Electropolymerization of a New Diketopyrrollopyrrole Derivative into Inherent Chiral Polymer Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Experimental Methods
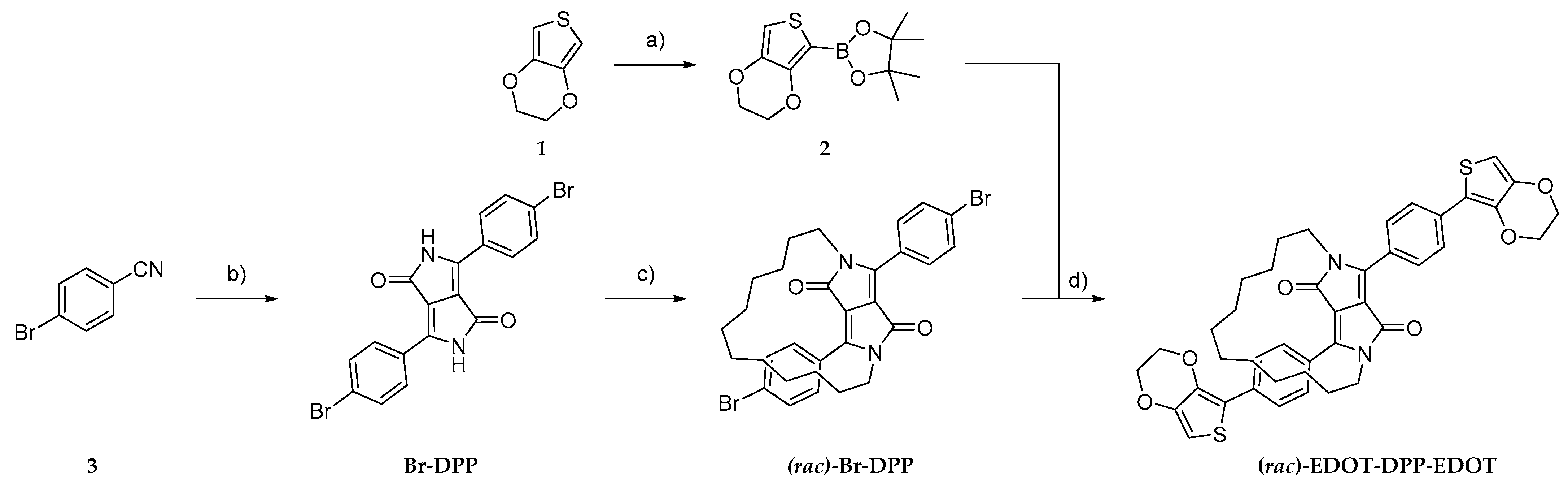
2.2. Synthesis of Compound (−)/(+)-EDOT-DPP-EDOT
2.3. Electrochemical Methods
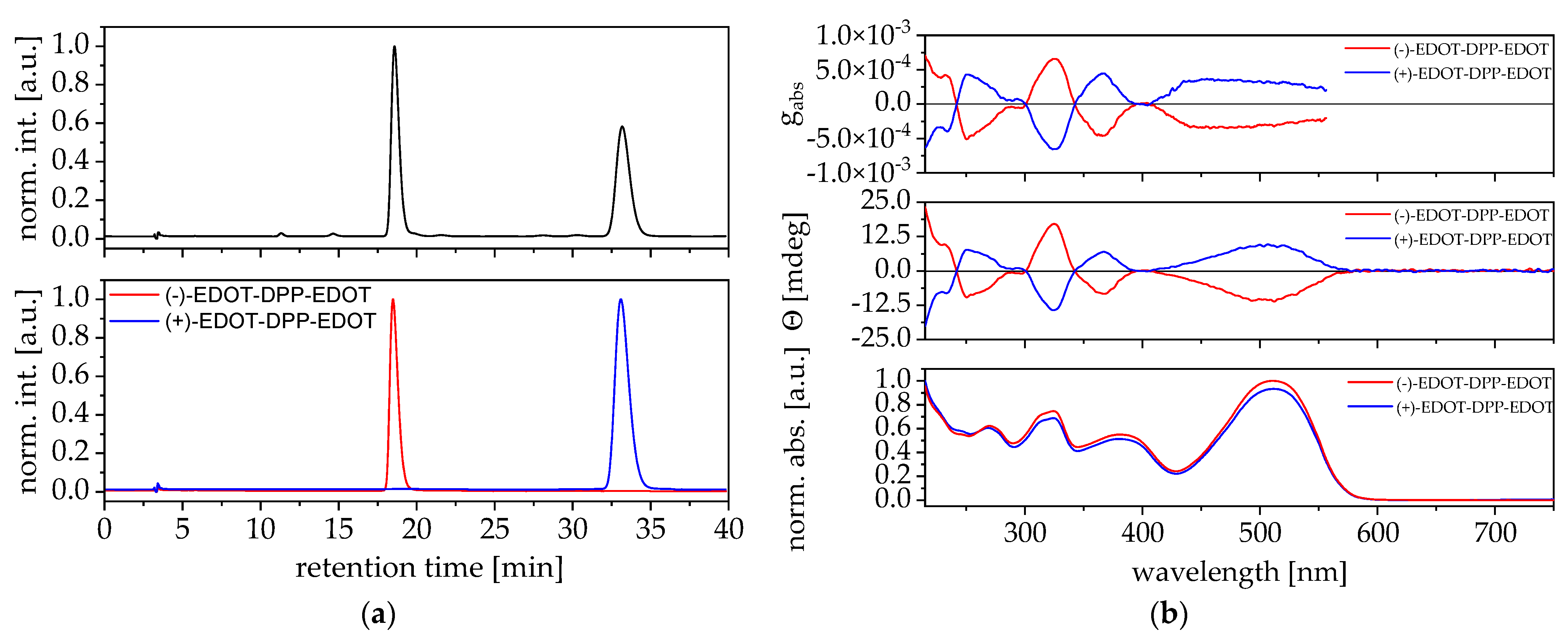
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosnier, S.; Karyakin, A. Electropolymerization: Concepts, Materials and Applications; Wiley-VCH: Weinheim, Germany, 2010; ISBN 9783527324149. [Google Scholar]
- Frontana Uribe, B.A.; Palma-Cando, A. Conducting Polymers. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2022; pp. 1–49. [Google Scholar]
- Rendón-Enríquez, I.; Palma-Cando, A.; Körber, F.; Niebisch, F.; Forster, M.; Tausch, M.W.; Scherf, U. Thin Polymer Films by Oxidative or Reductive Electropolymerization and Their Application in Electrochromic Windows and Thin-Film Sensors. Molecules 2023, 28, 883. [Google Scholar] [CrossRef] [PubMed]
- Erazo, E.A.; Ortiz, P.; Cortés, M.T. Tailoring the PEDOT:PSS Hole Transport Layer by Electrodeposition Method to Improve Perovskite Solar Cells. Electrochim. Acta 2023, 439, 141573. [Google Scholar] [CrossRef]
- Saito, Y.; Kitamura, T.; Wada, Y.; Yanagida, S. Application of Poly(3,4-Ethylenedioxythiophene) to Counter Electrode in Dye-Sensitized Solar Cells. Chem. Lett. 2002, 31, 1060–1061. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.W.; Lee, Y.J. Flexible Sensor with Electrophoretic Polymerized Graphene Oxide/PEDOT:PSS Composite for Voltammetric Determination of Dopamine Concentration. Sci. Rep. 2021, 11, 21101. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrogenerated Thin Films of Microporous Polymer Networks with Remarkably Increased Electrochemical Response to Nitroaromatic Analytes. ACS Appl. Mater. Interfaces 2015, 7, 11127–11133. [Google Scholar] [CrossRef]
- Räupke, A.; Palma-Cando, A.; Shkura, E.; Teckhausen, P.; Polywka, A.; Görrn, P.; Scherf, U.; Riedl, T. Highly Sensitive Gas-Phase Explosive Detection by Luminescent Microporous Polymer Networks. Sci. Rep. 2016, 6, 29118. [Google Scholar] [CrossRef]
- Quiroz-Arturo, H.; Reinoso, C.; Scherf, U.; Palma-Cando, A. Microporous Polymer-Modified Glassy Carbon Electrodes for the Electrochemical Detection of Metronidazole: Experimental and Theoretical Insights. Nanomaterials 2024, 14, 180. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical-Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-Based Microporous Polymer Networks via Chemical or Electrochemical Oxidative Coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef]
