Abstract
WO3/Ag/TiO2 composite photoelectrodes were formed via the high-temperature calcination of a WO3 film, followed by the sputtering of a very thin silver film and deposition of an overlayer of commercial TiO2 nanoparticles. These synthetic photoanodes were characterized in view of the oxidation of a model organic compound glucose combined with the generation of hydrogen at a platinum cathode. During prolonged photoelectrolysis under simulated solar light, these photoanodes demonstrated high and stable photocurrents of ca. 4 mA cm−2 due, on one hand, to the occurrence of the so-called photocurrent doubling and, on the other hand, to the plasmonic effect of Ag nanoparticles. The post-photoelectrolysis analyses of the electrolyte demonstrated the formation of high-value final glucose photo-reforming products, principally gluconic acid, erythrose and formic acid.
1. Introduction
Water splitting is regarded as a sustainable way of producing hydrogen by absorbing and transferring the energy from photons in sunlight. Shortly after the beginning of investigations into the production of hydrogen via photoelectrochemical water splitting, first reported by Fujishima and Honda [1], it was noted that the overall efficiency of the process is controlled by the sluggish counterpart oxygen evolution reaction occurring at the TiO2 photoanode. This observation prompted a series of studies on other more kinetically favorable substrates able to replace water oxidation. Apparently, the first such work was by Kawai and Sakata, who reported [2] using a RuO2/TiO2/Pt photocatalyst, carrying out the oxidation of saccharose and starch into CO2 with simultaneous hydrogen evolution. In such a system, the RuO2/TiO2/Pt particles operated as photoelectrochemical micro-cells with RuO2/TiO2 playing the role of a photoanode and Pt/TiO2 that of a cathode. That work demonstrated, for the first time, the preferential photo-oxidation of polysaccharides and, consequently, of glucose, promoting hydrogen generation both in photocatalytic and photoelectrochemical systems.
Practically, glucose can be obtained from agricultural waste biomass through hydrolysis of its major constituent cellulose and hemicellulose [3,4,5]. The possibility of conducting the conversion of glucose into fine organic products under milder reaction conditions than those required by selective catalytic glucose oxidation prompted a large number of investigations on its photo-reforming. The major part of photo-reforming studies relies on the use of photocatalysts in the form of suspensions of n-type semiconductor nanoparticles [2] where photo-oxidation and reduction reactions take place on the same particle. In most cases, these studies employed TiO2 and modified TiO2 photocatalysts able to capture UV and, in general, only a marginal portion of visible light [6,7,8,9,10,11,12,13,14]. Similarly, modified TiO2 results were reported using Pt-decorated NiTiO3 [15]. An important part of the work regarding photocatalytic processes applied to the conversion of biomass (including glucose) into value-added organic products has been recently reviewed by de Assis et al. [16].
Quite generally, a major problem associated with the use of photocatalyst suspensions with large surface areas is a slow reaction rate and the difficulty of separating the adsorbed final reaction products from the photocatalyst. In order to facilitate product desorption from the photocatalyst surface and increase selectivity, a mixture of water and polar aprotic solvent (acetonitrile) is used in some cases [9,17], which generates environmental problems. Finally, a mixture of photo-oxidation and reduction products is obtained in the unique compartment of the photocatalytic cell. The situation is different in a photoelectrochemical (PEC) system where the photoanode and the (photo)cathode are placed in two different cell compartments separated by a membrane. Consequently, the reduction products, such as hydrogen, may be collected independently in the cathode compartment. In contrast with the photocatalyst suspensions, the PEC configuration also offers the possibility of controlling the photoanodic process separately, e.g., by modifying the external bias voltage imposed on the PEC cell [18].
Only a limited number of published works have been devoted to glucose photo-reforming in PEC systems [19]. Esposito et al. [20], using thin-film WO3 photoanodes, observed a relatively low extent of glucose conversion into CO2, i.e., its total mineralization. However, intermediate oxidation products could not be identified by the authors. In a very recent work, Tian et al. [21], who employed black hydrogenated TiO2 [22] nanorods decorated with “single atom” platinum formed by atomic layer deposition, reported efficient glucose photo-reforming into mainly glucaric acid under simulated sunlight irradiation. Apparently, the performance of the Pt/def.TiO2 photoanode could be maintained over five repeated 5.5 h long glucose photoelectrolysis tests. However, despite the electrochemical reduction treatment of TiO2 nanorods leading to their black coloration, the effective absorption of visible light by the obtained Pt/def-TiO2 photoanodes appeared limited to ca. 430 nm, as shown by the reported incident photon-to-glucaric acid conversion efficiency (IPCE) spectrum.
