Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles
Abstract
1. Introduction
Platform Novelty
2. Materials and Methods
2.1. Materials
2.2. Bacterial Groups in This Study (Study’s Approach)
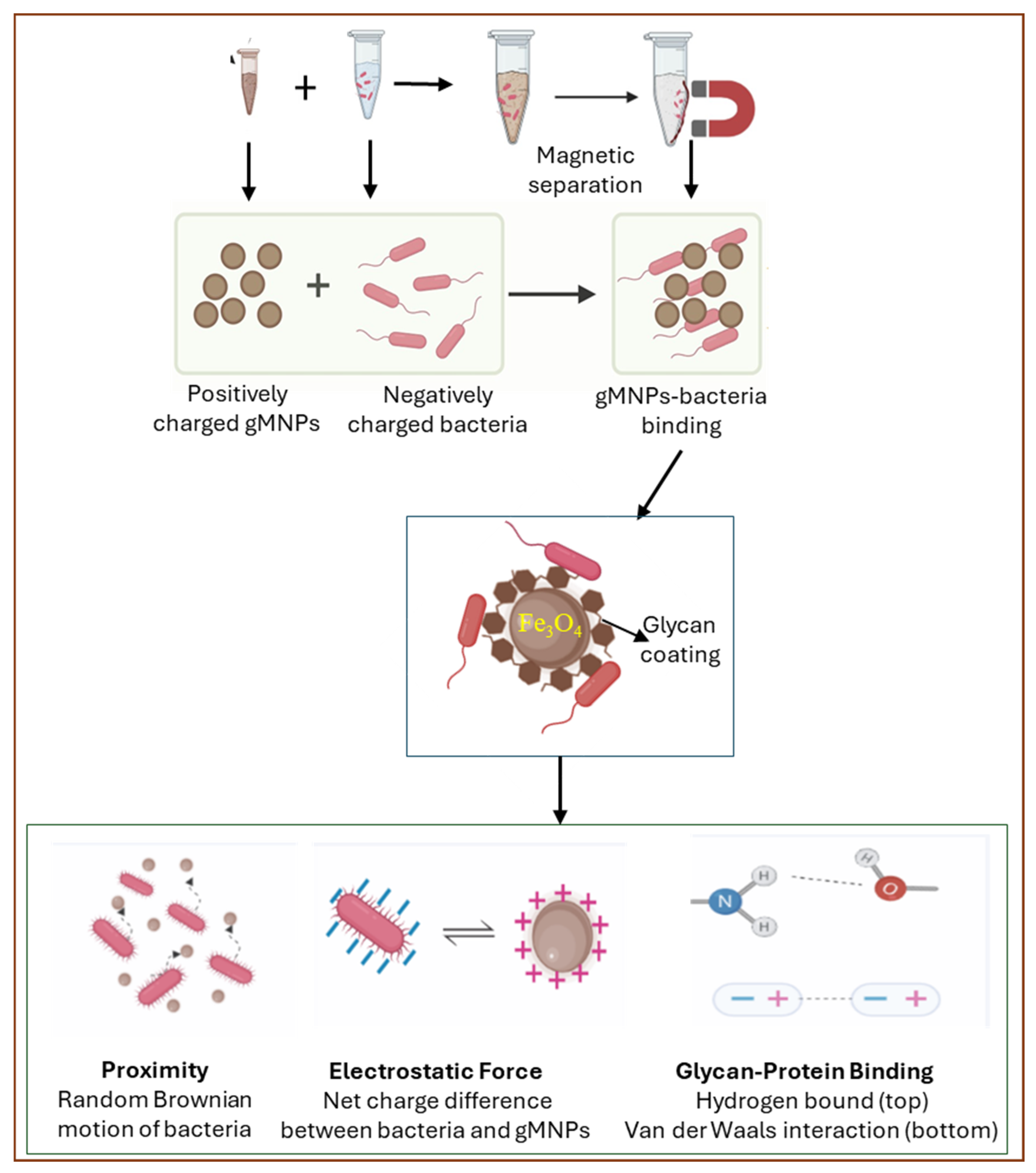
2.3. gMNP Synthesis and Its Binding Capacity
2.4. Zeta Potential (Cell Surface Charge)
2.5. Visualization of Bacterial Cells and gMNP–Cell Interaction
2.6. Statistical Analysis
3. Results and Discussion
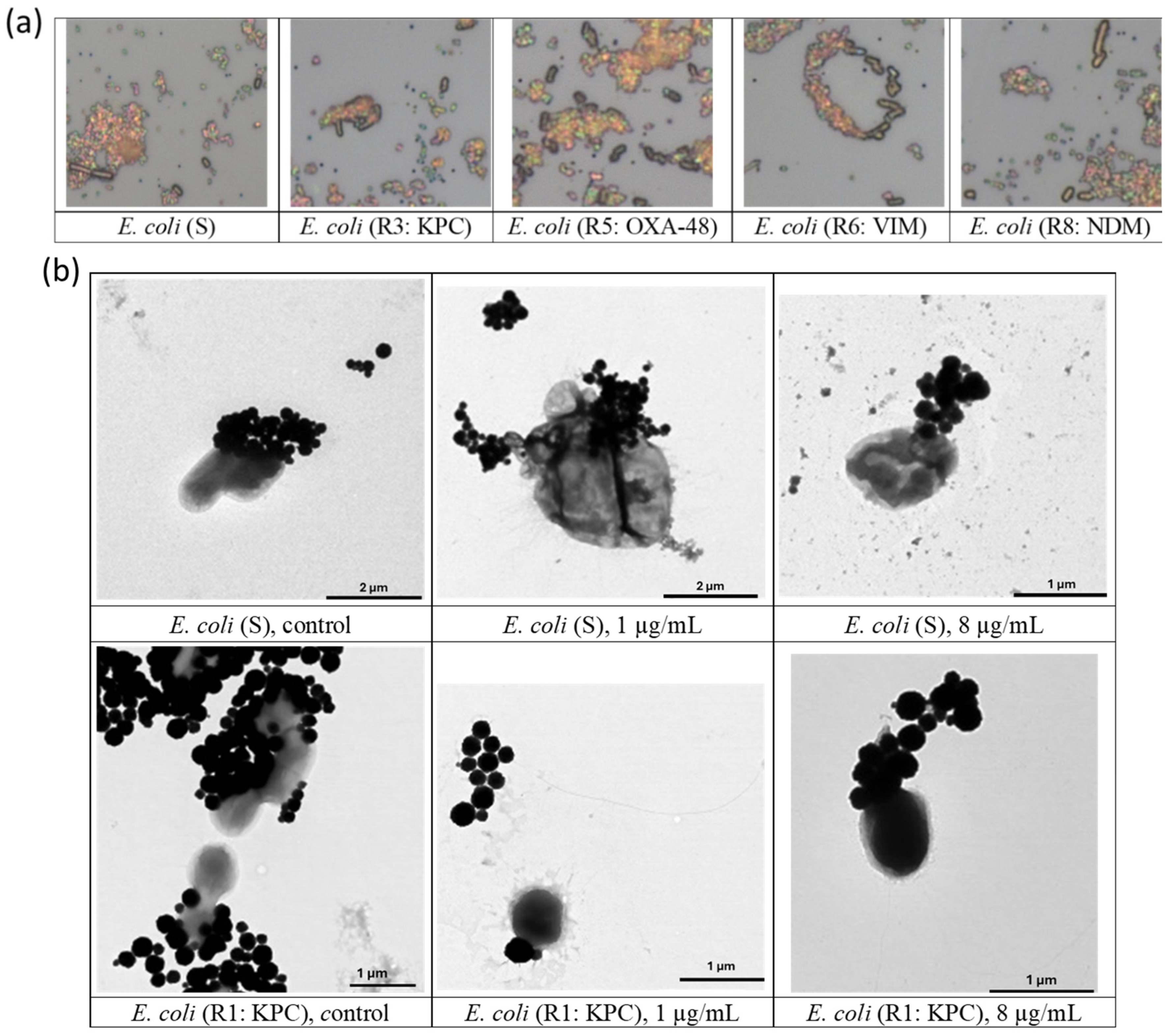
3.1. Visualization of Bacterial Cells
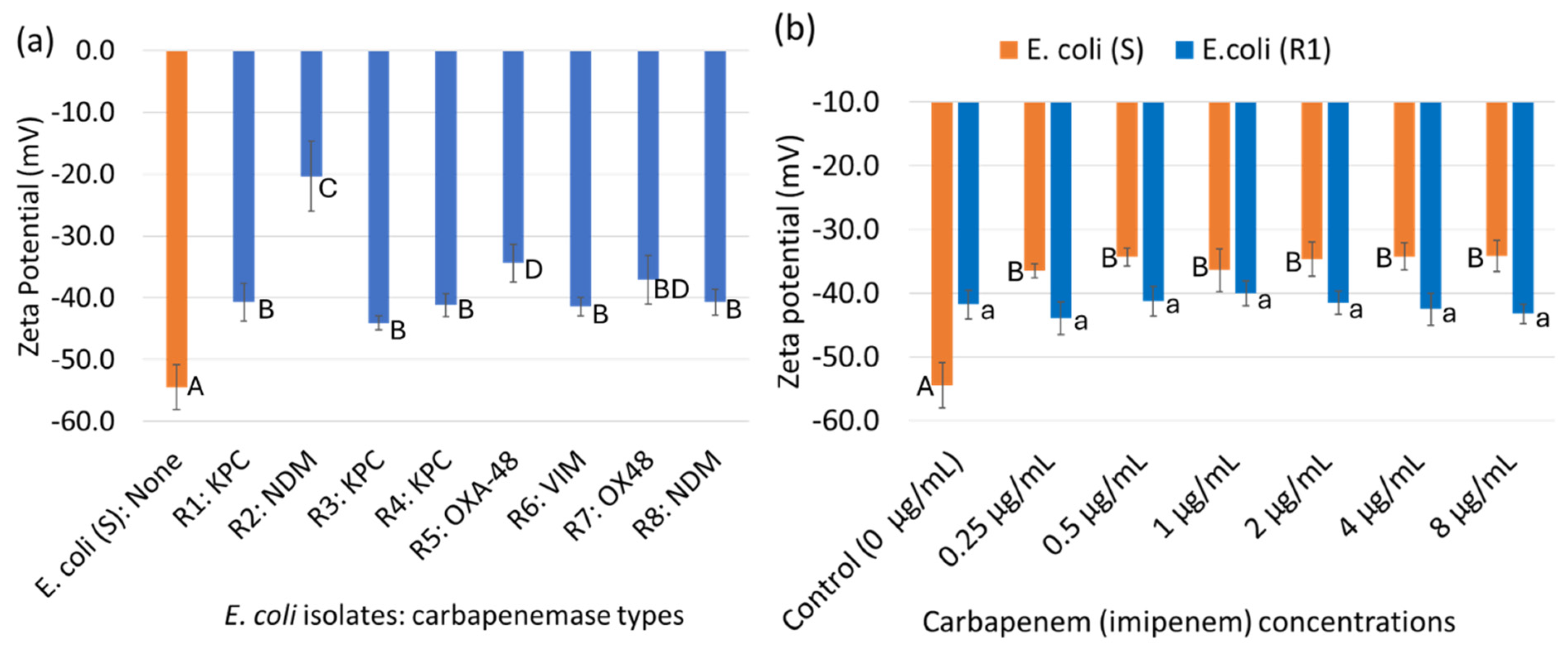
3.2. Cell Surface Charge (Zeta Potential) of Bacterial Cells
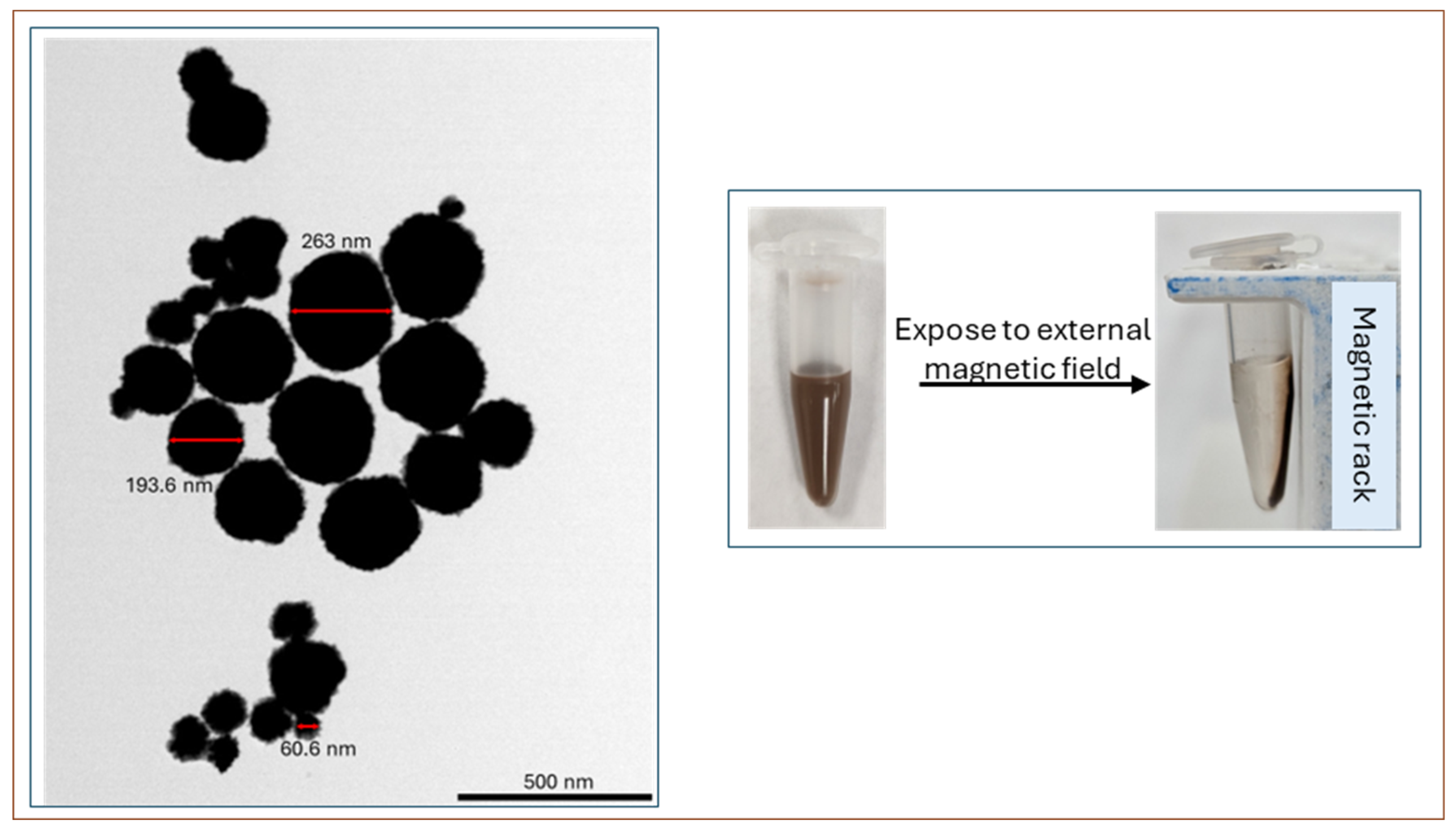
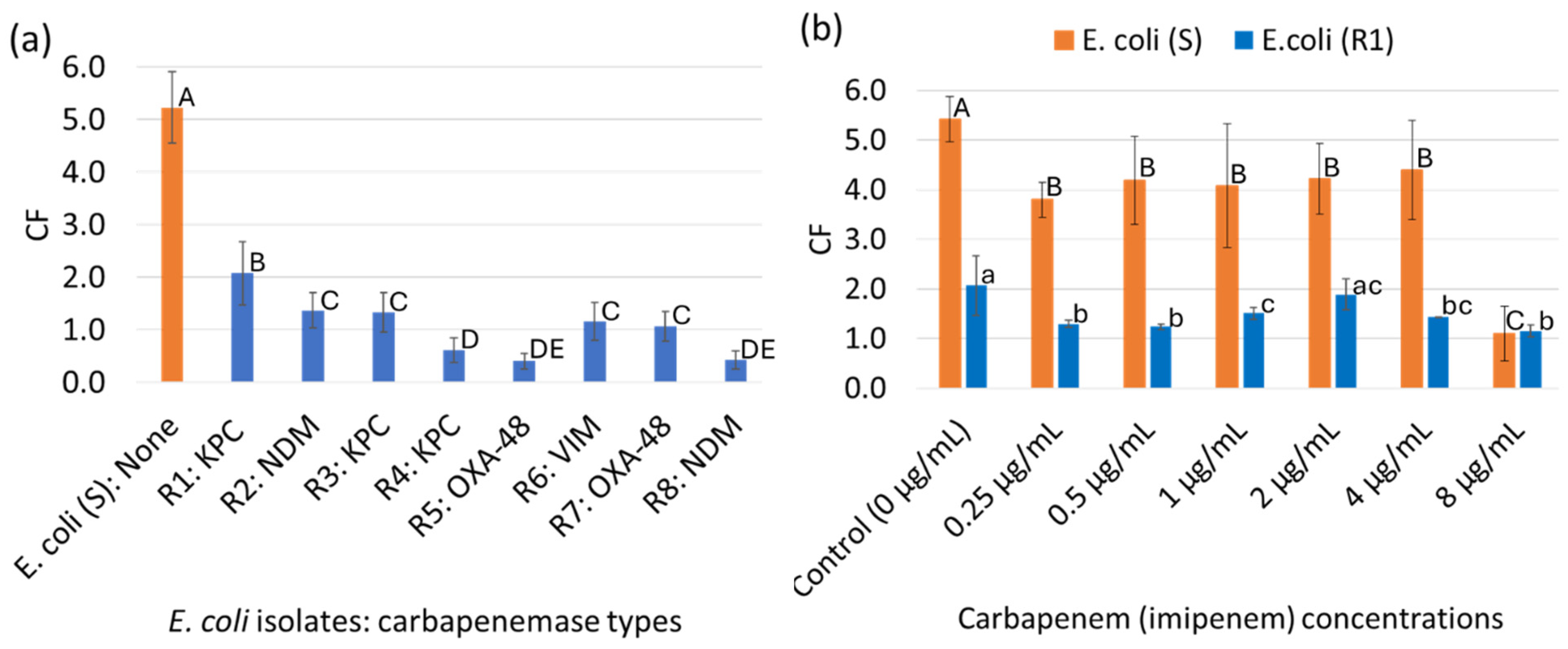
3.3. Characterization of the gMNPs and gMNP–Cell Binding Capacity (Concentration Factor)
3.4. Visualization of gMNP–Cell Interaction
3.5. Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The Role of the Natural Environment in the Emergence of Antibiotic Resistance in Gram-Negative Bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Caliskan-Aydogan, O.; Alocilja, E.C. A Review of Carbapenem Resistance in Enterobacterales and Its Detection Techniques. Microorganisms 2023, 11, 1491. [Google Scholar] [CrossRef]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Sandegren, L. Selection of Antibiotic Resistance at Very Low Antibiotic Concentrations. Upsala J. Med. Sci. 2014, 119, 103–107. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Evolution of Antibiotic Resistance at Non-Lethal Drug Concentrations. Drug Resist. Updates 2012, 15, 162–172. [Google Scholar] [CrossRef]
- ter Kuile, B.H.; Kraupner, N.; Brul, S. The Risk of Low Concentrations of Antibiotics in Agriculture for Resistance in Human Health Care. FEMS Microbiol. Lett. 2016, 363, fnw210. [Google Scholar] [CrossRef]
- Hughes, D.; I Andersson, D. Environmental and Genetic Modulation of the Phenotypic Expression of Antibiotic Resistance. FEMS Microbiol. Rev. 2017, 41, 374–391. [Google Scholar] [CrossRef]
- Reynoso, E.C.; Laschi, S.; Palchetti, I.; Torres, E. Advances in Antimicrobial Resistance Monitoring Using Sensors and Biosensors: A Review. Chemosensors 2021, 9, 232. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Virulence Mech. Bact. Pathog. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Taggar, G.; Rehman, M.A.; Boerlin, P.; Diarra, M.S. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Eljaaly, K.; Alhumaid, S.; Albayat, H.; Al-Adsani, W.; Sabour, A.A.; Alshiekheid, M.A.; Al-Jishi, J.M.; Khamis, F.; Alwarthan, S.; et al. An Overview on Phenotypic and Genotypic Characterisation of Carbapenem-Resistant Enterobacterales. Medicina 2022, 58, 1675. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Weiss, D.S. Heteroresistance: A Cause of Unexplained Antibiotic Treatment Failure? PLoS Pathog. 2019, 15, e1007726. [Google Scholar] [CrossRef]