- Sotzing, G.A.; Reynolds, J.R.; Steel, P.J. Poly(3,4-Ethylenedioxythiophene) (PEDOT) Prepared via Electrochemical Polymerization of EDOT, 2,2′-Bis(3,4-Ethylenedioxythiophene) (BiEDOT), and Their TMS Derivatives. Adv. Mater. 1997, 9, 795–798. [Google Scholar] [CrossRef]
- Palma-Cando, A.U.; Frontana-Uribe, B.A.; Maldonado, J.L.; Hernández, M.R. Control of Thickness of PEDOT Electrodeposits on Glass/ITO Electrodes from Organic Solutions and Its Use as Anode in Organic Solar Cells. Procedia Chem. 2014, 12, 92–99. [Google Scholar] [CrossRef]
- Nasybulin, E.; Wei, S.; Kymissis, I.; Levon, K. Effect of Solubilizing Agent on Properties of Poly(3,4-Ethylenedioxythiophene) (PEDOT) Electrodeposited from Aqueous Solution. Electrochim. Acta 2012, 78, 638–643. [Google Scholar] [CrossRef]
- Yan, J.; Sun, C.; Tan, F.; Hu, X.; Chen, P.; Qu, S.; Zhou, S.; Xu, J. Electropolymerized Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) (PEDOT:PSS) Film on ITO Glass and Its Application in Photovoltaic Device. Sol. Energy Mater. Sol. Cells 2010, 94, 390–394. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Saghaei, J.; Koodalingam, M.; Burn, P.L.; Gentle, I.R.; Pivrikas, A.; Shaw, P.E. Effect of PEDOT:PSS on the Performance of Solution-Processed Blue Phosphorescent Organic Light-Emitting Diodes with an Exciplex Host. Mater. Adv. 2022, 3, 1055–1063. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Liu, Q.; Li, L.; Kong, J. Electrodeposition of Three-Dimensional Network Nanostructure PEDOT/PANI for Simultaneous Voltammetric Detection of Ascorbic Acid, Dopamine and Uric Acid. ChemistrySelect 2020, 5, 1288–1293. [Google Scholar] [CrossRef]
- David, J.; Weiter, M.; Vala, M.; Vyňuchal, J.; Kučerík, J. Stability and Structural Aspects of Diketopyrrolopyrrole Pigment and Its N-Alkyl Derivatives. Dyes Pigment. 2011, 89, 137–143. [Google Scholar] [CrossRef]
- Gao, K.; Jo, S.B.; Shi, X.; Nian, L.; Zhang, M.; Kan, Y.; Lin, F.; Kan, B.; Xu, B.; Rong, Q.; et al. Over 12% Efficiency Nonfullerene All-Small-Molecule Organic Solar Cells with Sequentially Evolved Multilength Scale Morphologies. Adv. Mater. 2019, 31, 1807842. [Google Scholar] [CrossRef]
- Wienk, M.M.; Turbiez, M.; Gilot, J.; Janssen, R.A.J. Narrow-Bandgap Diketo-Pyrrolo-Pyrrole Polymer Solar Cells: The Effect of Processing on the Performance. Adv. Mater. 2008, 20, 2556–2560. [Google Scholar] [CrossRef]