In the present work, we investigated the oxidation of glucose into value-added organic products using a composite photoanode, consisting of mesoporous WO3 covered with a very thin Ag film and with an overlayer of TiO2 nanoparticles, operating in a PEC cell. We explored, in particular, the plasmonic effect of Ag nanoparticles, as well as the role of the composition of the employed supporting electrolyte. The fabricated composite photoanodes exhibit enhanced visible light absorption, rapid electron/hole separation, and hole transfer towards the electrode–solution interface.
2. Materials and Methods
2.1. Synthesis of WO3 Mesoporous Film
The WO3 films were prepared based on fresh tungstic acid using a sol–gel method. In brief, 0.5 M aqueous solution of Na2WO4 was eluted through a proton exchange resin: Dowex 50 WX2-200 (Saint Louis, MO, USA). The solution was collected in ethanol to prevent the condensation of tungstic acid to form polyoxoanions. After elution was complete, the solution was concentrated under vacuum to around ∼0.5 M before the addition of polyethylene glycol (PEG) 300 and kept under continuous stirring. The WO3/PEG ratio used in the precursor was 0.4 w/w. The films were obtained by a sequential layer-by-layer deposition and annealing method. The creation of a complex between H2WO4 and the hydrophilic polyethylene glycol delayed the formation of fully crystallized monoclinic tungsten trioxide below a temperature of ∼500 °C. The precursor solution was coated via the doctor blading method and briefly air-dried onto the F-doped SnO2 (FTO)-covered glass substrates, provided by Sigma Aldrich, at a resistance of 7 ohms/square. Then, films were annealed in the flow of oxygen for 30 min at 550 °C. The process of WO3 film preparation is presented in Scheme 1. The WO3 films used in this work had three layers of precursor solution, and each of them was annealed in O2 at 550 °C for 30 min [23]. The WO3 film final thickness employed to build hybrid photoanodes was ca. 1.4 µm, as determined by cross-sectional scanning electron microscopy (SEM), as shown in Figure 1a.
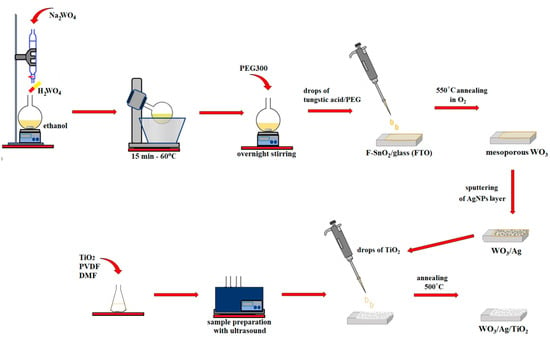
Scheme 1.
The preparation process of WO3/Ag/TiO2 film electrodes used in glucose photo-reforming experiments.
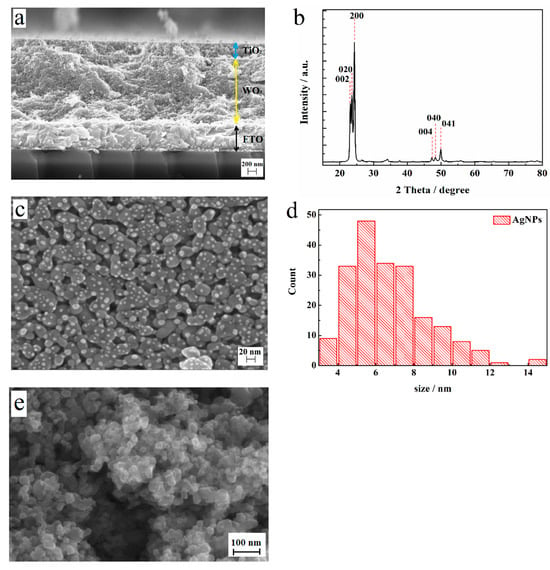
Figure 1.
(a) A cross-sectional SEM image of the WO3/Ag/TiO2 film electrode. (b) The X-ray diffraction pattern of a WO3 film annealed at 550 °C with a perfect monoclinic crystalline structure. (c) A top-view SEM image of the WO3 film covered with silver nanoparticles. (d) A particle size distribution histogram of Ag NPs. (e) A top-view SEM image of the WO3/Ag/TiO film.
