Gold Nanoparticle-Embedded Thiol-Functionalized Ti3C2Tx MXene for Sensitive Electrochemical Sensing of Ciprofloxacin
Abstract
1. Introduction
2. Materials and Methods
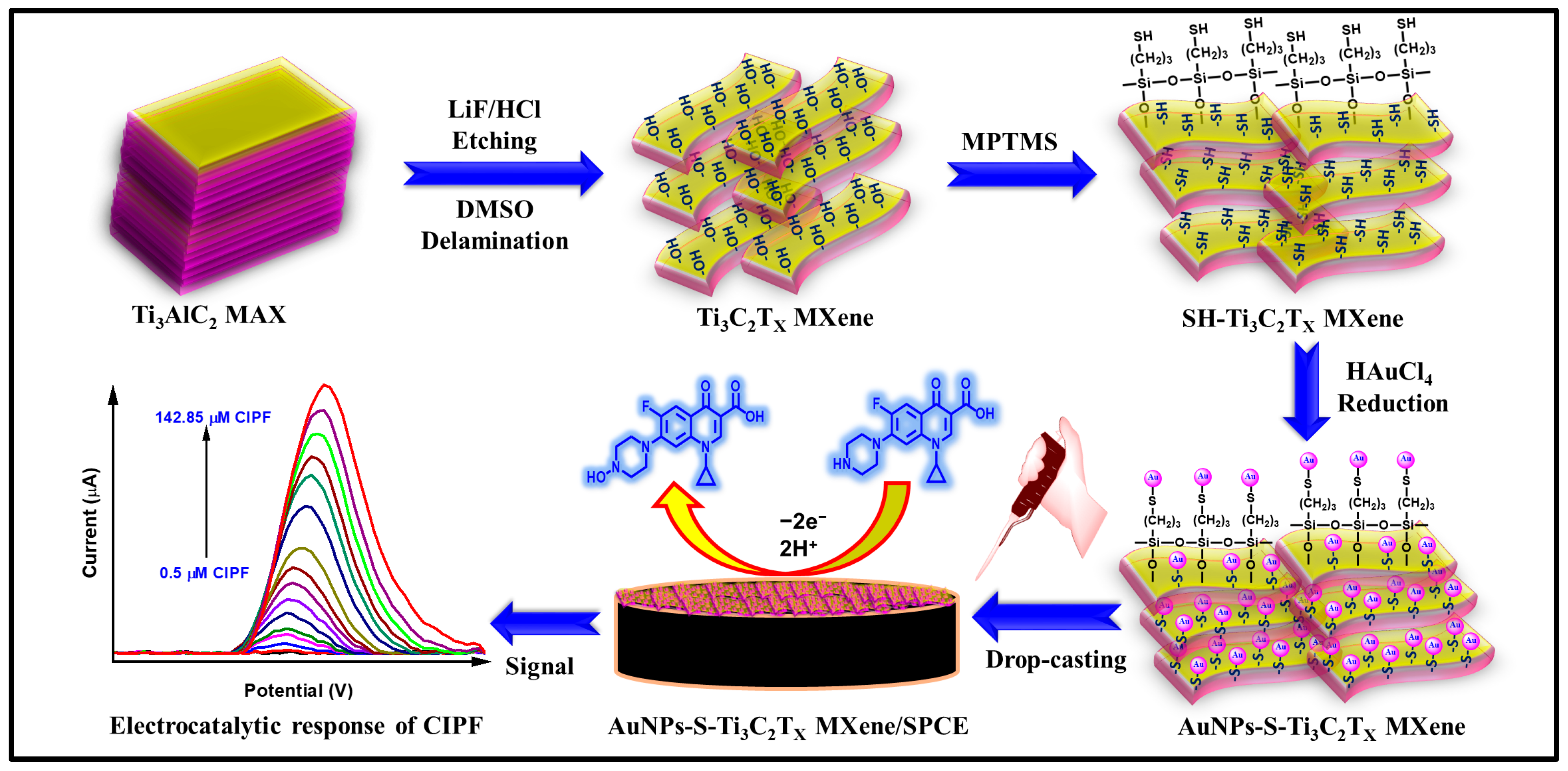
2.1. Synthesis of Thiol-Functionalized Ti3C2Tx MXene (SH-Ti3C2Tx MXene)
2.2. Synthesis of Gold Nanoparticle-Embedded Thiol-Functionalized Ti3C2Tx MXene (AuNPs-S-Ti3C2Tx MXene)
2.3. Fabrication of AuNPs-S-Ti3C2Tx MXene-Modified SPCE
3. Results and Discussion
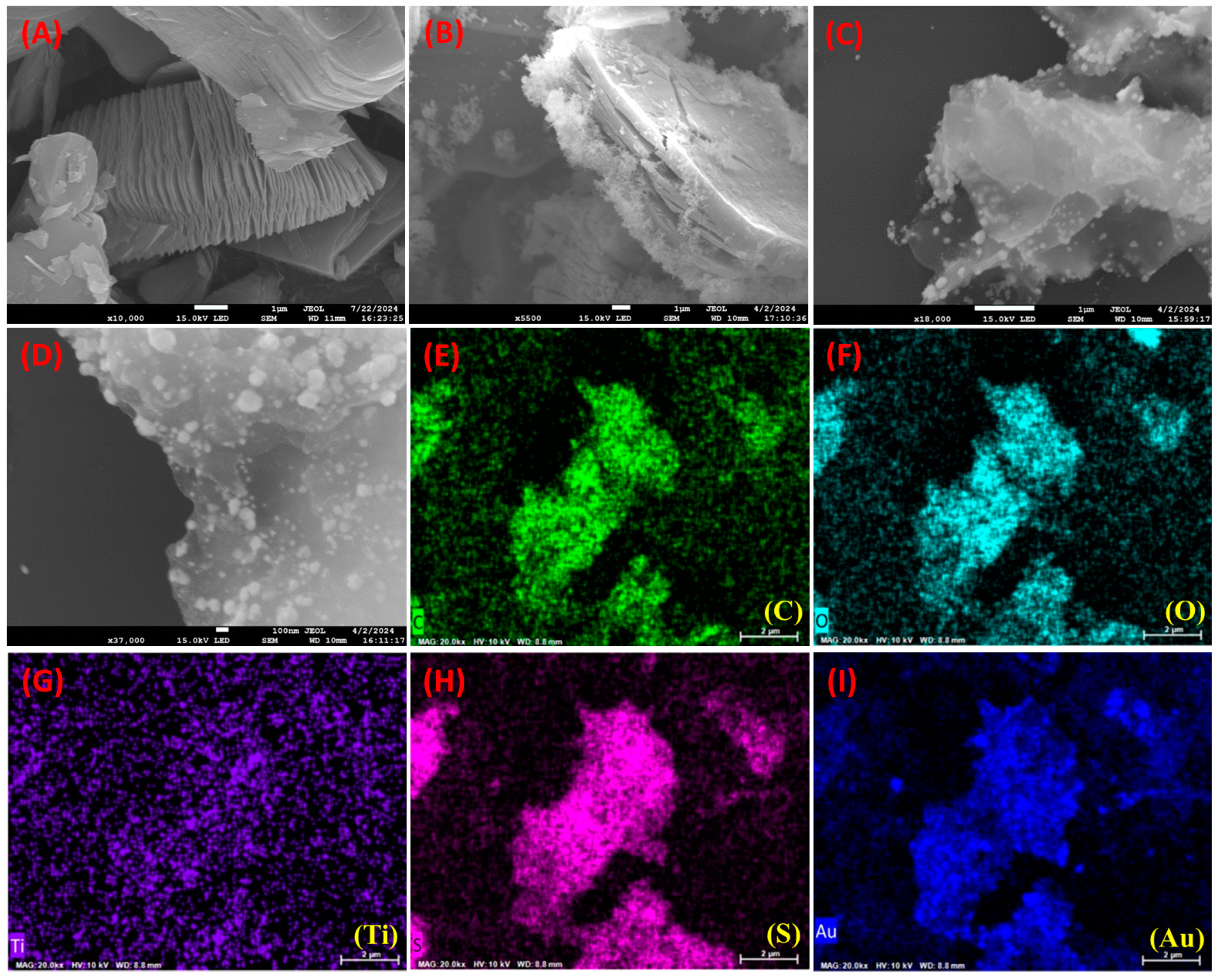
3.1. Surface Morphological Investigation of AuNPs-S-Ti3C2Tx MXene
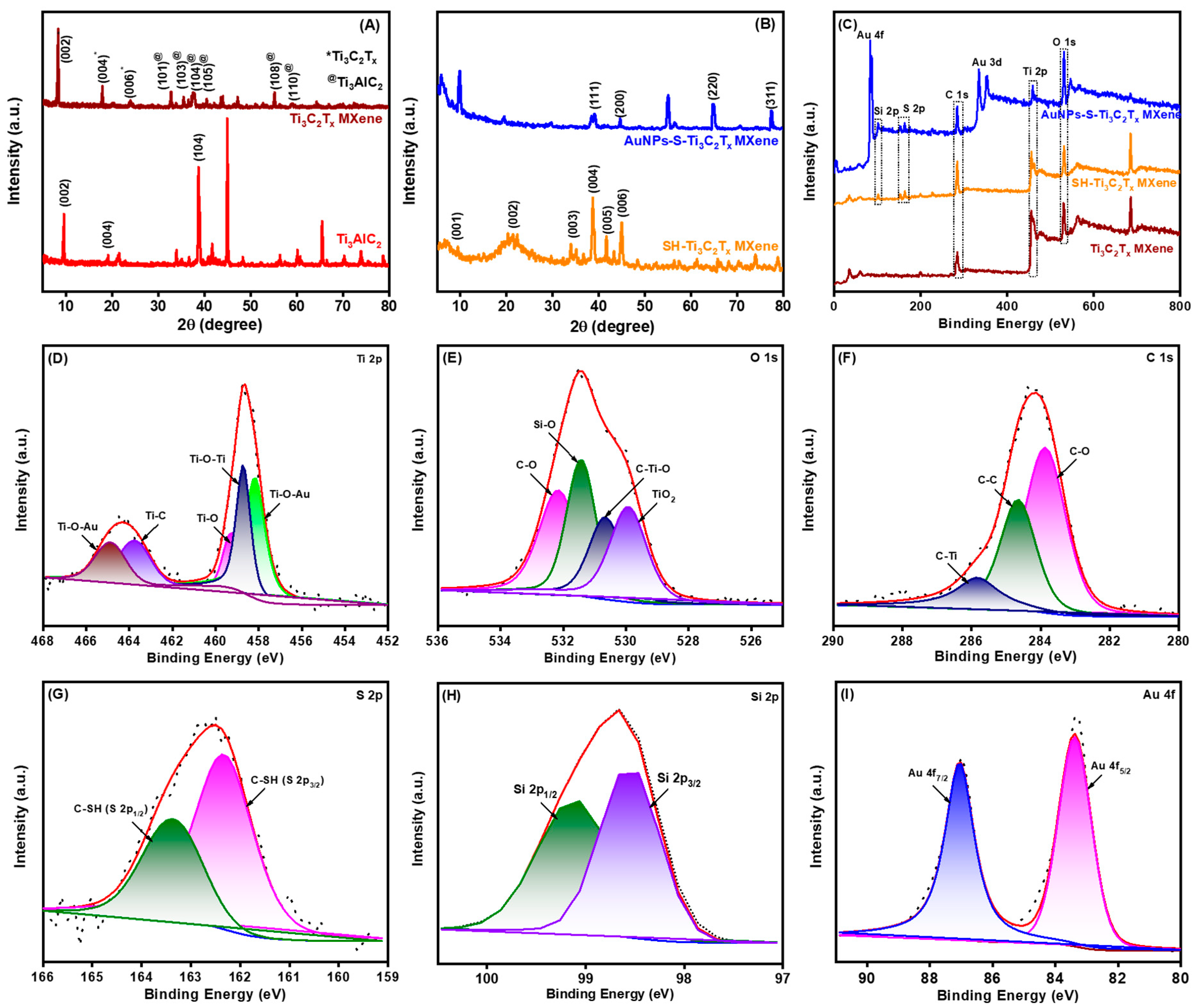
3.2. Structural Analysis of AuNPs-S-Ti3C2Tx MXene
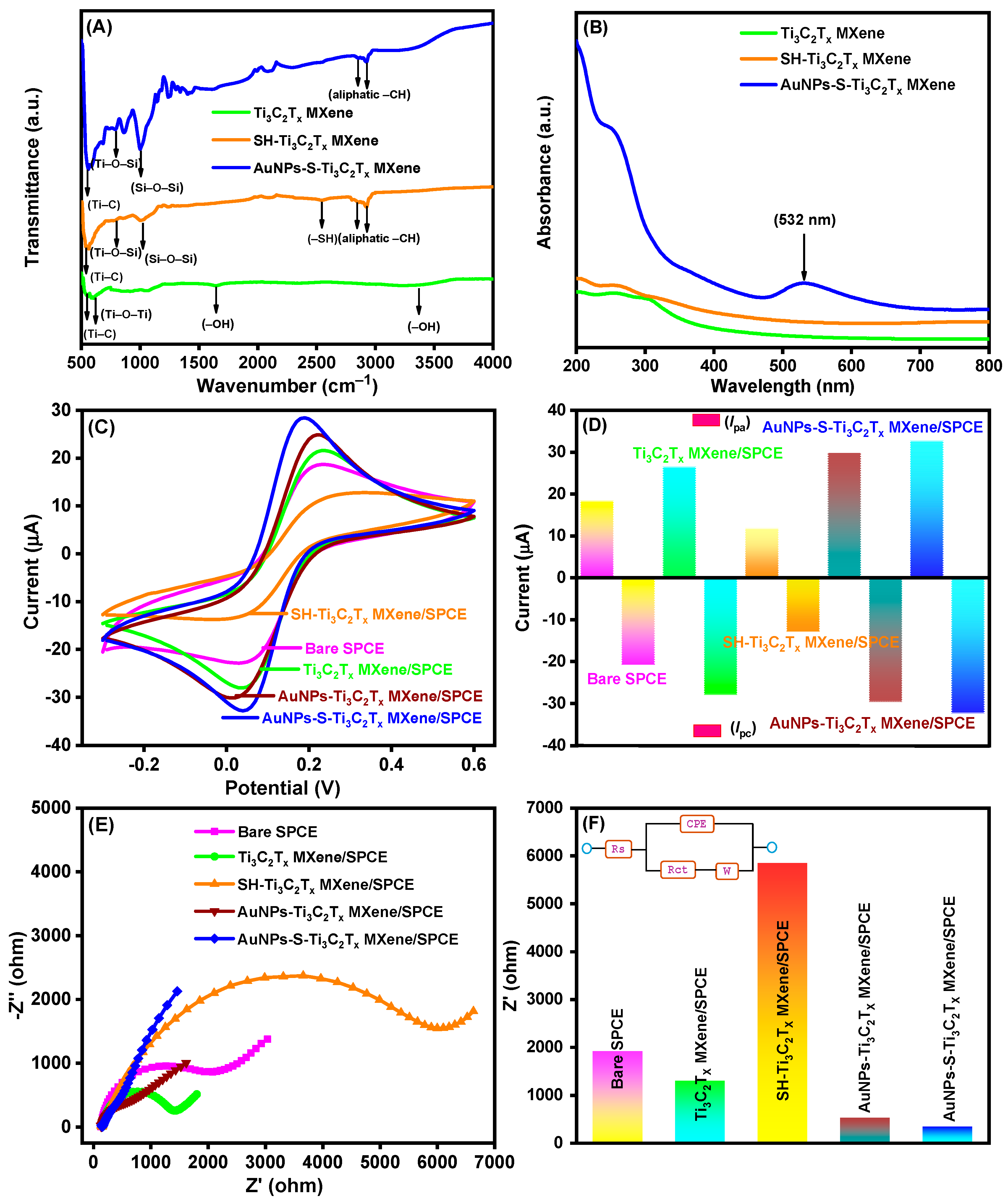
3.3. FTIR and UV–Visible Analysis of AuNPs-S-Ti3C2Tx MXene
3.4. Electrochemical Behavior of the AuNPs-S-Ti3C2Tx MXene-Modified SPCE
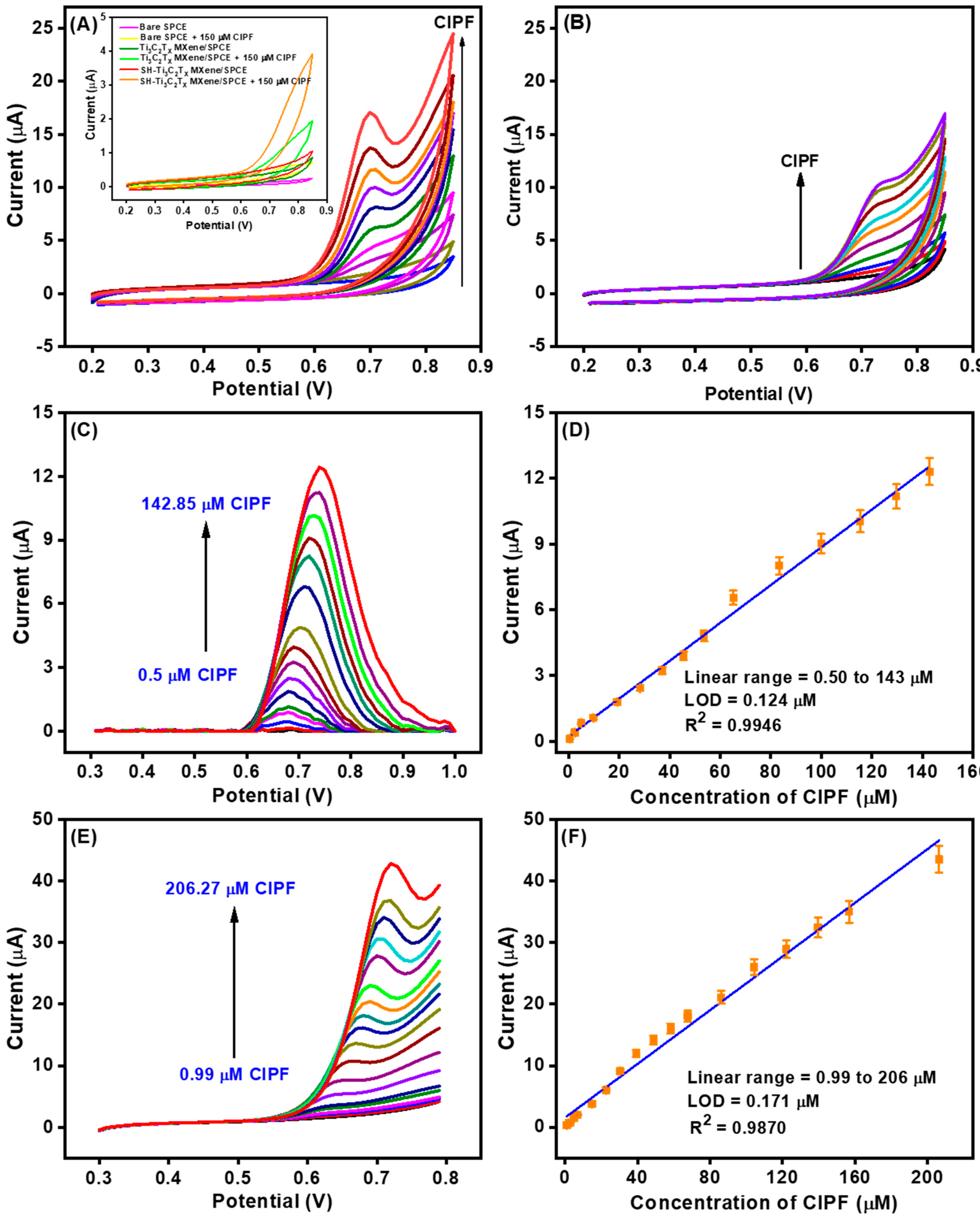
3.5. Electrocatalytic Oxidation of CIPF with the AuNPs-S-Ti3C2Tx MXene-Modified SPCE
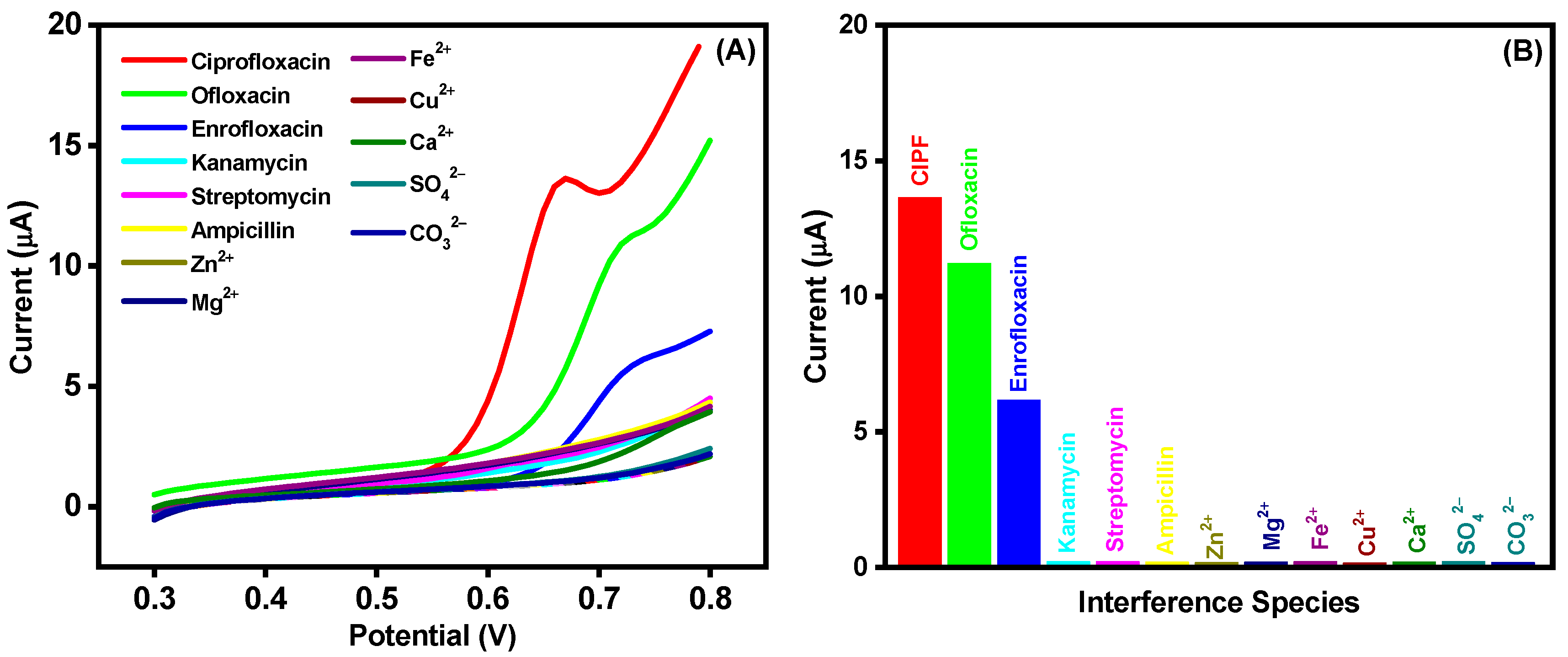
3.6. Selectivity, Stability, and Reproducibility of the AuNPs-S-Ti3C2Tx MXene/SPCE
3.7. Analysis of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crapnell, R.D.; Adarakatti, P.S.; Banks, C.E. Electroanalytical Overview: The Measurement of Ciprofloxacin. Sens. Diagn. 2024, 3, 40–58. [Google Scholar] [CrossRef]
- Rehman, A.; Patrick, W.M.; Lamont, I.L. Mechanisms of Ciprofloxacin Resistance in Pseudomonas aeruginosa: New Approaches to an Old Problem. J. Med. Microbiol. 2019, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Kumbhakar, P.; Mahapatra, P.L.; Sinha, S.K.; Lahiri, B.; Tiwary, C.S. Trace Detection of Ciprofloxacin in Milk by Label-Free Raman Enhancement Using Two-Dimensional Magnesiochromite. ACS Appl. Eng. Mater. 2023, 1, 2494–2502. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Li, W.; Xu, X.; Wang, B.; Xu, X.; Hussain, D.; Chen, D. Current Analytical Strategies for the Determination of Quinolone Residues in Milk. Food Chem. 2023, 430, 137072. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Cai, L.; Wang, H.; Zhang, B.; Fang, G.; Wang, S. A “Signal On/Off” Biomimetic Electrochemiluminescence Sensor Using Titanium Carbide Nanodots as Co-reaction Accelerator for Ultra-Sensitive Detection of Ciprofloxacin. Anal. Chim. Acta 2022, 1206, 339690. [Google Scholar] [CrossRef]