- Abdeta, A.; Bitew, A.; Fentaw, S.; Tsige, E.; Assefa, D.; Lejisa, T.; Kefyalew, Y.; Tigabu, E.; Evans, M. Phenotypic Characterization of Carbapenem Non-Susceptible Gram-Negative Bacilli Isolated from Clinical Specimens. PLoS ONE 2021, 16, e0256556. [Google Scholar] [CrossRef]
- Dankittipong, N.; Fischer, E.A.J.; Swanenburg, M.; Wagenaar, J.A.; Stegeman, A.J.; de Vos, C.J. Quantitative Risk Assessment for the Introduction of Carbapenem-Resistant Enterobacteriaceae (CPE) into Dutch Livestock Farms. Antibiotics 2022, 11, 281. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States; US Department of Health and Human Services, Centres for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Rebold, N.; Lagnf, A.M.; Alosaimy, S.; Holger, D.J.; Witucki, P.; Mannino, A.; Dierker, M.; Lucas, K.; Coyne, A.J.K.; El Ghali, A.; et al. Risk Factors for Carbapenem-Resistant Enterobacterales Clinical Treatment Failure. Microbiol. Spectr. 2023, 11, e0264722. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. WHO. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 6 September 2022).
- Capozzi, C.; Maurici, M.; Panà, A. Antimicrobial Resistance: It Is a Global Crisis, ‘a Slow Tsunami’. Ig. Sanita Pubblica 2019, 75, 429–450. [Google Scholar]
- About Carbapenem-resistant Enterobacterales; CDC: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/cre/about/index.html (accessed on 12 December 2022).
- Queenan, A.M.; Bush, K. Carbapenemases: The Versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Guerra, B.; Fischer, J.; Helmuth, R. An Emerging Public Health Problem: Acquired Carbapenemase-Producing Microorganisms Are Present in Food-Producing Animals, Their Environment, Companion Animals and Wild Birds. Veter-Microbiol. 2014, 171, 290–297. [Google Scholar] [CrossRef]
- Mills, M.C.; Lee, J. The Threat of Carbapenem-Resistant Bacteria in the Environment: Evidence of Widespread Contamination of Reservoirs at a Global Scale. Environ. Pollut. 2019, 255, 113143. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S.; Pitino, R. Carbapenemase-Producing Bacteria in Food-Producing Animals, Wildlife and Environment: A Challenge for Human Health. Ital. J. Food Saf. 2019, 8, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. Carbapenem-Resistant Enterobacteriaceae in Wildlife, Food-Producing, and Companion Animals: A Systematic Review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Wareham, D.W.; Guerra, B.; Teale, C. Carbapenemase-Producing Enterobacteriaceae and Non-Enterobacteriaceae from Animals and the Environment: An Emerging Public Health Risk of Our Own Making? J. Antimicrob. Chemother. 2014, 69, 287–291. [Google Scholar] [CrossRef]
