Enhanced Photocatalysis of Electrically Polarized Titania Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Titania Nanosheets
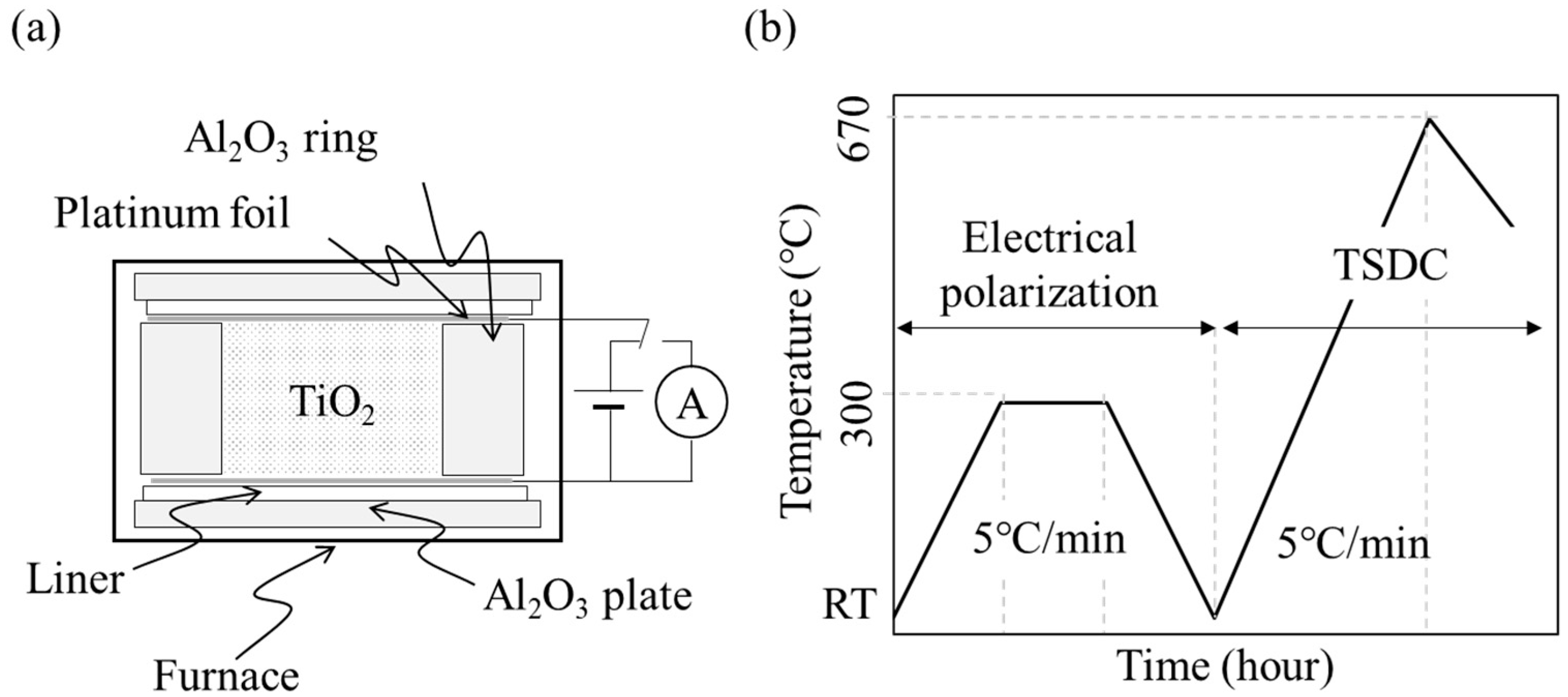
2.2. Electrical Polarization
2.3. Characterization
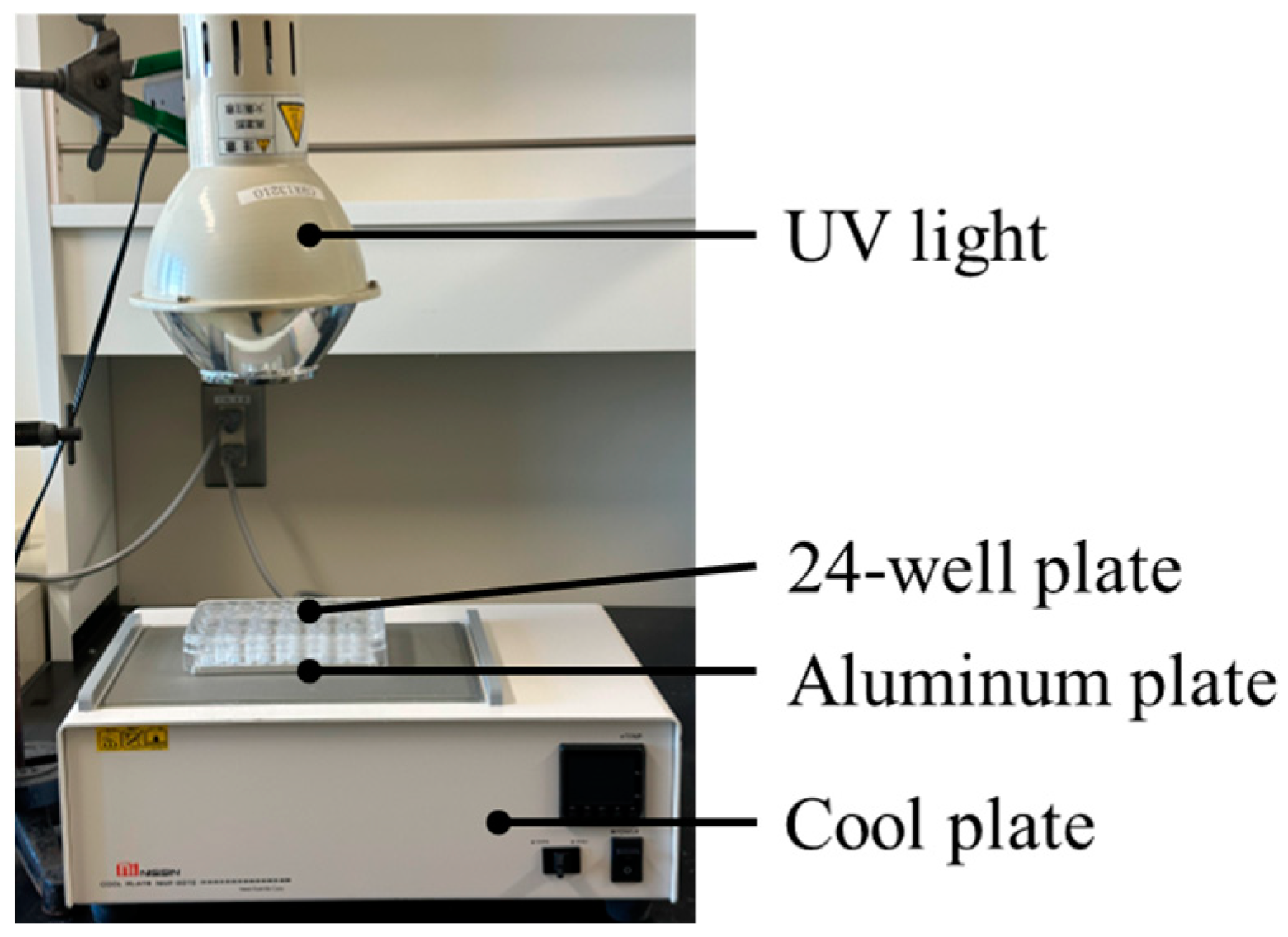
2.4. Dye-Degradation Assay
2.5. Measurement of ROS
2.6. Statistical Analysis
3. Results
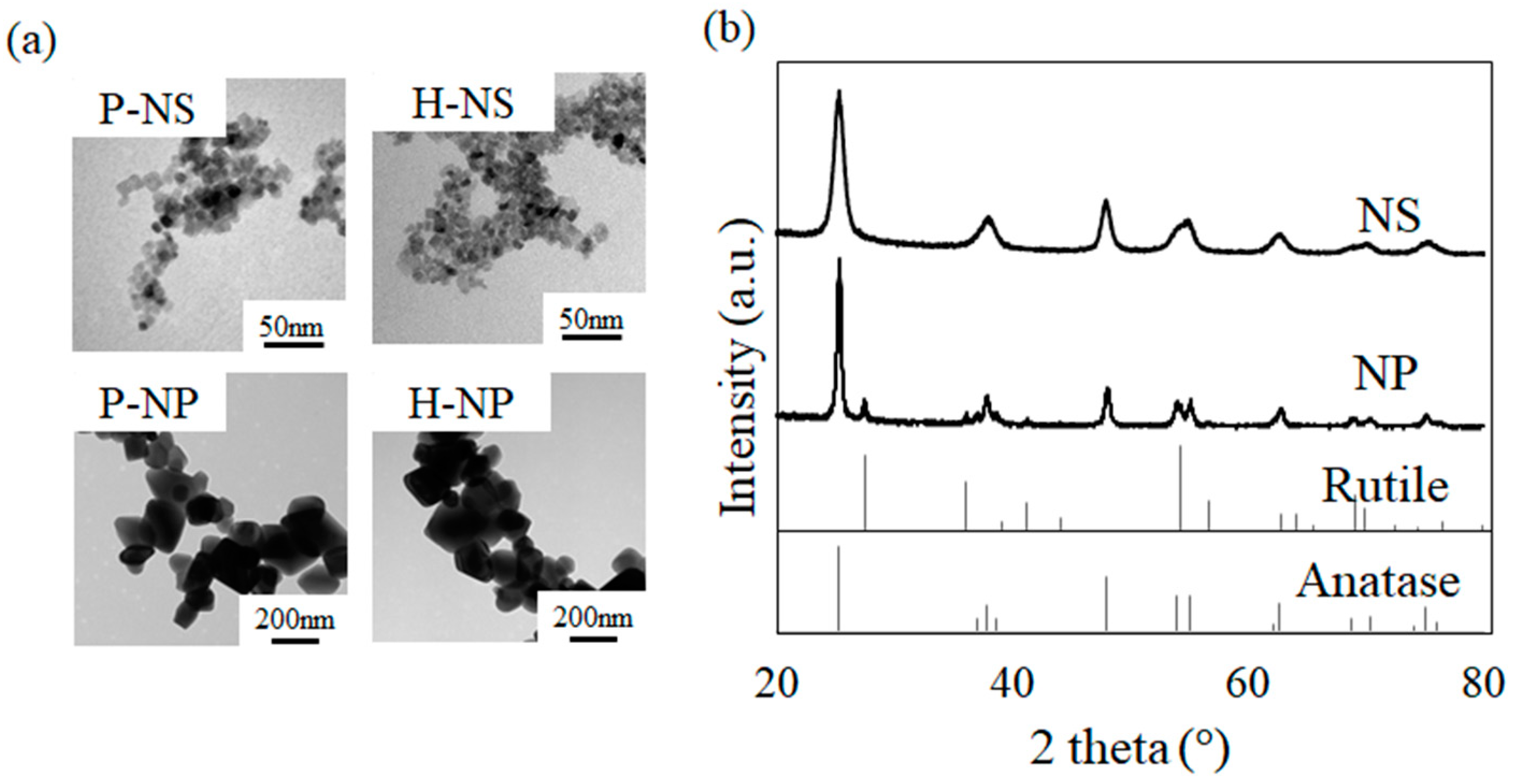
3.1. Crystal Morphology and Structure of Polarized Titania
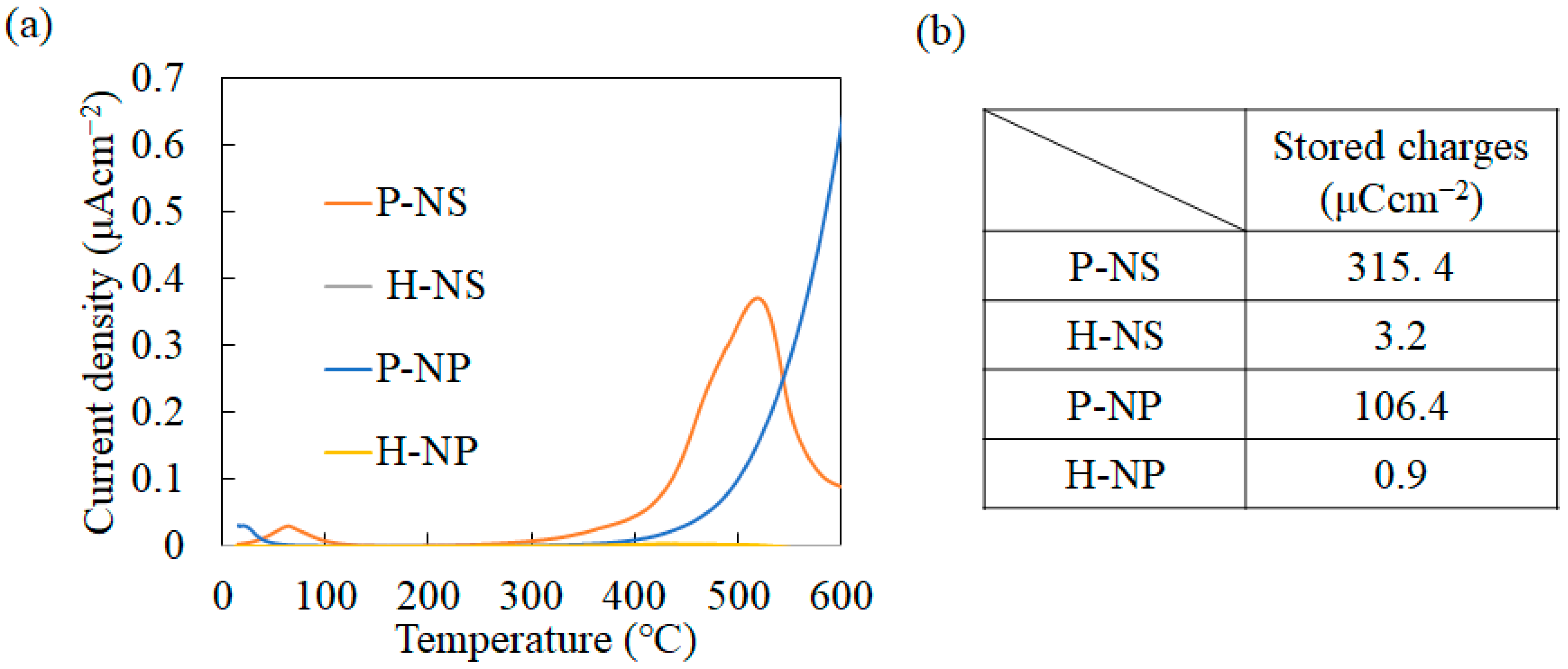
3.2. Surface Charges and Optical Bandgap
3.3. Effects of Electrical Polarization on Photocatalytic Properties of Titania Nanosheet
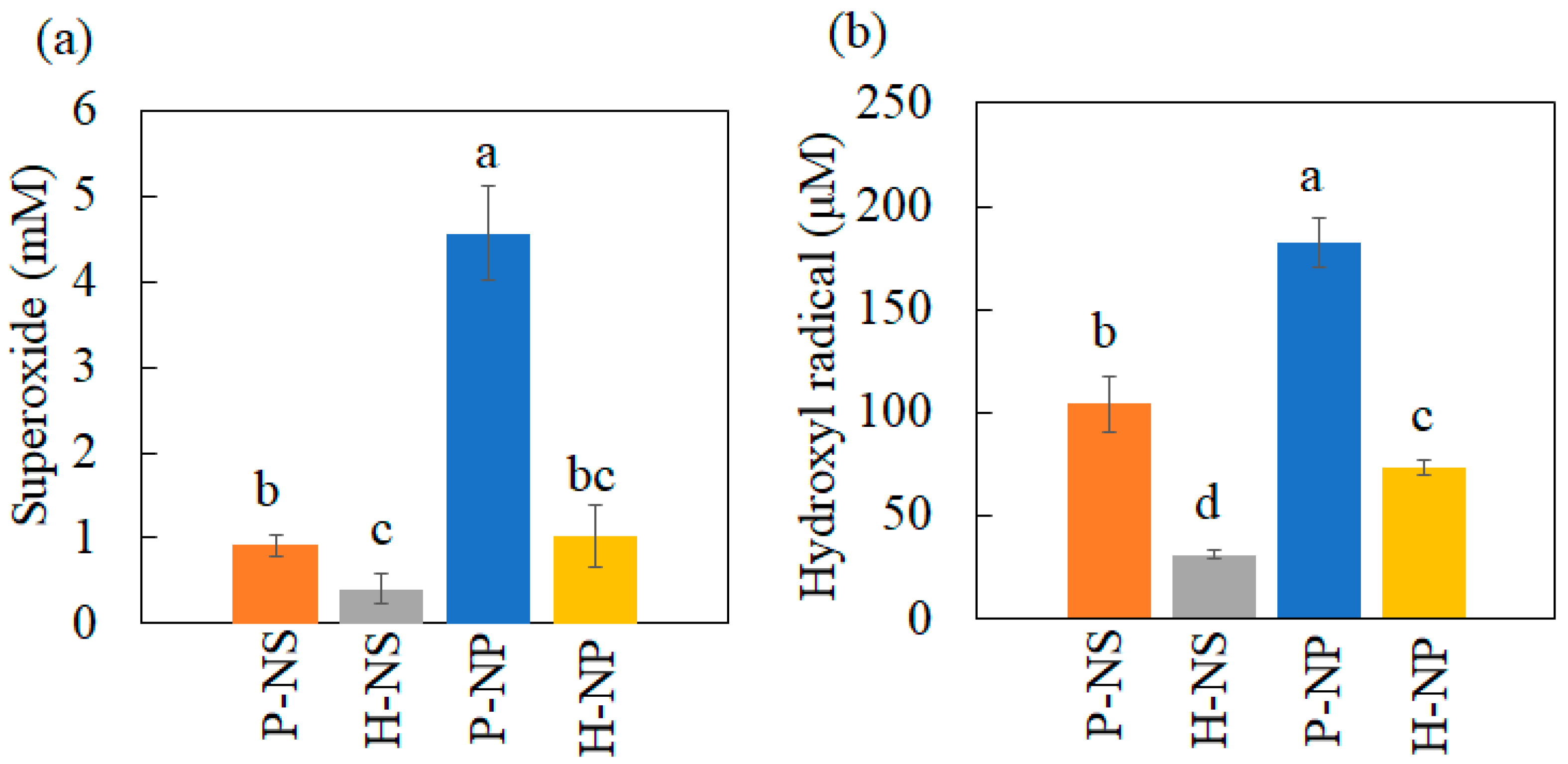
3.3.1. Production of Hydroxyl Radical and Superoxide Anion under UV Irradiation
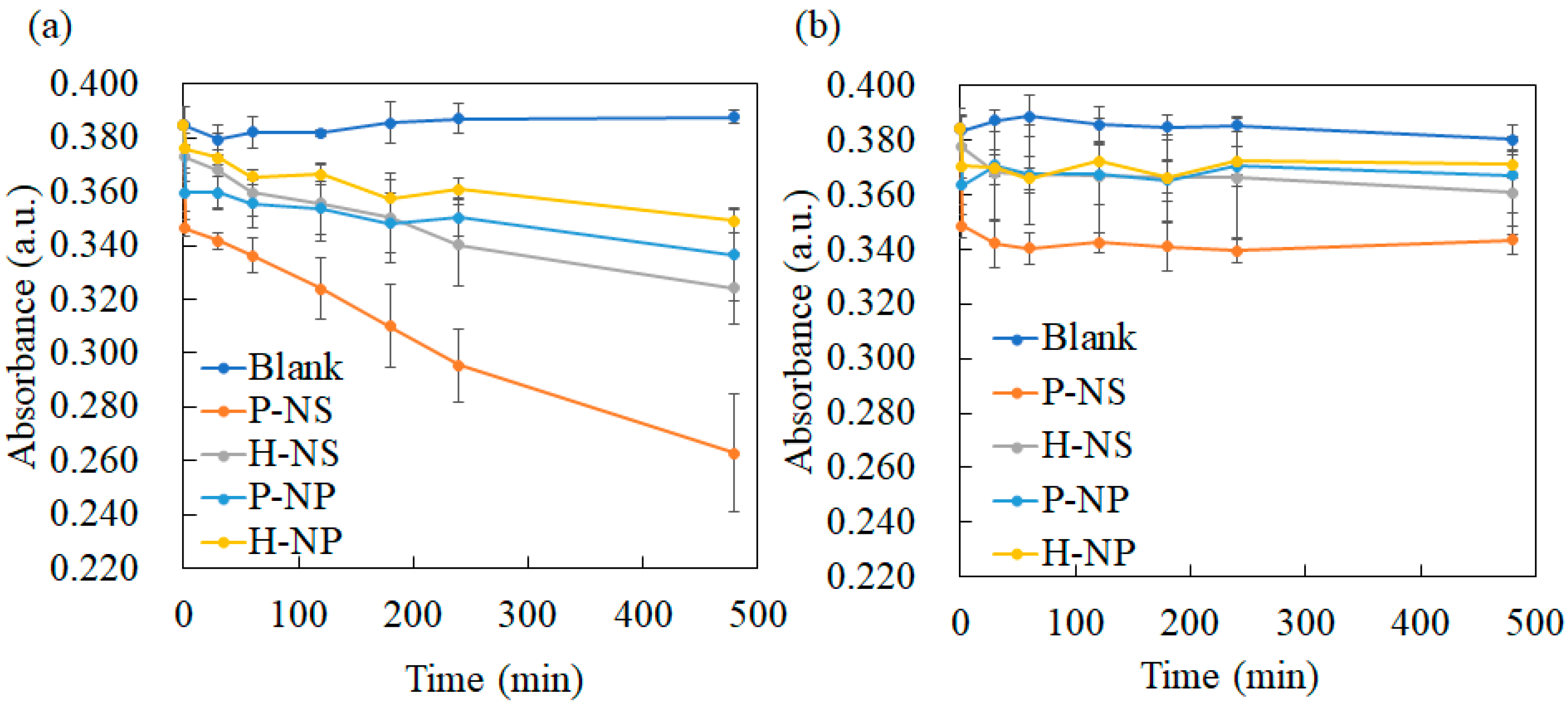
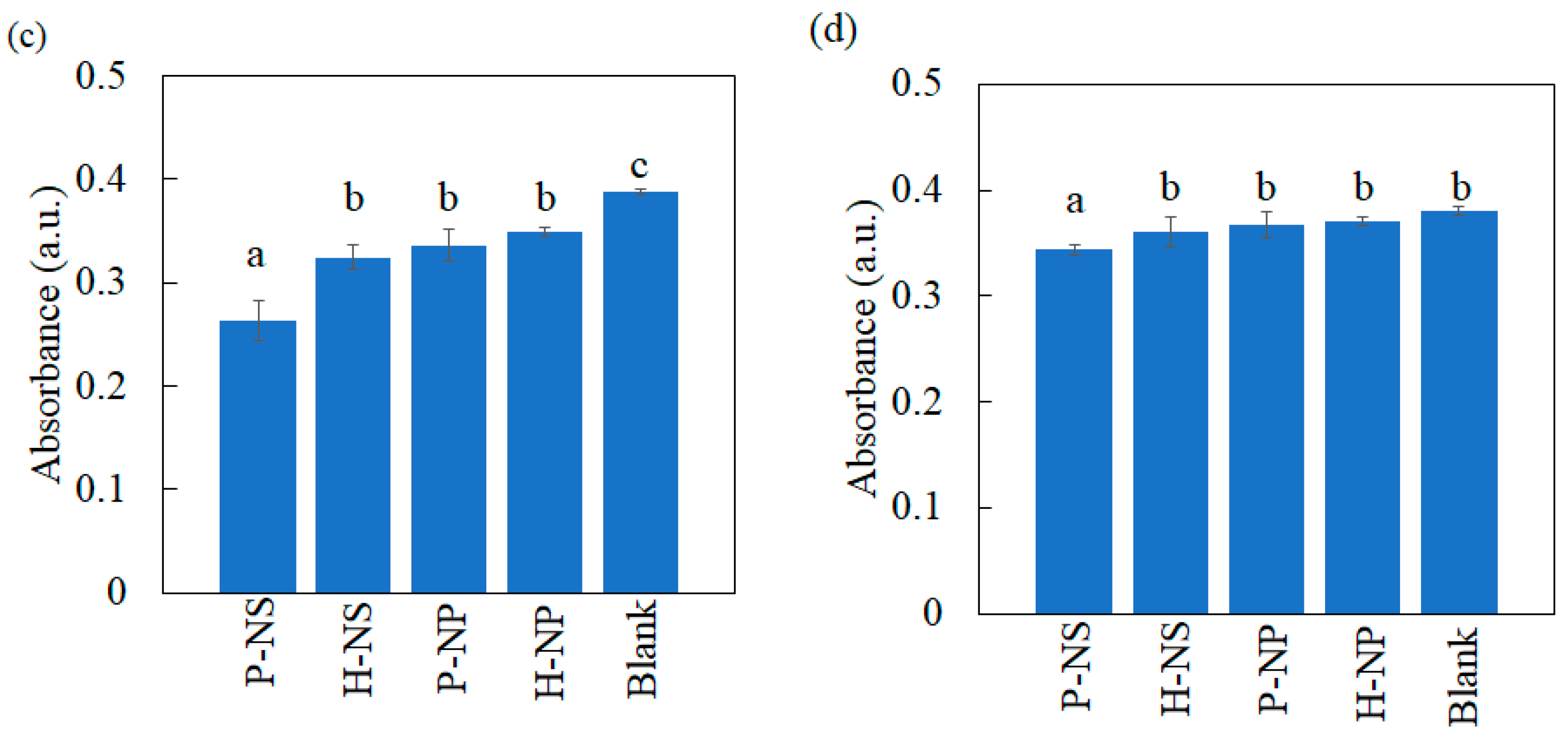
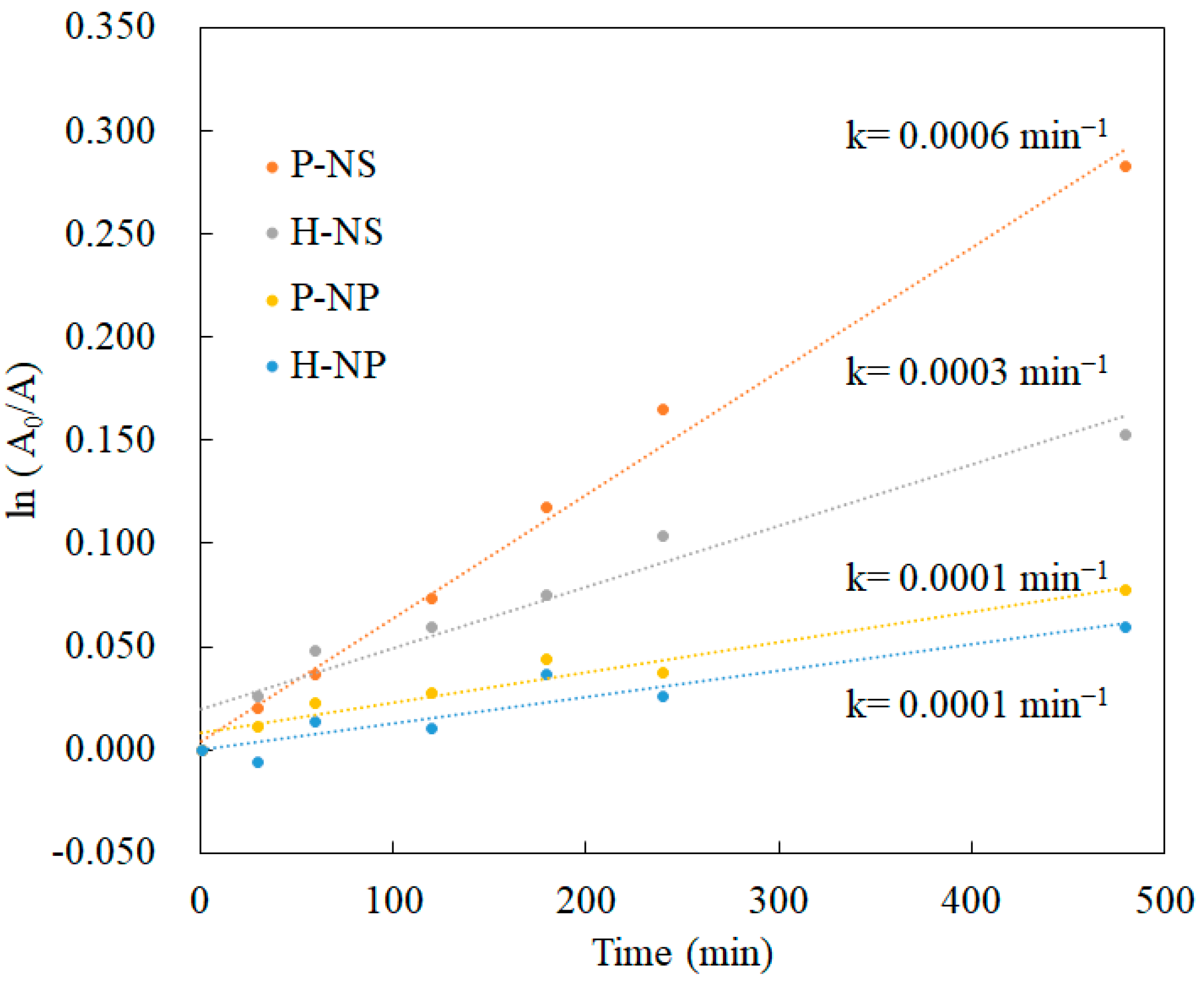