2.2. Preparation of Silver Nanoparticles
Silver nanoparticles were fabricated via the deposition of thin films by the electron beam physical vapor deposition technique (e-PVD, Lesker PVD 75, Pittsburgh, PA, USA). A thickness of 1 nm was chosen to form well-separated semi-spherical particles. The thickness and the evaporation rate of the layers were measured during the evaporation process with the use of two quartz crystal microbalance sensors (QCMs, Inficon, 6 MHz gold-coated crystals, Bad Ragaz, Switzerland) placed close to the crucible and the sample, respectively. The sensors were calibrated prior to the experiment. Silver catalyst formed islands with sizes ranging from a few to more than 10 nm, as shown on the SEM image in Figure 1c. The particle size distribution histogram of silver nanoparticles is presented in Figure 1d.
2.3. The TiO2 Overlayer
Subsequently, the WO3/Ag film was covered with a ca. 0.3 μm thick layer of P25 TiO2 (from Ovonics) nanoparticles, deposited following the previously described method [24]. The TiO2 layer of WO3/Ag/TiO2 photoanode is shown in the cross-sectional SEM image in Figure 1a and in the top-view SEM image in Figure 1e. Both images show a three-dimensional structure composed of TiO2 crystals of different sizes.
All SEM images, as well as the morphology of the silver-coated samples, were investigated using a field emission scanning electron microscope (SEM, Zeiss, Sigma HV, Oberkochen, Germany) equipped with the in-lens secondary electrons detector. The X-ray powder diffraction (XRD) and pattern of the WO3 films were obtained by a Siemens D500 diffractometer equipped with a high-resolution semiconductor Si/Li detector using Cu Kα radiation.
The photocurrent-potential (j-E) and incident photon-to-current conversion efficiency (IPCE) measurements were performed in a Teflon cell equipped with a quartz window (with 45 mL of anolyte). The composite photoanode was illuminated through the FTO substrate (exposed surface area 0.28 cm2). The simulated AM 1.5G (100 mW cm−2) illumination was obtained from an Oriel 150 W solar simulator. All electrochemical experiments were measured in a three-electrode system with a composite photoanode, separated by a Nafion membrane from a platinum grid cathode, and a reference electrode (Ag/AgCl, E = 0.197 V vs. SHE). The incident photon-to-current conversion efficiency measurements of the photoanodes were obtained using light from a 150 W xenon lamp passing through a photoelectric spectrometer featuring a monochromator with a bandwidth of 10 nm. The products of glucose photo-reforming were identified after the long-term light exposition (5 to 20 h) of the composite photoanode (ca. 0.9 cm2 surface area).
All chromatographic analyses were performed with a GC-MS-QP2010 Ultra gas chromatograph interfaced with a single quadrupole mass spectrometer equipped with AOC-5000 autosampler manufactured by Shimadzu, Canby, OR, USA. GCMS solution v4.41 software was used for data acquisition and processing.
The mineralization of glucose was monitored with a TOC-5050A total organic carbon analyzer connected with an ASI-5000A autosampler manufactured by Shimadzu, Kyoto, Japan.
3. Results
The consecutive steps involved in the WO3/Ag/TiO2 film preparation process are shown in Scheme 1.
Figure 1 presents the morphological (Figure 1a,c,d) and structural (Figure 1b) characteristics of the formed film. On the cross-sectional SEM image (Figure 1a), we can clearly identify the FTO substrate, the WO3 film, and the TiO2 overlayer. However, due to the use SEM magnification, the silver inter-layer cannot be distinguished. The SEM image in Figure 1c shows the typical morphology of the WO3 film composed of partly fused NPs with sizes between 30 and 50 nm and numerous pores of similar sizes. To achieve a quasi-spherical shape, the nano-island films were annealed at 150 °C for 5 min on a standard hot plate. The XRD pattern in Figure 1b for the monoclinic WO3 film reveals dominant (002), (020), and (200) peaks, as well as less intense (004), (040), and (041) peaks, indicating the preferential orientation of the crystallites parallel to the FTO substrate. Figure 1c also shows the layer of silver NPs deposited by sputtering on the surface of the WO3 film. As indicated by the histogram in Figure 1d, the major part of Ag NPs has sizes in the range of 4–8 nm.
