as Support for Cellulase Immobilization. Ind. Eng. Chem. Res. 2014, 53, 3448–3454. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.H.; He, Y.J.; Cui, G.L.; Abdulrazaq, M.A.; Yan, Y.J. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine-glutaraldehyde dendrimer modified magnetic nanoparticles. Chem. Eng. J. 2018, 351, 258–268. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Hegab, H.E.; Lvov, Y.; Snow, L.D.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. Springerplus 2016, 5, 48. [Google Scholar] [CrossRef]
- Juyal, V.K.; Pathak, A.; Panwar, M.; Thakuri, S.C.; Prakash, O.; Agrwal, A.; Nand, V. Schiff base metal complexes as a versatile catalyst: A review. J. Organomet. Chem. 2023, 999, 122825. [Google Scholar] [CrossRef]
- Ifko, D.; Vasic, K.; Knez, Z.; Leitgeb, M. (Magnetic) Cross-Linked Enzyme Aggregates of Cellulase from T. reesei: A Stable and Efficient Biocatalyst. Molecules 2023, 28, 1305. [Google Scholar] [CrossRef]
- Ahmad, T.; Phul, R.; Khan, H. Iron Oxide Nanoparticles: An Efficient Nano-catalyst. Curr. Org. Chem. 2019, 23, 994–1004. [Google Scholar] [CrossRef]
- Roy, S.D.; Das, K.C.; Dhar, S.S. Conventional to green synthesis of magnetic iron oxide nanoparticles; its application as catalyst, photocatalyst and toxicity: A short review. Inorg. Chem. Commun. 2021, 134, 109050. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, D.; Wang, G.; Zhao, C.W.; Ma, Y.H.; Yang, W.T. Immobilization of cellulase on styrene/maleic anhydride copolymer nanoparticles with improved stability against pH changes. Chem. Eng. J. 2018, 336, 152–159. [Google Scholar] [CrossRef]
- Ingle, A.P.; Rathod, J.; Pandit, R.; da Silva, S.S.; Rai, M. Comparative evaluation of free and immobilized cellulase for enzymatic hydrolysis of lignocellulosic biomass for sustainable bioethanol production. Cellulose 2017, 24, 5529–5540. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef]
- Abraham, R.E.; Verma, M.L.; Barrow, C.J.; Puri, M. Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels 2014, 7, 90. [Google Scholar] [CrossRef]
- Cótica, L.F.; Santos, I.A.; Girotto, E.M.; Ferri, E.V.; Coelho, A.A. Surface spin disorder effects in magnetite and poly(thiophene)-coated magnetite nanoparticles. J. Appl. Phys. 2010, 108, 064325. [Google Scholar] [CrossRef]
- Robatjazi, S.M.; Shojaosadati, S.A.; Khalilzadeh, R.; Farahani, E.V.; Balochi, N. Immobilization of magnetic modified Flavobacterium ATCC 27551 using magnetic field and evaluation of the enzyme stability of immobilized bacteria. Bioresour. Technol. 2012, 104, 6–11. [Google Scholar] [CrossRef]
- Yildiz, E.A.; Pepe, Y.; Erdener, D.; Karatay, A.; Boyacioglu, B.; Ünver, H.; Yapar, G.; Demir, N.; Yildiz, M.; Elmali, A. Effect of group electronegativity on spectroscopic, biological, chromogenic sensing and optical properties of 2-formyl-benzene sulfonic acid sodium salt-based Schiff bases. J. Mol. Struct. 2023, 1286, 135611. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Yuan, J.G.; Wang, Q.; Fan, X.R.; Wang, P. Covalent Immobilization of Cellulases onto a Water-Soluble-Insoluble Reversible Polymer. Appl. Biochem. Biotechnol. 2012, 166, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jiang, J.F.; Liu, L.L.; Shen, J.; Qi, L. Enzyme Reactor Based on Reversible pH-Controlled Catalytic Polymer Porous Membrane. ACS Appl. Mater. Interfaces 2019, 11, 15133–15140. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A. Chitosan based metal-chelated copolymer nanoparticles: Laccase immobilization and phenol degradation studies. Int. Biodeterior. Biodegrad. 2017, 125, 235–242. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Brundle, C.R.; Crist, B.V.; Lahiri, N.; Rosso, K.M. Combined multiplet theory and experiment for the Fe 2p and 3p XPS of FeO and Fe2O3. J. Chem. Phys. 2021, 154, 094709. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Li, S.; Liu, R.T.; Zhang, W.; Xu, H.J.; Hu, Y. Immobilization of Interfacial Activated Candida rugosa Lipase onto Magnetic Chitosan Using Dialdehyde Cellulose as Cross-Linking Agent. Front. Bioeng. Biotechnol. 2022, 10, 946117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.Y. Enzyme immobilization on cellulose matrixes. J. Bioact. Compat. Polym. 2016, 31, 553–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.L.; Li, D.; Yuan, Z.H. Preparation and properties of an immobilized cellulase on the reversibly soluble matrix Eudragit L-100. Biocatal. Biotransformation 2010, 28, 313–319. [Google Scholar] [CrossRef]


| Substrate | Enzyme | Km (mg/mL) | kcat (s−1) | kcat/Km (mL/mg/s) |
|---|---|---|---|---|
| CMC | Free EG5C-1 | 5.3 | 196.2 | 37.0 |
| Immobilized EG5C-1 | 8.2 | 232.8 | 28.4 | |
| Filter paper | Free EG5C-1 | 77.5 | 2.7 | 0.035 |
| Immobilized EG5C-1 | 86.4 | 2.8 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, J.; Wu, B.; Gao, Z.; He, B. Immobilization and Characterization of a Processive Endoglucanase EG5C-1 from Bacillus subtilis on Melamine–Glutaraldehyde Dendrimer-Functionalized Magnetic Nanoparticles. Nanomaterials 2024, 14, 340. https://doi.org/10.3390/nano14040340
Li X, Chen J, Wu B, Gao Z, He B. Immobilization and Characterization of a Processive Endoglucanase EG5C-1 from Bacillus subtilis on Melamine–Glutaraldehyde Dendrimer-Functionalized Magnetic Nanoparticles. Nanomaterials. 2024; 14(4):340. https://doi.org/10.3390/nano14040340
Chicago/Turabian StyleLi, Xiaozhou, Jie Chen, Bin Wu, Zhen Gao, and Bingfang He. 2024. "Immobilization and Characterization of a Processive Endoglucanase EG5C-1 from Bacillus subtilis on Melamine–Glutaraldehyde Dendrimer-Functionalized Magnetic Nanoparticles" Nanomaterials 14, no. 4: 340. https://doi.org/10.3390/nano14040340
APA StyleLi, X., Chen, J., Wu, B., Gao, Z., & He, B. (2024). Immobilization and Characterization of a Processive Endoglucanase EG5C-1 from Bacillus subtilis on Melamine–Glutaraldehyde Dendrimer-Functionalized Magnetic Nanoparticles. Nanomaterials, 14(4), 340. https://doi.org/10.3390/nano14040340