3.3.2. Decolorization Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.Y.; Guo, Y.; Zhang, L.; Xu, R.; Xing, Z.Q.; Wang, S.; Zhang, X. Interaction of bovine serum albumin with acridine orange (CI Basic Orange 14) and its sonodynamic damage under ultrasonic irradiation. Dye. Pigment. 2009, 80, 271–278. [Google Scholar] [CrossRef]
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron-hole recombination time at TiO2 single-crystal surfaces: Influence of surface band bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Liu, X.G.; Dong, G.J.; Li, S.P.; Lu, G.X.; Bi, Y.P. Direct observation of charge separation on anatase TiO2 crystals with selectively etched {001} facets. J. Am. Chem. Soc. 2016, 138, 2917–2920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.M.; Bao, J.H.; Fang, J.S.; Zhao, S.; Zhang, Y.W.; Sheng, X.; Chen, W. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, H.; Liu, J.; Bi, C.; Tian, J.; Zong, K.; Wang, B.; Ding, P.; Wang, X.; Chu, P.K.; et al. Stacking Engineering of Heterojunctions in Half-Metallic Carbon Nitride for Efficient CO2 Photoreduction. Adv. Sci. 2023, 10, 2307192. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Sato, K.; Takami, S.; Numako, C.; Umetsu, M.; Soga, K.; Nakayama, M.; Sasaki, R.; Tanaka, T.; Ogino, C.; et al. Particle size for photocatalytic activity of anatase TiO2 nanosheets with highly exposed {001} facets. RSC Adv. 2013, 3, 19268–19271. [Google Scholar] [CrossRef]
- Hayashi, K.; Nozaki, K.; Tan, Z.Q.; Fujita, K.; Nemoto, R.; Yamashita, K.; Miura, H.; Itaka, K.; Ohara, S. Enhanced antibacterial property of facet-engineered TiO2 nanosheet in presence and absence of ultraviolet irradiation. Materials 2020, 13, 78. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Quan, S.; Xia, M.; Wang, Q.; Zhang, W.; Yang, J.M. Effect of surface charge status of amorphous porous coordination polymer particles on the adsorption of organic dyes from an aqueous solution. J. Colloid Interface Sci. 2018, 525, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Pamu, D. A