Figure 2 shows the absorbance spectra of FTO substrates covered with additional constituent layers depicting their input to the overall absorptivity of the working photoanode. The addition of Ag nanoparticles at the WO3/TiO2 interface resonantly increases absorbance at around 430 nm, proving the excitation of the localized surface plasmons. The resonant nature of the phenomenon is clearly seen when presented as a relative absorbance related to the reference FTO/WO3/TiO2 sample that is presented in Figure 2b. The increased slope around 500 nm comes from the asymmetric size distribution of the nanoparticles shown in Figure 1d, while the short-wavelength side of the curve is also affected by the absorption edge of the TiO2. It is worth mentioning that the parameters of the resonance, like spectral position, height, and width, depend on the material composition, size, and size distribution of the nanoparticles, which can be controlled simply by the nominal thickness of the deposited layers and the evaporation conditions.
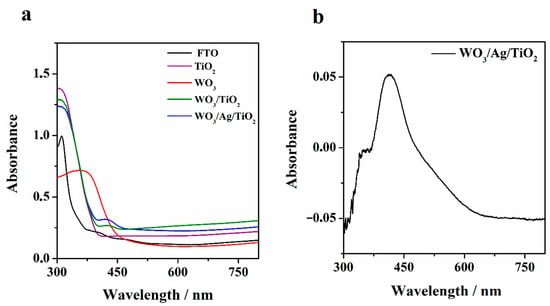
Figure 2.
(a) Absorbance spectra of FTO substrates: bare (black), with a TiO2 layer (violet), with a WO3 layer (red), with a TiO2 and WO3 bilayer (green), and with Ag nanoparticles placed between the WO3 and TiO2 layers. (b) Relative absorbance spectra of the Ag-enhanced photoanode related to the reference FTO/WO3/TiO2 sample.
In order to highlight the role of silver nano-catalyst, the photoelectrochemical characterization and prolonged glucose photoelectrolysis experiments have been performed for the WO3/Ag/TiO2 photoanode, as well as for a similar hybrid photoanode, WO3/TiO2, without silver and for bare WO3 and TiO2. Figure 3 represents the IPCE spectra measured for four electrodes in the solution of the NaCl/Na2SO4 supporting electrolyte with 0.01 mol L−1 of glucose. We note significantly larger IPCEs for the WO3/Ag/TiO2 electrode over the whole 350–490 nm range of wavelengths. The fact that both curves, WO3/Ag/TiO2 and WO3/TiO2, are almost parallel may suggest that the presence of silver enhances light reflection by the TiO2 overlayer and, at the same time, acts as an electrocatalyst, reducing charge carrier recombination at the surface of WO3.
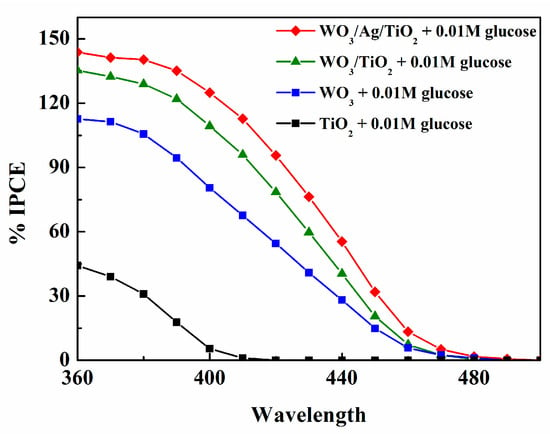
Figure 3.
IPCE spectra for WO3, TiO2, WO3/Ag/TiO2, and WO3/TiO2 photoanodes measured at 0.6 V vs. Ag/AgCl in a 0.01 M NaCl/Na2SO4 electrolyte of pH 7 containing 0.01 M glucose.
The latter hypothesis is consistent with the photocurrent vs. time plots recorded during 5 h long electrolysis of glucose shown in Figure 4.
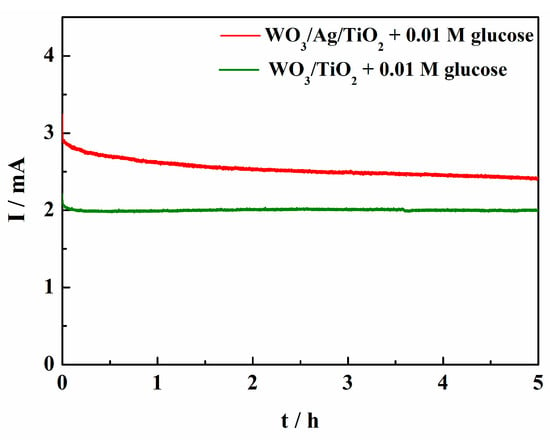
Figure 4.