- Caliskan-Aydogan, O.; Sharief, S.A.; Alocilja, E.C. Rapid Isolation of Low-Level Carbapenem-Resistant E. coli from Water and Foods Using Glycan-Coated Magnetic Nanoparticles. Biosensors 2023, 13, 902. [Google Scholar] [CrossRef]
- Caliskan-Aydogan, O.; Alocilja, E.C. Parallel Biosensor Platform for the Detection of Carbapenemase-Producing E. coli in Spiked Food and Water Samples. Food Control. 2024, 163, 110485. [Google Scholar] [CrossRef]
- Suh, S.H.; Jaykus, L.A.; Brehm-Stecher, B. Advances in Separation and Concentration of Microorganisms From Food Samples; Woodhead Publishing Limited: Sawston, UK, 2013. [Google Scholar] [CrossRef]
- Dester, E.; Alocilja, E. Current Methods for Extraction and Concentration of Foodborne Bacteria with Glycan-Coated Magnetic Nanoparticles: A Review. Biosensors 2022, 12, 112. [Google Scholar] [CrossRef]
- Pitt, W.G.; Alizadeh, M.; Husseini, G.A.; McClellan, D.S.; Buchanan, C.M.; Bledsoe, C.G.; Robison, R.A.; Blanco, R.; Roeder, B.L.; Melville, M.; et al. Rapid separation of bacteria from blood—Review and outlook. Biotechnol. Prog. 2016, 32, 823–839. [Google Scholar] [CrossRef]
- Bohara, R.A.; Pawar, S.H. Innovative Developments in Bacterial Detection with Magnetic Nanoparticles. Appl. Biochem. Biotechnol. 2015, 176, 1044–1058. [Google Scholar] [CrossRef]
- Krishna, V.D.; Wu, K.; Su, D.; Cheeran, M.C.; Wang, J.-P.; Perez, A. Nanotechnology: Review of Concepts and Potential Application of Sensing Platforms in Food Safety. Food Microbiol. 2018, 75, 47–54. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering Nanomaterials-Based Biosensors for Food Safety Detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef]
- Olsvik, O.; Popovic, T.; Skjerve, E.; Cudjoe, K.S.; Hornes, E.; Ugelstad, J.; Uhlén, M. Magnetic Separation Techniques in Diagnostic Microbiology. Clin. Microbiol. Rev. 1994, 7, 43–54. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic Nanoparticles for Environmental and Biomedical Applications: A Review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Benoit, P.W.; Donahue, D.W. Methods for Rapid Separation and Concentration of Bacteria in Food that Bypass Time-Consuming Cultural Enrichment. J. Food Prot. 2003, 66, 1935–1948. [Google Scholar] [CrossRef]
- Boodoo, C.; Dester, E.; Sharief, S.A.; Alocilja, E.C. Influence of Biological and Environmental Factors in the Extraction and Concentration of Foodborne Pathogens Using Glycan-Coated Magnetic Nanoparticles. J. Food Prot. 2023, 86, 100066. [Google Scholar] [CrossRef]