comprehensive review on electrical properties of hydroxyapatite based ceramic composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 539–563. [Google Scholar] [CrossRef]
- Saxena, A.; Kakimoto, K.; Dubey, A.K. Polarization induced dielectric and electrical response of electrovector hydroxyapatite and ferroelectric sodium potassium niobate ceramics. J. Phys. D 2020, 53, 395402. [Google Scholar] [CrossRef]
- Sans, J.; Arnau, M.; Fontana-Escartín, A.; Turon, P.; Alemán, C. Permanently polarized materials: An approach for designing materials with customized electrical properties. Chem. Mater. 2023, 35, 3765–3780. [Google Scholar] [CrossRef]
- Yamashita, K.; Oikawa, N.; Umegaki, T. Acceleration and deceleration of bone-like crystal growth on ceramic hydroxyapatite by electric poling. Chem. Mater. 1996, 8, 2697–2700. [Google Scholar] [CrossRef]
- Ubele-Kalnina, D.; Nakamura, M.; Gross, K.A. Inter-Laboratory Study on Measuring the Surface Charge of Electrically Polarized Hydroxyapatite. J. Funct. Biomater. 2023, 14, 100. [Google Scholar] [CrossRef]
- Belik, A.A.; Morozov, V.A.; Deyneko, D.V.; Savon, A.E.; Baryshnikova, O.V.; Zhukovskaya, E.S.; Dorbakov, N.G.; Katsuya, Y.; Tanaka, M.; Stefanovich, S.Y.; et al. Antiferroelectric properties and site occupations of R3+ cations in Ca8MgR(PO4)7 luminescent host materials. J. Alloys Compd. 2017, 699, 928–937. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mat. Sci. Eng. C-Mat. 2019, 104, 109883. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.S.; Singh, A.; Kumar, D.; Dubey, A.K. Electro-mechanical and Polarization-Induced Antibacterial Response of 45S5 Bioglass-Sodium Potassium Niobate Piezoelectric Ceramic Composites. ACS Biomater. Sci. Eng. 2020, 6, 3055–3069. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Yang, W.; Wang, X. Implementation of ferroelectric materials in photocatalytic and photoelectrochemical water splitting. Nanoscale Horiz. 2020, 5, 1174–1187. [Google Scholar] [CrossRef]