- Ji, Y.; Xiao, C.; Wang, Q.; Zhang, J.; Li, C.; Wu, Y.; Wei, Z.; Zhan, X.; Hu, W.; Wang, Z.; et al. Asymmetric Diketopyrrolopyrrole Conjugated Polymers for Field-Effect Transistors and Polymer Solar Cells Processed from a Nonchlorinated Solvent. Adv. Mater. 2016, 28, 943–950. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.; Li, P.; Wen, J.; He, J.; Gao, X. Enhancement of the Thermoelectric Performance of DPP Based Polymers by Introducing One 3,4-Ethylenedioxythiophene Electron-Rich Building Block. J. Mater. Chem. C Mater. 2020, 8, 10859–10867. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Chen, C.; Wang, Y.; Zhang, Z.; Tang, L.; Sun, Q.; Xue, S.; Yang, W. 1,4-Diketo-Pyrrolo[3,4-c]Pyrroles (DPPs) Based Insoluble Polymer Films with Lactam Hydrogens as Renewable Fluoride Anion Chemosensor. Polymer 2018, 149, 266–272. [Google Scholar] [CrossRef]
- Jia, L.; Hao, J.; Wang, S.; Yang, L.; Liu, K. Sensitive Detection of 4-Nitrophenol Based on Pyridine Diketopyrrolopyrrole-Functionalized Graphene Oxide Direct Electrochemical Sensor. RSC Adv. 2023, 13, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Leenaers, P.J.; Wienk, M.M.; Janssen, R.A.J. Structural Design of Asymmetric Diketopyrrolopyrrole Polymers for Organic Solar Cells Processed from a Non-Halogenated Solvent. Org. Electron. 2020, 86, 105914. [Google Scholar] [CrossRef]
- Naik, M.A.; Venkatramaiah, N.; Kanimozhi, C.; Patil, S. Influence of Side-Chain on Structural Order and Photophysical Properties in Thiophene Based Diketopyrrolopyrroles: A Systematic Study. J. Phys. Chem. C 2012, 116, 26128–26137. [Google Scholar] [CrossRef]
- Lange, G.; Tieke, B. New Deeply Coloured and Fluorescent Polymers with 1,4-Dioxo-3,6-Diphenylpyrrolo[3,4-c]Pyrrole Units in the Main Chain. Macromol. Chem. Phys. 1999, 200, 106–112. [Google Scholar] [CrossRef]
- Dhar, J.; Venkatramaiah, N.; Anitha, A.; Patil, S. Photophysical, Electrochemical and Solid State Properties of Diketopyrrolopyrrole Based Molecular Materials: Importance of the Donor Group. J. Mater. Chem. C Mater. 2014, 2, 3457–3466. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, K.; Tieke, B. Electrochemical Polymerization. of Bis(3,4-Ethylenedioxythiophene)- Substituted 1,4-Diketo-3,6-Diphenyl-Pyrrolo[3,4-c]Pyrrole (DPP) Derivative. Macromol. Chem. Phys. 2009, 210, 431–439. [Google Scholar] [CrossRef]
- Zhu, Y. New Diketopyrrolopyrrole(DPP)-Based Conjugated Polymers Prepared upon Palladium Catalyzed Polymerization and Electropolymerization Reactions. Ph.D. Thesis, University of Cologne, Cologne, Germany, 2006. [Google Scholar]