- Peirano, G.; Bradford, P.A.; Kazmierczak, K.M.; Badal, R.E.; Hackel, M.; Hoban, D.J.; Pitout, J.D. Global Incidence of Carbapenemase-Producing Escherichia coli ST131. Emerg. Infect. Dis. 2014, 20, 1928–1931. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Hoelle, J.; Johnson, J.R.; Johnston, B.D.; Kinkle, B.; Boczek, L.; Ryu, H.; Hayes, S. Survey of US Wastewater for Carbapenem-Resistant Enterobacteriaceae. J. Water Health 2019, 17, 219–226. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Hamza, D.; Dorgham, S.; Ismael, E.; El-Moez, S.I.A.; Elhariri, M.; Elhelw, R.; Hamza, E. Emergence of β-Lactamase- and Carbapenemase- Producing Enterobacteriaceae at Integrated Fish Farms. Antimicrob. Resist. Infect. Control. 2020, 9, 67. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Cusack, T.; Ashley, E.; Ling, C.; Rattanavong, S.; Roberts, T.; Turner, P.; Wangrangsimakul, T.; Dance, D. Impact of CLSI and EUCAST Breakpoint Discrepancies on Reporting of Antimicrobial Susceptibility and AMR Surveillance. Clin. Microbiol. Infect. 2019, 25, 910–911. [Google Scholar] [CrossRef] [PubMed]
- Caliskan-Aydogan, O.; Kline, C.Z.; Alocilja, E.C. Cell Morphology as Biomarker of Carbapenem Exposure. J. Antibiot. 2024, 77, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Shrestha, S.; Pote, N.; Alocilja, E.C. Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods. Biosensors 2018, 9, 1. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Petersen, M.; Wang, S.; Lu, X. Detection and Characterization of Antibiotic-Resistant Bacteria Using Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 762. [Google Scholar] [CrossRef]
- Galvan, D.D.; Yu, Q. Surface-Enhanced Raman Scattering for Rapid Detection and Characterization of Antibiotic-Resistant Bacteria. Adv. Health Mater. 2018, 7, e1701335. [Google Scholar] [CrossRef]
- Schaechter, M.; MaalOe, O.; Kjeldgaard, N.O. Dependency on Medium and Temperature of Cell Size and Chemical Composition during Balanced Growth of Salmonella typhimurium. J. Gen. Microbiol. 1958, 19, 592–606. [Google Scholar] [CrossRef]
- Young, K.D. Bacterial Morphology: Why Have Different Shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar] [CrossRef]
- Young, K.D. Bacterial Shape: Two-Dimensional Questions and Possibilities. Annu. Rev. Microbiol. 2010, 64, 223–240. [Google Scholar] [CrossRef]
- Amir, A. Cell Size Regulation in Bacteria. Phys. Rev. Lett. 2014, 112, 208102. [Google Scholar] [CrossRef]