- Nagai, A.; Yamazaki, Y.; Ma, C.F.; Nozaki, K.; Toyama, T.; Yamashita, K. Response of osteoblast-like MG63 cells to TiO2 layer prepared by micro-arc oxidation and electric polarization. J. Eur. Ceram. Soc. 2012, 32, 2647–2652. [Google Scholar] [CrossRef]
- Ma, C.F.; Xiong, X.B.; Li, H.J.; Huang, J.F.; Lu, J.H.; Hu, Z.B. Response of osteoblast to CVIC/C and HA coating on CVIC/C in vitro. Rare Met. Mater. Eng. 2004, 33, 1274–1277. [Google Scholar]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef]
- Kowaka, Y.; Nozaki, K.; Mihara, T.; Yamashita, K.; Miura, H.; Tan, Z.; Ohara, S. Development of TiO2 nanosheets with high dye degradation performance by regulating crystal growth. Materials 2023, 16, 1229. [Google Scholar] [CrossRef]
- Sahni, M.; Locke, B.R. Quantification of hydroxyl radicals produced in aqueous phase pulsed electrical discharge reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Masudy, P.S.; Tanhaei, M.; Farahani, H.D.A.M.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Katal R. Chem. Eng. J. 2020, 384, 123384. [Google Scholar]
- Bi, X.; Du, G.; Kalam, A.; Sun, D.; Yu, Y.; Su, Q.; Xu, B.; Al-Sehemi, A.G. Tuning oxygen vacancy content in TiO2 nanoparticles to enhance the photocatalytic performance. Chem. Eng. Sci. 2021, 234, 116440. [Google Scholar] [CrossRef]
- Fu, R.; Wu, Z.; Pan, Z.; Gao, Z.; Li, Z.; Kong, X.; Li, L. Fluorine-Induced Surface Metallization for ammonia Synthesis underPhotoexcitation up to 1550 nm. Angew. Chem. Int. Ed. Engl. 2021, 60, 11173–11179. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (•OH) generation processes in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Nakamura, S.; Takeda, H.; Yamashita, K. Proton transport polarization and depolarization of hydroxyapatite ceramics. J. Appl. Phys. 2001, 89, 5386–5392. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihara, T.; Nozaki, K.; Kowaka, Y.; Jiang, M.; Yamashita, K.; Miura, H.; Ohara, S. Enhanced Photocatalysis of Electrically Polarized Titania Nanosheets. Nanomaterials 2024, 14, 171. https://doi.org/10.3390/nano14020171
Mihara T, Nozaki K, Kowaka Y, Jiang M, Yamashita K, Miura H, Ohara S. Enhanced Photocatalysis of Electrically Polarized Titania Nanosheets. Nanomaterials. 2024; 14(2):171. https://doi.org/10.3390/nano14020171
Chicago/Turabian StyleMihara, Tomoyuki, Kosuke Nozaki, Yasuyuki Kowaka, Mengtian Jiang, Kimihiro Yamashita, Hiroyuki Miura, and Satoshi Ohara. 2024. "Enhanced Photocatalysis of Electrically Polarized Titania Nanosheets" Nanomaterials 14, no. 2: 171. https://doi.org/10.3390/nano14020171
APA StyleMihara, T., Nozaki, K., Kowaka, Y., Jiang, M., Yamashita, K., Miura, H., & Ohara, S. (2024). Enhanced Photocatalysis of Electrically Polarized Titania Nanosheets. Nanomaterials, 14(2), 171. https://doi.org/10.3390/nano14020171