- Ponnappa, S.P.; Liu, Q.; Umer, M.; MacLeod, J.; Jickson, J.; Ayoko, G.; Shiddiky, M.J.A.; O’Mullane, A.P.; Sonar, P. Naphthalene Flanked Diketopyrrolopyrrole: A New Conjugated Building Block with Hexyl or Octyl Alkyl Side Chains for Electropolymerization Studies and Its Biosensor Applications. Polym. Chem. 2019, 10, 3722–3739. [Google Scholar] [CrossRef]
- Han, H.; Choi, J.H.; Ahn, J.; Lee, H.; Choi, C.; Jung, W.; Yeom, J.; Hwang, D.K.; Sung, B.J.; Lim, J.A. Chiral Diketopyrrolopyrrole-Based Conjugated Polymers with Intramolecular Rotation–Isomeric Conformation Asymmetry for Near-Infrared Circularly Polarized Light-Sensing Organic Phototransistors. ACS Appl. Mater. Interfaces 2023, 15, 57447–57460. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Y.; Duan, X.; Zhu, X.; Sun, H.; Xu, J. Chiral PEDOT-Based Enantioselective Electrode Modification Material for Chiral Electrochemical Sensing: Mechanism and Model of Chiral Recognition. Anal. Chem. 2017, 89, 9695–9702. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.; Aronica, L.A.; Pescitelli, G.; Di Bari, L. Chiral Diketopyrrolo[3,4-c]Pyrrole-Based Oligothiophenes: Synthesis and Characterization of Aggregated States in Solution and Thin Films. Chirality 2024, 36, e23608. [Google Scholar] [CrossRef] [PubMed]
- Otaki, M.; Komaba, K.; Goto, H. Synthesis of Polythiophene-Based Chiral Magnetic Block Copolymers with High Stereoregularity. ACS Appl. Polym. Mater. 2022, 5, 311–319. [Google Scholar] [CrossRef]
- Vacek, J.; Hrbáč, J.; Strašák, T.; Církva, V.; Sýkora, J.; Fekete, L.; Pokorný, J.; Bulíř, J.; Hromadová, M.; Crassous, J.; et al. Anodic Deposition of Enantiopure Hexahelicene Layers. ChemElectroChem 2018, 5, 2080–2088. [Google Scholar] [CrossRef]
- Goto, H.; Akagi, K. Preparation of Poly(3,4-Ethylenedioxythiophene) in a Chiral Nematic Liquid-Crystal Field. Macromol. Rapid Commun. 2004, 25, 1482–1486. [Google Scholar] [CrossRef]
- Hassan Omar, O.; La Gatta, S.; Tangorra, R.R.; Milano, F.; Ragni, R.; Operamolla, A.; Argazzi, R.; Chiorboli, C.; Agostiano, A.; Trotta, M.; et al. Synthetic Antenna Functioning as Light Harvester in the Whole Visible Region for Enhanced Hybrid Photosynthetic Reaction Centers. Bioconjug. Chem. 2016, 27, 1614–1623. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, W.; Huang, Y.; Yang, J.; Wang, W. Narrow Band-Gap Donor-Acceptor Copolymers Based on Diketopyrrolopyrrole and Diphenylethene: Synthesis, Characterization and Application in Field Effect Transistor. Dyes Pigment. 2016, 127, 37–44. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Wang, L. Packing-Dependent Polymorphism: A Stimuli-Responsive Macrocyclic Diketopyrrolopyrrole. Dyes Pigment. 2022, 198, 110024. [Google Scholar] [CrossRef]