- Harris, L.K.; Theriot, J.A. Surface Area to Volume Ratio: A Natural Variable for Bacterial Morphogenesis. Trends Microbiol. 2018, 26, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S.-I. Measurement and Visualization of Cell Membrane Surface Charge in Fixed Cultured Cells Related with Cell Morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; O’Driscoll, N.H.; Lamb, A.J. Morphological and Ultrastructural Changes in Bacterial Cells as an Indicator of Antibacterial Mechanism of Action. Cell. Mol. Life. Sci. 2016, 73, 4471–4492. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; García-Curiel, A.; Pichardo, C.; Pachón-Ibáñez, M.; Jiménez-Mejías, M.; Pachón, J. Morphological Changes Induced by Imipenem and Meropenem at Sub-Inhibitory Concentrations in Acinetobacter Baumannii. Clin. Microbiol. Infect. 2004, 10, 931–934. [Google Scholar] [CrossRef]
- Horii, T.; Kobayashi, M.; Sato, K.; Ichiyama, S.; Ohta, M. An In-Vitro Study of Carbapenem-Induced Morphological Changes and Endotoxin Release in Clinical Isolates of Gram-Negative Bacilli. J. Antimicrob. Chemother. 1998, 41, 435–442. [Google Scholar] [CrossRef]
- Chang, T.-W.; Weinstein, L. Morphological Changes in Gram-Negative Bacilli Exposed to Cephalothin. J. Bacteriol. 1964, 88, 1790–1797. [Google Scholar] [CrossRef]
- Toprak, E.; Veres, A.; Michel, J.-B.; Chait, R.; Hartl, D.L.; Kishony, R. Evolutionary Paths to Antibiotic Resistance under Dynamically Sustained Drug Selection. Nat. Genet. 2012, 44, 101–105. [Google Scholar] [CrossRef]
- Codjoe, F.S.; Donkor, E.S. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef]
- Smith, H.Z.; Kendall, B. Carbapenem Resistant Enterobacteriaceae; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Murtha, A.N.; Kazi, M.I.; Schargel, R.D.; Cross, T.; Fihn, C.; Cattoir, V.; Carlson, E.E.; Boll, J.M.; Dörr, T. High-Level Carbapenem Tolerance Requires Antibiotic-Induced Outer Membrane Modifications. PLOS Pathog. 2022, 18, e1010307. [Google Scholar] [CrossRef]
- Wojnicz, D.; Kłak, M.; Adamski, R.; Jankowski, S. Influence of Subinhibitory Concentrations of Amikacin and Ciprofloxacin on Morphology and Adherence Ability of Uropathogenic Strains. Folia Microbiol. 2007, 52, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Blair, K.M.; Salama, N.R. Staying in Shape: The Impact of Cell Shape on Bacterial Survival in Diverse Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.P.F.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta Potential beyond Materials Science: Applications to Bacterial Systems and to the Development of Novel Antimicrobials. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- Soon, R.L.; Nation, R.L.; Cockram, S.; Moffatt, J.H.; Harper, M.; Ben Adler, B.; Boyce, J.D.; Larson, I.; Li, J. Different Surface Charge of Colistin-Susceptible and -Resistant Acinetobacter baumannii Cells Measured with Zeta Potential as a Function of Growth Phase and Colistin Treatment. J. Antimicrob. Chemother. 2011, 66, 126–133. [Google Scholar] [CrossRef]