The photo-currents of glucose oxidation measured during 5 h long electrolysis conducted at 0.6 V vs. Ag/AgCl in a 0.01 M NaCl/Na2SO4 electrolyte of pH 7, using WO3/Ag/TiO2 and WO3/TiO2 photoanodes irradiated with simulated AM 1.5 G solar light.
In Figure 5, we present photocurrent-potential (j-E) plots for the WO3/TiO2 and WO3/Ag/TiO2 photoanodes in a 0.01 M NaCl/Na2SO4 electrolyte of pH 7. Under simulated solar AM 1.5G (100 mW cm−2) irradiation, both photoanodes reached the plateau of ca. 2.2 mA cm−2. A table reporting a comparison of our results with the papers cited in the present article is presented in Table S1. The addition of 0.01 mol L−1 of glucose to the supporting electrolyte led to very large increases in both photocurrents, with a larger value of ca. 4.1 mA cm−2 obtained for the WO3/Ag/TiO2. This measured value is much higher than the photocurrent obtained for WO3/TiO2, as well as those for bare WO3 and TiO2 photoanodes. An interesting result was also provided by measurements performed for the WO3/Ag/TiO2 photoanode in a 0.01 M NaCl/Na2SO4 electrolyte of pH 7 with 0.01 mol L−1 of glucose under 3 sun illumination (Figure 6), where an impressive increase in the photocurrent, during the backside illumination, up to ca. 16 mA cm2 was observed. As mentioned above, this is clearly related to the light reflection by the quasi-continuous layer of silver nanoparticles and by the TiO2 overlayer.
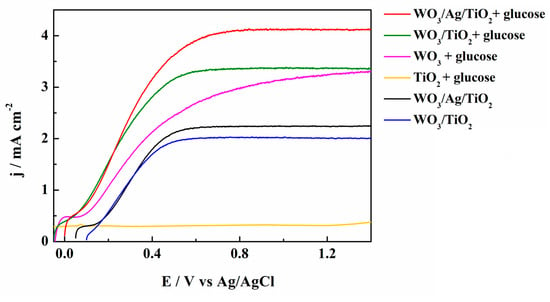
Figure 5.
Photocurrent densities vs. imposed potential (j-E) plots in a 0.01 M NaCl/Na2SO4 electrolyte of pH 7 containing 0.01 M glucose using WO3, TiO2, WO3/TiO2 and WO3/Ag/TiO2 recorded under simulated AM 1.5G irradiation.
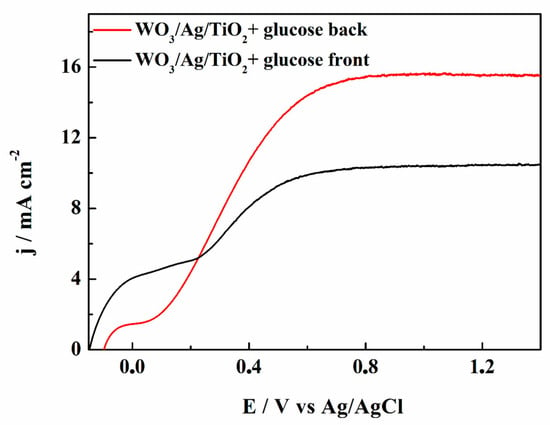
Figure 6.
Photocurrent-potential plots for the WO3/Ag/TiO2 electrode recorded in a 0.01 M NaCl/Na2SO4 electrolyte of pH 7 with 0.01 M glucose under 3 sun illumination from the front side (black curve) and from the back side (red curve).
The IPCEs largely exceeding 100% shown in Figure 3 require an additional comment regarding the mechanism of glucose photo-oxidation. As in the case of numerous other organic compounds [25], glucose photo-oxidation involves so-called photocurrent doubling [18]. This means that the initial step of the reaction, involving the transfer of the positive hole generated in the valence band of WO3 to the adsorbed molecule of glucose, is followed by the injection, carried out by the formed radical species, of an electron to the conduction band of WO3. This corresponds well to the formation of gluconic acid—a two-electron oxidation product. Gluconic acid was by far the most prominent glucose photo-oxidation product obtained after the 5 h long electrolysis, as shown in Figure 4, with much higher Faradaic yields obtained for the WO3/Ag/TiO2 photoanode (cf. Figure 7b). The presence of significant amounts of arabinose and erythrose in the analyzed post-electrolysis solution suggests that the main glucose photo-reforming pathway was pathway (A) represented in the proposed reaction mechanism shown in Figure 8. We note that the formation of arabinose and erythrose, involving an exchange of two additional electrons, is accompanied by the elimination of formic acid.