- Chuiprasert, J.; Srinives, S.; Boontanon, N.; Polprasert, C.; Ramungul, N.; Lertthanaphol, N.; Karawek, S.; Boontanon, S.K. Electrochemical Sensor Based on a Composite of Reduced Graphene Oxide and Molecularly Imprinted Copolymer of Polyaniline–Poly(o-Phenylenediamine) for Ciprofloxacin Determination: Fabrication, Characterization, and Performance Evaluation. ACS Omega 2023, 8, 2564–2574. [Google Scholar] [CrossRef]
- Dionisio, R.; Daniel, D.; Alkimin, G.D.; Nunes, B. Multi-Parametric Analysis of Ciprofloxacin Toxicity at Ecologically Relevant Levels: Short-and Long-Term Effects on Daphnia Magna. Environ. Toxicol. Pharmacol. 2020, 74, 103295. [Google Scholar] [CrossRef]
- Vybiralova, Z.; Nobilis, M.; Zoulova, J.; Kvetina, J.; Petr, P. High-Performance Liquid Chromatographic Determination of Ciprofloxacin in Plasma Samples. J. Pharm. Biomed. Anal. 2005, 37, 851–858. [Google Scholar] [CrossRef]
- Oun, Z.N.A.; Turkie, N.S. New Turbidimetric Method for Determination of Ciprofloxacin Hydrochloride in Pharmaceutical Drugs Using Continuous Flow Injection Manifold Design with CIP.HCL-Sodium Nitro Prusside System. Eurasian Chem. Commun. 2021, 3, 743–754. [Google Scholar]
- Nagaralli, B.S.; Seetharamappa, J.; Melwanki, M.B. Sensitive Spectrophotometric Methods for the Determination of Amoxycillin, Ciprofloxacin and Piroxicam in Pure and Pharmaceutical Formulations. J. Pharm. Biomed. Anal. 2022, 29, 859–864. [Google Scholar] [CrossRef]
- Attia, M.S.; Essawy, A.A.; Youssef, A.O. Europium-Sensitized and Simultaneous pH-Assisted Spectrofluorimetric Assessment of Ciprofloxacin, Norfloxacin and Gatifloxacin in Pharmaceutical and Serum Samples. J. Photochem. Photobiol. A Chem. 2012, 236, 26–34. [Google Scholar] [CrossRef]