- Iqbal, A.; Jost, M.; Kirchmayr, R.; Pfenninger, J.; Rochat, A. The synthesis and properties of 1,4-diketo-pyrrolo[3,4-c]pyrroles. Bull. Des Sociétés Chim. Belg. 1988, 97, 615–644. [Google Scholar] [CrossRef]
- Ruggli, P. Über Einen Ring Mit Dreifacher Bindung. Justus Liebigs Ann. Chem. 1912, 392, 92–100. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of Electronic Circular Dichroism in Configurational and Conformational Analysis of Organic Compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Kirkus, M.; Wang, L.; Mothy, S.; Beljonne, D.; Cornil, J.; Janssen, R.A.J.; Meskers, S.C.J. Optical Properties of Oligothiophene Substituted Diketopyrrolopyrrole Derivatives in the Solid Phase: Joint J- and H-Type Aggregation. J. Phys. Chem. A 2012, 116, 7927–7936. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Wen, T.-C.; Gopalan, A. Electrochemical and Spectroelectrochemical Evidences for Copolymer Formation Between 2-Aminodiphenylamine and Aniline. J. Electrochem. Soc. 2001, 148, E427. [Google Scholar] [CrossRef][Green Version]



| Polymer | Roughness [nm] | Average Thickness [nm] |
|---|---|---|
| P-(rac)-EDOT-DPP-EDOT | 105 | ~990 |
| P-(−)-EDOT-DPP-EDOT | 18 | ~280 |
| P-(+)-EDOT-DPP-EDOT | 18 | ~265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebisch, F.; Scherf, U.; Palma-Cando, A. Electropolymerization of a New Diketopyrrollopyrrole Derivative into Inherent Chiral Polymer Films. Nanomaterials 2024, 14, 1776. https://doi.org/10.3390/nano14221776
Niebisch F, Scherf U, Palma-Cando A. Electropolymerization of a New Diketopyrrollopyrrole Derivative into Inherent Chiral Polymer Films. Nanomaterials. 2024; 14(22):1776. https://doi.org/10.3390/nano14221776
Chicago/Turabian StyleNiebisch, Felix, Ullrich Scherf, and Alex Palma-Cando. 2024. "Electropolymerization of a New Diketopyrrollopyrrole Derivative into Inherent Chiral Polymer Films" Nanomaterials 14, no. 22: 1776. https://doi.org/10.3390/nano14221776
APA StyleNiebisch, F., Scherf, U., & Palma-Cando, A. (2024). Electropolymerization of a New Diketopyrrollopyrrole Derivative into Inherent Chiral Polymer Films. Nanomaterials, 14(22), 1776. https://doi.org/10.3390/nano14221776