- Matta, L.L.; Alocilja, E.C. Carbohydrate Ligands on Magnetic Nanoparticles for Centrifuge-Free Extraction of Pathogenic Contaminants in Pasteurized Milk. J. Food Prot. 2018, 81, 1941–1949. [Google Scholar] [CrossRef]
- Wilson, W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of Methods for Assessing Bacterial Cell Surface Charge Properties Based on Zeta Potential Measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment Efficiency of Gold Nanoparticles by Gram-Positive and Gram-Negative Bacterial Strains Governed by Surface Charges. J. Nanoparticle Res. 2019, 21, 186. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Development, Surface Modification and Applications in Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Matta, L.L.; Alocilja, E.C. Emerging Nano-Biosensing with Suspended MNP Microbial Extraction and EANP Labeling. Biosens. Bioelectron. 2018, 117, 781–793. [Google Scholar] [CrossRef]
- Sharief, S.A.; Caliskan-Aydogan, O.; Alocilja, E.C. Carbohydrate-Coated Nanoparticles for PCR-Less Genomic Detection of Salmonella from Fresh Produce. Food Control. 2023, 150, 109770. [Google Scholar] [CrossRef]
- Sharief, S.A.; Caliskan-Aydogan, O.; Alocilja, E. Carbohydrate-Coated Magnetic and Gold Nanoparticles for Point-of-Use Food Contamination Testing. Biosens. Bioelectron. X 2023, 13, 100322. [Google Scholar] [CrossRef]
- Matta, L.L.; Harrison, J.M.; Deol, G.S.; Alocilja, E.C. Carbohydrate-Functionalized Nanobiosensor for Rapid Extraction of Pathogenic Bacteria Directly from Complex Liquids with Quick Detection Using Cyclic Voltammetry. IEEE Trans. Nanotechnol. 2018, 17, 1006–1013. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Shi, L.; Shao, L.; Fu, P.; Wang, K.; Xiao, R.; Wang, S.; Gu, B. Rapid Identification and Antibiotic Susceptibility Test of Pathogens in Blood Based on Magnetic Separation and Surface-Enhanced Raman Scattering. Microchim. Acta 2019, 186, 475. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Schurr, M.J.; Leblanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of Bacterial Pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Finlay, B.B. Molecular Mechanisms of Escherichia coli Pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Khan, M.A.; Steiner, T.S. Mechanisms of Emerging Diarrheagenic Escherichia coli Infection. Curr. Infect. Dis. Rep. 2002, 4, 112–117. [Google Scholar] [CrossRef]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Liu, C.; Chen, Z.; Zhou, D. Molecular Pathogenesis of Klebsiella pneumoniae. Future Microbiol. 2016, 9, 1071–1081. [Google Scholar] [CrossRef]