- Zhou, X.; Xing, D.; Zhu, D.; Tang, Y.; Jia, L. Development and Application of a Capillary Electrophoresis–Electrochemiluminescent Method for the Analysis of Enrofloxacin and its Metabolite Ciprofloxacin in Milk. Talanta 2008, 75, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.W.; Li, L.Q.; Chen, X.Y. Flow-Injection Enhanced Chemiluminescence Method for Determination of Ciprofloxacin in Pharmaceutical Preparations and Biological Fluids. Anal. Bioanal. Chem. 2006, 384, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, X.; Liu, Y.; Li, Q.; Zeng, Z.; Maiyalagan, T.; Mao, S. Nanocomposites of Zr (IV)-Based Metal–Organic Frameworks and Reduced Graphene Oxide for Electrochemically Sensing Ciprofloxacin in Water. ACS Appl. Nano Mater. 2019, 2, 2367–2376. [Google Scholar] [CrossRef]
- Pollap, A.; Baran, K.; Kuszewska, N.; Kochana, J. Electrochemical Sensing of Ciprofloxacin and Paracetamol in Environmental Water Using Titanium Sol Based Sensor. J. Electroanal. Chem. 2020, 878, 114574. [Google Scholar] [CrossRef]
- Gayen, P.; Chaplin, B.P. Selective Electrochemical Detection of Ciprofloxacin with a Porous Nafion/Multiwalled Carbon Nanotube Composite Film Electrode. ACS Appl. Mater. Interfaces 2015, 8, 1615–1626. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, S.; Wang, X.; Zhang, Y.; Yue, Z.; Li, C.; Ma, Y. An Electrochemical Sensor Based on MnO2/ZnO Composites for the Detection of Ciprofloxacin in Honey. Microchem. J. 2023, 194, 109355. [Google Scholar] [CrossRef]
- Bagheri, H.; Khoshsafar, H.; Amidi, S.; Ardakani, Y.H. Fabrication of an Electrochemical Sensor Based on Magnetic Multi-Walled Carbon Nanotubes for the Determination of Ciprofloxacin. Anal. Methods 2016, 8, 3383–3390. [Google Scholar] [CrossRef]
- Sadeghi, S.; Javanshiri-Ghasemabadi, J. Bimetallic Metal Organic Framework/Ni doped ZnO Nanomaterials Modified Carbon Paste Electrode for Selective Electrochemical Determination of Ciprofloxacin. RSC Adv. 2024, 14, 7836–7849. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Sukardi, D.K.A.; Sari, A.P.; Tomisaki, M.; Wafiroh, S.; Hartati, S.; Arramel; Wong, Y.H.; Woi, P.M.; Juan, J.C. Fabrication and Characterization of rGO-SnO2 Nanocomposite for Electrochemical Sensor of Ciprofloxacin. Sens. Int. 2024, 5, 100276. [Google Scholar] [CrossRef]