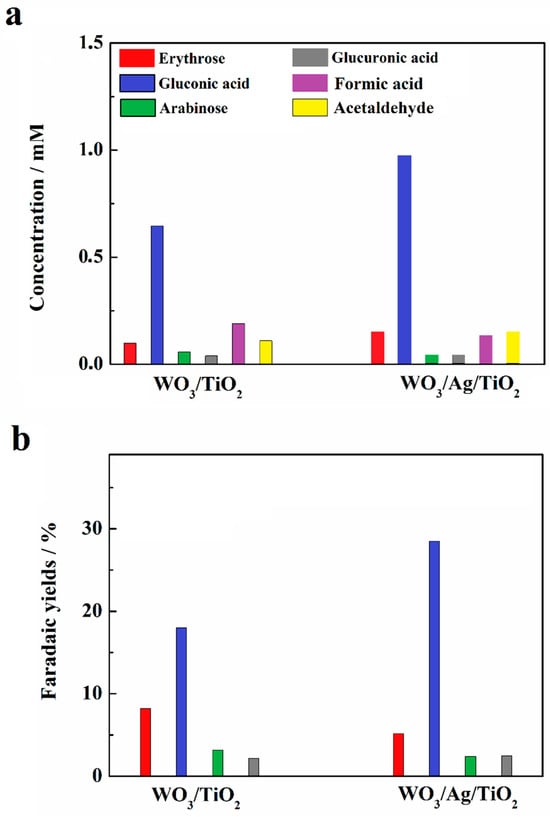
Figure 7.
Concentrations (a) and Faradaic yields (b) of glucose photo-reforming products collected after electrolysis performed following the conditions depicted in the legend of Figure 3.
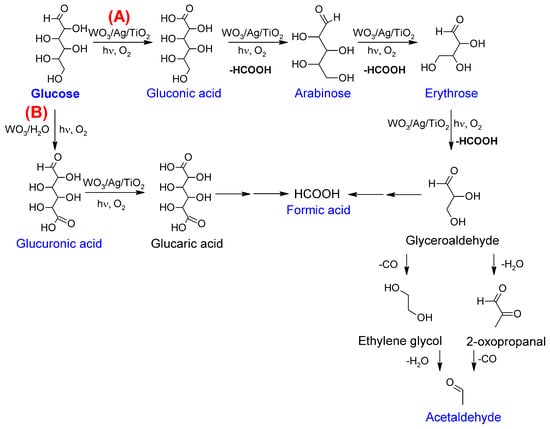
Figure 8.
The proposed reaction pathway of glucose reforming over the WO3/Ag/TiO2 photoanode; products quantified in this work are shown in blue. (A) main pathway; (B) parallel pathway.
As shown in Figure 7, the analyses also revealed other photoelectrolysis products, namely glucuronic acid and acetaldehyde. The formation of acetaldehyde was previously reported during the photo-oxidation of glycerol at TiO2-based photocatalysts [26]. The comparison of our analytical data with the literature [7,15,27,28,29,30] led us to identify the pathway (B) as a parallel route for the glucose photo-oxidation reaction (Figure 8).
Analyses of the extent of glucose remaining in the solution at the end of photoelectrolyses showed conversions of 26% and 30%—the larger one was for the WO3/Ag/TiO2 photoanode. On the other hand, total organic carbon (TOC) analyses indicated the absence of glucose mineralization into carbon dioxide at both photoanodes.