- Rizi, K.S.; Ghazvini, K.; Farsiani, H. Clinical and Pathogenesis Overview of Enterobacter Infections. Rev. Clin. Med. 2020, 6, 146–154. [Google Scholar] [CrossRef]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-Bond Mechanism of the Bacterial Adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef]
- Safina, G.; Vanlier, M.; Danielsson, B. Flow-Injection Assay of the Pathogenic Bacteria Using Lectin-Based Quartz Crystal Microbalance Biosensor. Talanta 2008, 77, 468–472. [Google Scholar] [CrossRef]
- Mi, F.; Guan, M.; Hu, C.; Peng, F.; Sun, S.; Wang, X. Application of Lectin-Based Biosensor Technology in the Detection of Foodborne Pathogenic Bacteria: A Review. Analyst 2021, 146, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Briceno, R.K.; Sergent, S.R.; Benites, S.M.; Alocilja, E.C. Nanoparticle-Based Biosensing Assay for Universally Accessible Low-Cost Tb Detection with Comparable Sensitivity as Culture. Diagnostics 2019, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of Unamplified E. coli O157 DNA Extracted from Large Food Samples Using a Gold Nanoparticle Colorimetric Biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Dester, E.F. Extraction, Concentration, and Detection of Foodborne Pathogens Using Glycan-Coated Magnetic Nanoparticles and a Gold Nanoparticle Colorimetric Biosensor; Michigan State University: East Lansing, MI, USA, 2022. [Google Scholar]
- Matta, L.L. Biosensing Total Bacterial Load in Liquid Matrices to Improve Food Supply Chain Safety Using Carbohydrate-Functionalized Magnetic Nanoparticles for Cell Capture and Gold Nanoparticles for Signaling. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2018. [Google Scholar]
- Chen, C.; Chen, L.; Yi, Y.; Chen, C.; Wu, L.-F.; Song, T. Killing of Staphylococcus aureus via Magnetic Hyperthermia Mediated by Magnetotactic Bacteria. Appl. Environ. Microbiol. 2016, 82, 2219–2226. [Google Scholar] [CrossRef]
- Boodoo, C.; Dester, E.; David, J.; Patel, V.; Kc, R.; Alocilja, E.C. Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples. Biosensors 2023, 13, 608. [Google Scholar] [CrossRef]
- Lau, H.K.; Clotilde, L.M.; Lin, A.P.; Hartman, G.L.; Lauzon, C.R. Comparison of IMS Platforms for Detecting and Recovering Escherichia coli O157 and Shigella flexneri in Foods. JALA J. Assoc. Lab. Autom. 2013, 18, 178–183. [Google Scholar] [CrossRef]
- Liu, D. “Escherichia coli,” Encyclopedia of Microbiology; Schmidt, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Yu, D.; Banting, G.; Neumann, N.F. A Review of the Taxonomy, Genetics, and Biology of the Genus Escherichia and the Type Species Escherichia coli. Can. J. Microbiol. 2021, 67, 553–571. [Google Scholar] [CrossRef]