- Chuiprasert, J.; Srinives, S.; Boontanon, N.; Polprasert, C.; Ramungul, N.; Karawek, A.; Boontanon, S.K. Ciprofloxacin Electrochemical Sensor Using Copper-Iron Mixed Metal Oxides Nanoparticles/Reduced Graphene Oxide Composite. ACS Omega 2024, 9, 23172–23183. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, J.; Chen, L.; Lin, H.; Han, D.; Bao, Y.; Wang, W.; Niu, L. A Wearable Electrochemical Biosensor Utilizing Functionalized Ti3C2Tx MXene for the Real-Time Monitoring of Uric Acid Metabolite. Anal. Chem. 2024, 96, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qin, W.; Li, X.; Feng, L.; Gu, C.; Chen, J.; Tian, Z.; Chen, J.; Yang, M.; Qiao, H.; et al. Molecularly Imprinted Electrochemical Sensors Based on Ti3C2Tx-MXene and Graphene Composite Modifications for Ultrasensitive Cortisol Detection. Anal. Chem. 2023, 95, 16079–16088. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Wang, W.; Chen, M.; Wan, Q.; Wang, C.; Knopp, D.; Tang, D. CRISPR-Cas12a-Driven MXene-PEDOT:PSS Piezoresistive Wireless Biosensor. Nano Energy 2021, 82, 105711. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensor: A Review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Eswaran, M.; Shuck, C.E.; Senthilkumar, S.; Elumalai, S.; Dhanusuraman, R.; Ponnusamy, V.K. Facile Synthesis of Polyaniline/Titanium Carbide (MXene) Nanosheets/Palladium Nanocomposite for Efficient Electrocatalytic Oxidation of Methanol for Fuel Cell Application. Fuel 2021, 303, 121329. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in Energy Conversion and Storage Systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Su, M.; Lan, H.; Tian, L.; Jiang, M.; Cao, X.; Zhu, C.; Yu, C. Ti3C2Tx-Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for Serotonin in Human Biofluids. Sens. Actuators B Chem. 2022, 367, 132019. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimi-Maleh, H. In Situ Synthesis of Label-Free Electrochemical Aptasensor-Based Sandwich-Like AuNPs/PPy/Ti3C2Tx for Ultrasensitive Detection of Lead Ions as Hazardous Pollutants in Environmental Fluids. Chemosphere 2023, 324, 138302. [Google Scholar] [CrossRef]
- Zeng, R.; Lin, L.; Gong, H.; Li, Y.; Xu, J.; Huang, L.; Wang, W.; Lin, S.; Tang, D.; Guo, S. Tuning the Rate-Determining Step of Uric Acid Electrooxidation over Single-Atom-Site Metal Centers for High-Performing Sensing. Chem. Catal. 2023, 3, 100514. [Google Scholar] [CrossRef]