4. Conclusions
In summary, we demonstrated the efficient photoelectrochemical oxidation of glucose into principally gluconic acid with a high faradaic yield of almost 30%, achieved using the WO3/Ag/TiO2 photoanode. The latter photoanode attained much larger photocurrents due to the combination of the optical and electrocatalytic effects induced by the presence of silver. A careful analysis of all formed organic products led us to propose a glucose photo-oxidation mechanism.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano14242001/s1, Figure S1. (a) SEM micrographs of Ag nanoparticles on glass/FTO/WO3 substrates deposited with nominal thickness labeled in the left upper corner in red. (b) Histograms of NPs diameter for subsequent nominal thicknesses of Ag layer. Figure S2. The absorbance (Abs) of samples with Ag nanoparticles deposited on glass/FTO/WO3 substrates subtracted from the absorbance (Absref) of reference samples, i.e., those without nanoparticles. The resonant shape of the presented curves and the nanoparticle size-dependent position of the peaks in the blue spectral region indicate that the observed maxima are associated with LSPR excitation on the silver nanoparticles. Figure S3. Chromatogram of the reacted sample, illustrating the formation of acetaldehyde and formic acid as products of glucose reforming. Figure S4. Chromatogram of the reacted sample, illustrating the formation of monosaccharides and uronic acids as products of glucose reforming. Note that due to co-elution, gluconic acid, and erythrose were quantified using the characteristic fragmentation ions. For the analysis of products, the glucose peak was removed to avoid oversaturating the MS detector. Table S1. PEC performances of the photoanode used in this work compared with those reported by other authors cited in the article. References [31,32] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.A. and K.J.-P.; methodology, J.A. and K.J.-P., B.W., P.W. and K.M.; formal analysis, J.A.; investigation, K.J.-P., B.W. and P.W.; resources, J.A., B.W., P.W. and K.M.; data curation, K.J.-P., B.W. and P.W.; writing—original draft preparation, J.A.; writing—review and editing, K.J.-P., B.W., P.W. and K.M.; visualization, K.J.-P., B.W. and P.W.; supervision, J.A.; project administration, J.A.; funding acquisition, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish National Science Center, OPUS grant No. UMO-2019/33/B/ST4/02718 (J.A.).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Granone, L.I.; Sieland, F.; Zheng, N.; Dillerta, R.; Bahnemann, D.W. Photocatalytic Conversion of Biomass into Valuable Products: A Meaningful Approach? Green Chem. 2018, 20, 1169–1192. [Google Scholar] [CrossRef]
- Da Vià, L.; Recchi, C.; Gonzalez-Yanez, E.O.; Davies, T.E.; Lopez-Sanchez, J.A. Visible light selective photocatalytic conversion of glucose by TiO2. Appl. Catal. B Environ. 2017, 202, 281–288. [Google Scholar] [CrossRef]
- Bellardita, M.; Garcia-Lopez, E.I.; Marci, G.; Megna, B.; Pomilla, F.R.; Palmisano, L. Photocatalytic conversion of glucose in aqueous suspensions of heteropolyacid-TiO2 composites. RSC Adv. 2015, 5, 59037–59047. [Google Scholar] [CrossRef]
- Chong, R.; Li, J.; Ma, Y.; Zhang, B.; Han, H.; Li, C. Selective conversion of aqueous glucose to value-added sugar aldose on TiO2-based photocatalysts. J. Catal. 2014, 314, 101–108. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Kurzydlowski, K.; Grzonka, J.; Chernyayeva, O.; Lisovytskiy, D. Low-temperature ultrasound-promoted synthesis of Cr–TiO2-supported photocatalysts for valorization of glucose and phenol degradation from liquid phase. Appl. Catal. B Environ. 2013, 134–135, 136–144. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Bielejewska, A. High-value chemicals obtained from selective photo-oxidation of glucose in the presence of nanostructured titanium photocatalysts. Bioresour. Technol. 2011, 102, 11254–11257. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Sampaio, M.J.; Marques, R.R.N.; Ferreira, L.A.; Tavares, P.B.; Silva, A.M.T.; Faria, J.L. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B Environ. 2015, 178, 82–90. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Yamamoto, D.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic hydrogen production with aid of simultaneous metal deposition using titanium dioxide from aqueous glucose solution. Int. J. Hydrogen Energy 2013, 38, 5517–5524. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Al-Mazroai, L.S.; Dickinson, A.; Greaves, J.; James, D.; Millard, L.; Pedrono, F. Sustainable H2 gas production by photocatalysis. J. Photochem. Photobiol. A Chem. 2010, 216, 115–118. [Google Scholar] [CrossRef]