- Desmarchelier, P.; Fegan, N. “Pathogens in Milk: Escherichia coli”, Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Rivas, L.; Mellor, G.E.; Gobius, K.; Fegan, N. Detection and Typing Strategies for Pathogenic Escherichia coli; Springer Nature: Dordrecht, GX, Netherlands, 2015; ISBN 9781461493778. [Google Scholar]
- Grande, R.; Puca, V.; Muraro, R. Antibiotic Resistance and Bacterial Biofilm. Expert Opin. Ther. Patents 2020, 30, 897–900. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- El-Gamal, M.I.; Brahim, I.; Hisham, N.; Aladdin, R.; Mohammed, H.; Bahaaeldin, A. Recent Updates of Carbapenem Antibiotics. Eur. J. Med. Chem. 2017, 131, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Rousseau, A.; Martins, C.; Blot, M.; Buisson, M.; Mahy, S.; Chavanet, P.; Piroth, L. Comparative Review of Imipenem/Cilastatin versus Meropenem. Med. Et Mal. Infect. 2020, 50, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Shabalkin, I.D.; Komlev, A.S.; Tsymbal, S.A.; Burmistrov, O.I.; Zverev, V.I.; Krivoshapkin, P.V. Multifunctional Tunable ZnFe2 O4@MnFe2O4 Nanoparticles for Dual-Mode MRI and Combined Magnetic Hyperthermia with Radiotherapy Treatment. J. Mater. Chem. B 2023, 11, 1068–1078. [Google Scholar] [CrossRef] [PubMed]

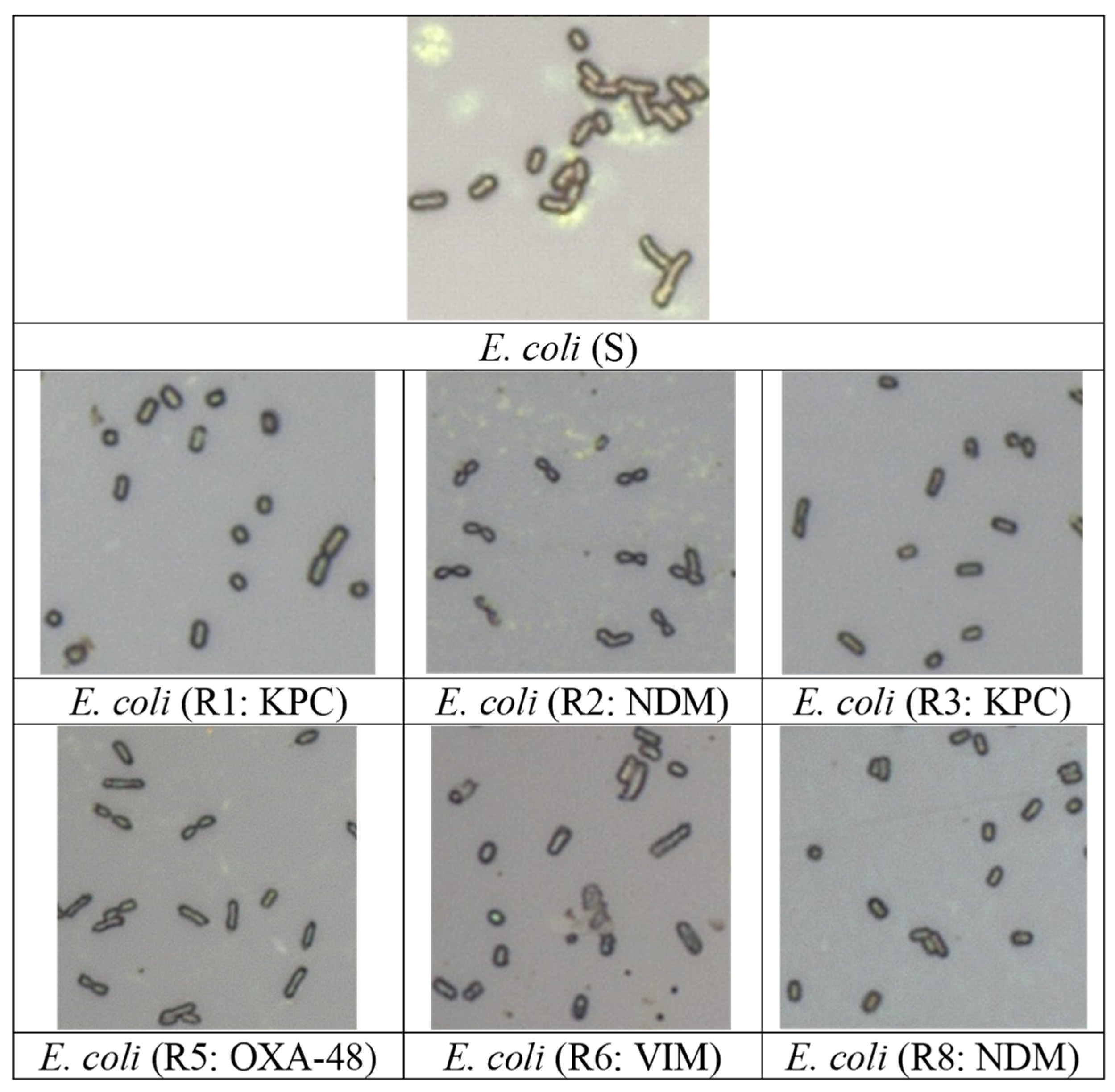
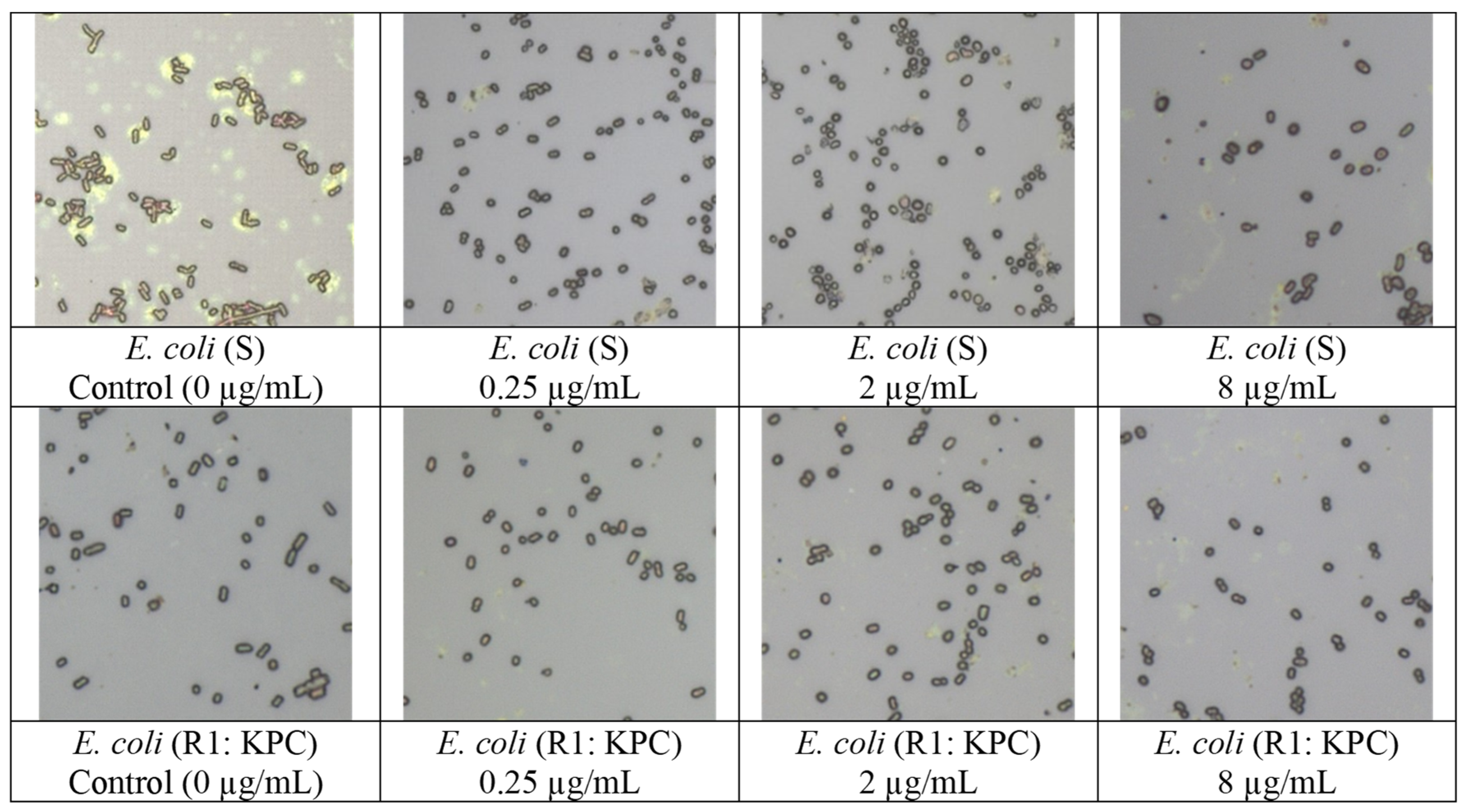
| (1) Carbapenem-Susceptible (S) E. coli vs. Carbapenem-Resistant (R) E. coli | (2) Carbapenem-Exposed E. coli vs. Unexposed E. coli | ||
|---|---|---|---|
| Reference Isolate | Resistant isolates | No antibiotic treatment (Control, 0 μg/mL) | Antibiotic- treatment 0.25 and 0.5 μg/mL, 1 and 2 μg/mL, 4 and 8 μg/mL, |
| E. coli (S) | E. coli (R) R1: KPC (ATCC) * R2: NDM * R3: KPC R4: KPC R5: OXA-48 R6: VIM R7: OXA-48 R8: NDM | E. coli (S) E. coli (R1: KPC) | E. coli (S) E. coli (R1: KPC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caliskan-Aydogan, O.; Zaborney Kline, C.; Alocilja, E.C. Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles. Nanomaterials 2024, 14, 2010. https://doi.org/10.3390/nano14242010
Caliskan-Aydogan O, Zaborney Kline C, Alocilja EC. Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles. Nanomaterials. 2024; 14(24):2010. https://doi.org/10.3390/nano14242010
Chicago/Turabian StyleCaliskan-Aydogan, Oznur, Chloe Zaborney Kline, and Evangelyn C. Alocilja. 2024. "Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles" Nanomaterials 14, no. 24: 2010. https://doi.org/10.3390/nano14242010
APA StyleCaliskan-Aydogan, O., Zaborney Kline, C., & Alocilja, E. C. (2024). Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles. Nanomaterials, 14(24), 2010. https://doi.org/10.3390/nano14242010