- Naz, G.; Salem, M.A.; Sharma, B.P.; Mekkey, S.D.; Soomro, R.A.; Karakuş, S.; El-Bahy, Z.M. Ti3C2Tx-Au Hybrid Composites-Based Electrochemical Biosensors for Calreticulin Biomarker Detection. Microchem. J. 2023, 194, 109307. [Google Scholar] [CrossRef]
- Sakthivel, R.; Lin, Y.C.; Yu, M.C.; Dhawan, U.; Liu, X.; Chen, J.C.; Tung, C.W.; Chung, R.J. A Sensitive Sandwich-Type Electrochemical Immunosensor Using Nitrogen-Doped Graphene/Metal-Organic Framework-Derived CuMnCoOx and Au/MXene for the Detection of Breast Cancer Biomarker. Colloids Surf. B Biointerface 2024, 234, 113755. [Google Scholar] [CrossRef]
- Zeng, R.; Qiu, M.; Wan, Q.; Huang, Z.; Liu, X.; Tang, D.; Knopp, D. Smartphone-Based Electrochemical Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 15155–15161. [Google Scholar] [CrossRef] [PubMed]
- Jandas, P.J.; Prabakaran, K.; Luo, J.; Fu, C.; Fu, Y.Q.; Derry, H.M.G. Ti3C2Tx MXene-Au Nanoparticles Doped Polyimide Thin Film as a Transducing Bioreceptor for Real-Time Acoustic Detection of Carcinoembryonic Antigen. Sens. Actuators A Phys. 2021, 331, 112998. [Google Scholar]
- Bhat, K.S.; Byun, S.; Alam, A.; Ko, M.; An, J.; Lim, S. A Fast and Label-Free Detection of Hydroxymethylated DNA Using a Nozzle-Jet Printed AuNPs@Ti3C2 MXene-Based Electrochemical Sensor. Talanta 2022, 244, 123421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, H.; Hou, J.; Hou, C.; Huo, D. Sulfhydryl-Functionalized 3D MXene-AuNPs Enabled Electrochemical Sensors for the Selective Determination of Pb2+, Cu2+ and Hg2+ in Grain. Food Chem. 2024, 446, 138770. [Google Scholar] [CrossRef]
- Wang, W.; Gunasekaran, S. MXene/Gold Nanoparticles Heterostructure as Catalase Mimic for Colorimetric Detection of Penicillin G. Chem. Eng. J. 2024, 482, 148693. [Google Scholar] [CrossRef]
- Luo, L.; Guo, X.; Xi, X.; Bao, T.; Li, Y.; Wu, Z.; Zhang, X.; Wang, S.; Wen, W. Pt/Ti3C2Tx Nanozyme-Amplified Colorimetric Lateral Flow Biosensor for Dual-Readout Detection of HIV-DNA. Sens. Actuators B Chem. 2023, 381, 133444. [Google Scholar] [CrossRef]