- Kampouri, S.; Stylianou, K.C. Dual-Functional Photocatalysis for Simultaneous Hydrogen Production and Oxidation of Organic Substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Parrino, F.; Bellardita, M.; García-López, E.I.; Marcì, G.; Loddo, V.; Palmisano, L. Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Nwosu, U.; Zhao, H.; Kibria, M.; Hu, J. Unlocking Selective Pathways for Glucose Photoreforming by Modulating Reaction Conditions. ACS Sustain. Chem. Eng. 2022, 10, 5867–5874. [Google Scholar] [CrossRef]
- de Assis, G.C.; Silva, I.M.A.; dos Santos, T.G.; dos Santos, T.V.; Meneghetti, M.R.; Meneghetti, S.M.P. Photocatalytic processes for biomass conversion. Catal. Sci. Technol. 2021, 11, 2354–2360. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A. Room Temperature Versatile Conversion of Biomass-Derived Compounds by Means of Supported TiO2 Photocatalysts. J. Mol. Catal. A Chem. 2013, 366, 156–162. [Google Scholar] [CrossRef]
- Jakubow-Piotrowska, K.; Witkowski, B.; Augustynski, J. Photoelectrocatalytic hydrogen generation coupled with reforming of glucose into valuable chemicals using a nanostructured WO3 photoanode. Commun. Chem. 2022, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.M.; Di Franco, F.; Loddo, V.; Bellardita, M.; Santamaria, M. Photoelectrolysis of glucose and fructose containing solution in PGM-free cells for hydrogen and valuable chemicals production. Int. J. Hydrogen Energy 2024, 87, 1277–1287. [Google Scholar] [CrossRef]
- Esposito, D.V.; Forest, R.; Chang, Y.; Gaillard, N.; McCandless, B.E.; Hou, S.; Lee, K.H.; Birkmire, R.W.; Chen, J.G. Photoelectrochemical reforming of glucose for hydrogen production using a WO3-based tandem cell device. Energy Environ. Sci. 2012, 5, 9091–9099. [Google Scholar] [CrossRef]
- Tian, Z.; Da, Y.; Wang, M.; Dou, X.; Cui, X.; Chen, J.; Jiang, R.; Xi, S.; Cui, B.; Luo, Y.; et al. Selective photoelectrochemical oxidation of glucose to glucaric acid by single atom Pt decorated defective TiO2. Nat. Commun. 2023, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmannn, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Augustynski, J. Charge carrier transport in nanostructured anatase TiO2 films assisted by the self-doping of nanoparticles. J. Phys. Chem. 1998, 102, 7820–7828. [Google Scholar] [CrossRef]
- Solarska, R.; Santato, C.; Jorand-Sartoretti, C.; Ulmann, M.; Augustynski, J. Photoelectrolytic oxidation of organic species at mesoporous tungsten trioxide film electrodes under visible light illumination. J. Appl. Electrochem. 2005, 35, 715–721. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Karamerou, E.E.; Kondarides, D.I. Kinetics and mechanism of glycerol photo-oxidation and photo-reforming reactions in aqueous TiO2 and Pt/TiO2 suspensions. Catal. Today 2013, 209, 91–98. [Google Scholar] [CrossRef]
- Lan, L.; Daly, H.; Sung, R.; Tuna, F.; Skillen, N.; Robertson, P.K.J.; Hardacre, C.; Fan, X. Mechanistic Study of Glucose Photo-reforming over TiO2-Based Catalysts for H2 Production. ACS Catal. 2023, 13, 8574–8587. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Palmisano, L. Photocatalytic formation of H2 and value-added chemicals in aqueous glucose (Pt)-TiO2 suspension. Int. J. Hydrogen Energy 2016, 41, 5934–5947. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, P.; Wu, X.; Wang, A.; Zheng, D.; Wang, S.; Chen, Z.; Larter, S.; Li, Y.; Su, B.-L.; et al. Plasmon enhanced glucose photoreforming for arabinose and gas fuel co-production over 3DOM TiO2-Au. Appl. Catal. B Environ. 2021, 291, 120055. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Nasillo, G.; Palmisano, L. Photocatalytic Solar Light H2 Production by Aqueous Glucose Reforming. Eur. J. Inorg. Chem. 2018, 2018, 4522–4532. [Google Scholar] [CrossRef]
- Colombini, M.P.; Andreotti, A.; Bonaduce, I.; Modugno, F.; Ribechini, E. Analytical strategies for characterizing organic paint media using gas chromatography/mass spectrometry. ACC Chem. Res. 2010, 43, 715–727. [Google Scholar] [CrossRef]
- Zhou, D.; Hou, Q.; Liu, W.; Ren, X. Rapid determination of formic and acetic acids in biomass hydrolysate by headspace gas chromatography. J. Ind. Eng. Chem. 2017, 47, 281–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).