- Qu, W.; Zhao, H.; Zhang, Q.; Xia, D.; Tang, Z.; Chen, Q.; He, C.; Shu, D. Multifunctional Au/Ti3C2 Photothermal Membrane with Antibacterial Ability for Stable and Efficient Solar Water Purification under the Full Spectrum. ACS Sustain. Chem. Eng. 2021, 9, 11372–11387. [Google Scholar] [CrossRef]
- Mari, E.; Duraisamy, M.; Eswaran, M.; Sellappan, S.; Won, K.; Chandra, P.; Tsai, P.C.; Huang, P.C.; Chen, Y.H.; Lin, Y.C.; et al. Highly Electrochemically Active Ti3C2Tx MXene/MWCNT Nanocomposite for the Simultaneous Sensing of Paracetamol, Theophylline, and Caffeine in Human Blood Samples. Microchim. Acta 2024, 191, 212. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Muthumalai, K.; Nagaraja, T.; Rajendrakumar, R.T.; Das, S.R. Chemically Exfoliated Titanium Carbide MXene for Highly Sensitive Electrochemical Sensors for Detection of 4-Nitrophenols in Drinking Water. ACS Omega 2022, 7, 42644–42654. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Niu, K.; Du, H.; Li, R.; Chen, J.; Lu, X. Ultrasensitive Rapid Detection of Antibiotic Resistance Genes by Electrochemical Ratiometric Genosensor Based on 2D Monolayer Ti3C2@AuNPs. Biosens. Bioelectron. 2023, 240, 115643. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guan, S.; Zhou, X.; Chang, Q.; Jiang, G. Co-Intercalation of CTAB Favors the Preparation of Ti3C2Tx/PANI Composite with Improved Electrochemical Performance. Ionics 2021, 27, 2501–2508. [Google Scholar] [CrossRef]
- Finocchio, E.; Macis, E.; Raiteri, R.; Busca, G. Adsorption of Trimethoxysilane and of 3-Mercaptopropyltrimethoxysilane on Silica and on Silicon Wafers from Vapor Phase: An IR study. Langmuir 2007, 23, 2505–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, W.; Wang, S.; Liu, C.; Shi, H.; Liang, L.; Pi, K. Surface Functionalization of Ti3C2TX and its Application in Aqueous Polymer Nanocomposites for Reinforcing Corrosion Protection. Compos. Part B 2021, 217, 108900. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Makaryan, T.; Melikyan, A.; Minassian, H.; Gogotsi, Y.; Yoshimura, M. One-Step Solution Processing of Ag, Au and Pd@MXene Hybrids for SERS. Sci. Rep. 2016, 6, 32049. [Google Scholar] [CrossRef]
- Elumalai, S.; Mani, V.; Jeromiyas, N.; Ponnusamy, V.K.; Yoshimura, M. A Composite Film Prepared from Titanium Carbide Ti3C2Tx (MXene) and Gold Nanoparticles for Voltammetric Determination of Uric Acid and Folic Acid. Microchim. Acta 2020, 187, 33. [Google Scholar] [CrossRef]
- Xu, J.T.; Hu, H.T.; Chen, Z.; Feng, X.Z.; Han, G.C.; Kraatz, H.B. One-Step Construction of MWCNTs@MoS2@Chitosan Film for Ciprofloxacin Sensing and its Oxidation Mechanism. Microchem. J. 2024, 205, 111184. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Ruthenium Doped Cu-MOF as an Efficient Sensing Platform for the Voltammetric Detection of Ciprofloxacin. Microchem. J. 2023, 188, 108481. [Google Scholar] [CrossRef]
- Adane, W.D.; Chandravanshi, B.S.; Tessema, M. A Simple, Ultrasensitive and Cost-Effective Electrochemical Sensor for the Determination of Ciprofloxacin in Various Types of Samples. Sens. Bio-Sens. Res. 2023, 39, 100547. [Google Scholar] [CrossRef]
- De Souza, C.C.; Alves, G.F.; Lisboa, T.P.; Matos, M.A.C.; Matos, R.C. Low-Cost Paper-Based Electrochemical Sensor for the Detection of Ciprofloxacin in Honey and Milk Samples. J. Food Compos. Anal. 2022, 112, 104700. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Lee, S.; Yoon, T.H.; Singh, M.; Lee, D.; Won, K. Disposable Electrochemical Sensors Based on Reduced Graphene Oxide/Polyaniline/Poly(Alizarin Red S)-Modified Integrated Carbon Electrodes for the Detection of Ciprofloxacin in Milk. Microchim. Acta 2024, 191, 507. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.A.; Singh, S.; Nate, Z.; Pawar, C.; Chauhan, R.; Thapliyal, N.B.; Karpoormath, R.; Patel, R. One-Pot Synthesis of β-Cyclodextrin Modified Silver Nanoparticles for Highly Sensitive Detection of Ciprofloxacin. J. Pharm. Biomed. Anal. 2021, 203, 114219. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Su, T.; Li, Z.K.; Chu, T.S.; Zhu, M.L.; Yan, Y.B.; Zhang, B.W.; Xuan, F.Z. Elastic Properties and Tensile Strength of 2D Ti3C2Tx MXene Monolayers. Nat. Commun. 2024, 15, 1566. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Chilcott, R.; Luo, S.; Sinitskii, A. Bifunctional Amine- and Thiol-Modified Ti3C2Tx MXene for Trace Detection of Heavy Metals. Langmuir 2022, 38, 12924–12934. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Perspective: What Constitutes a Quality Paper in Electroanalysis? Talanta Open 2021, 4, 100065. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion Coefficients of Ferri-and Ferrocyanide Ions in Aqueous Media, Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
| Electrodes | Method | Linear Range (µM) | LOD (µM) | Sensitivity (µA/µM) | Samples | Ref. |
|---|---|---|---|---|---|---|
| MIP/rGO/GCE | DPV | 0.001–0.5 | 0.00005 | 5.78 | Water | [6] |
| NH2-UiO-66/RGO | ASV | 0.02–1 | 0.00667 | 10.86 | Water | [14] |
| TiO2/PB/AuNPs/CMK-3/Nafion/GE | CV | 1–10 | 0.108 | 15.93 | Water | [15] |
| Cu/Ce-MOF/NZP/CPE | DPV | 0.75–100 | 0.142 | 1.29 | Milk, urine, and water | [19] |
| rGO-SnO2/SPE | SWV | 30–100 | 2.03 | 9.348 | Water and milk | [20] |
| MWCNT/MoS2/CS | DPV | 0.5–1200 | 0.16 | - | Water | [48] |
| Ru-Cu-TMA/GCE | DPV | 2.5–100 | 0.00329 | 0.0524 | Water | [49] |
| ChCl/CPE | SWV | 0.005–200 | 0.00036 | - | Water | [50] |
| PBE | DPV | 9.90–220 | 4.96 | - | Milk and honey | [51] |
| ERGO/PANI/PARS/SPCE | LSV | 0.01–69.8 | 0.0021 | 0.4833 | Milk | [52] |
| Ag-B-CD/GCE | DPV | 0.0001–0.05 | 0.000028 | - | Water | [53] |
| AuNPs-S-Ti3C2Tx MXene/SPCE | DPV LSV | 0.5–143 0.99–206 | 0.124 0.171 | 0.0863 0.2182 | Milk and water | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elancheziyan, M.; Singh, M.; Won, K. Gold Nanoparticle-Embedded Thiol-Functionalized Ti3C2Tx MXene for Sensitive Electrochemical Sensing of Ciprofloxacin. Nanomaterials 2024, 14, 1655. https://doi.org/10.3390/nano14201655
Elancheziyan M, Singh M, Won K. Gold Nanoparticle-Embedded Thiol-Functionalized Ti3C2Tx MXene for Sensitive Electrochemical Sensing of Ciprofloxacin. Nanomaterials. 2024; 14(20):1655. https://doi.org/10.3390/nano14201655
Chicago/Turabian StyleElancheziyan, Mari, Manisha Singh, and Keehoon Won. 2024. "Gold Nanoparticle-Embedded Thiol-Functionalized Ti3C2Tx MXene for Sensitive Electrochemical Sensing of Ciprofloxacin" Nanomaterials 14, no. 20: 1655. https://doi.org/10.3390/nano14201655
APA StyleElancheziyan, M., Singh, M., & Won, K. (2024). Gold Nanoparticle-Embedded Thiol-Functionalized Ti3C2Tx MXene for Sensitive Electrochemical Sensing of Ciprofloxacin. Nanomaterials, 14(20), 1655. https://doi.org/10.3390/nano14201655







