Photocatalytic Degradation of Pharmaceutical Residues from Water and Sewage Effluent Using Different TiO2 Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Photocatalysis
2.3. Extraction and Analytical Procedures
3. Results and Discussion
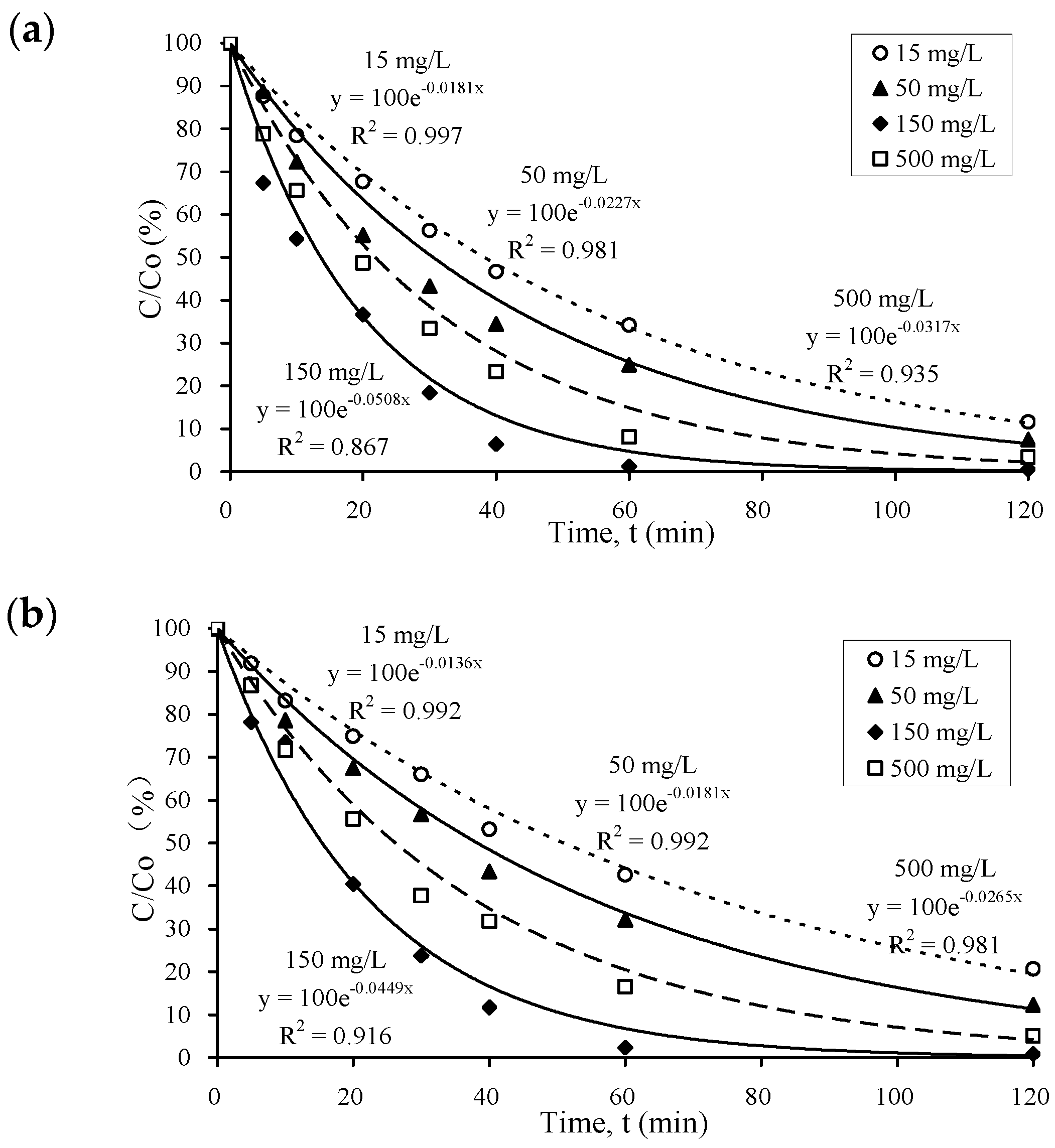
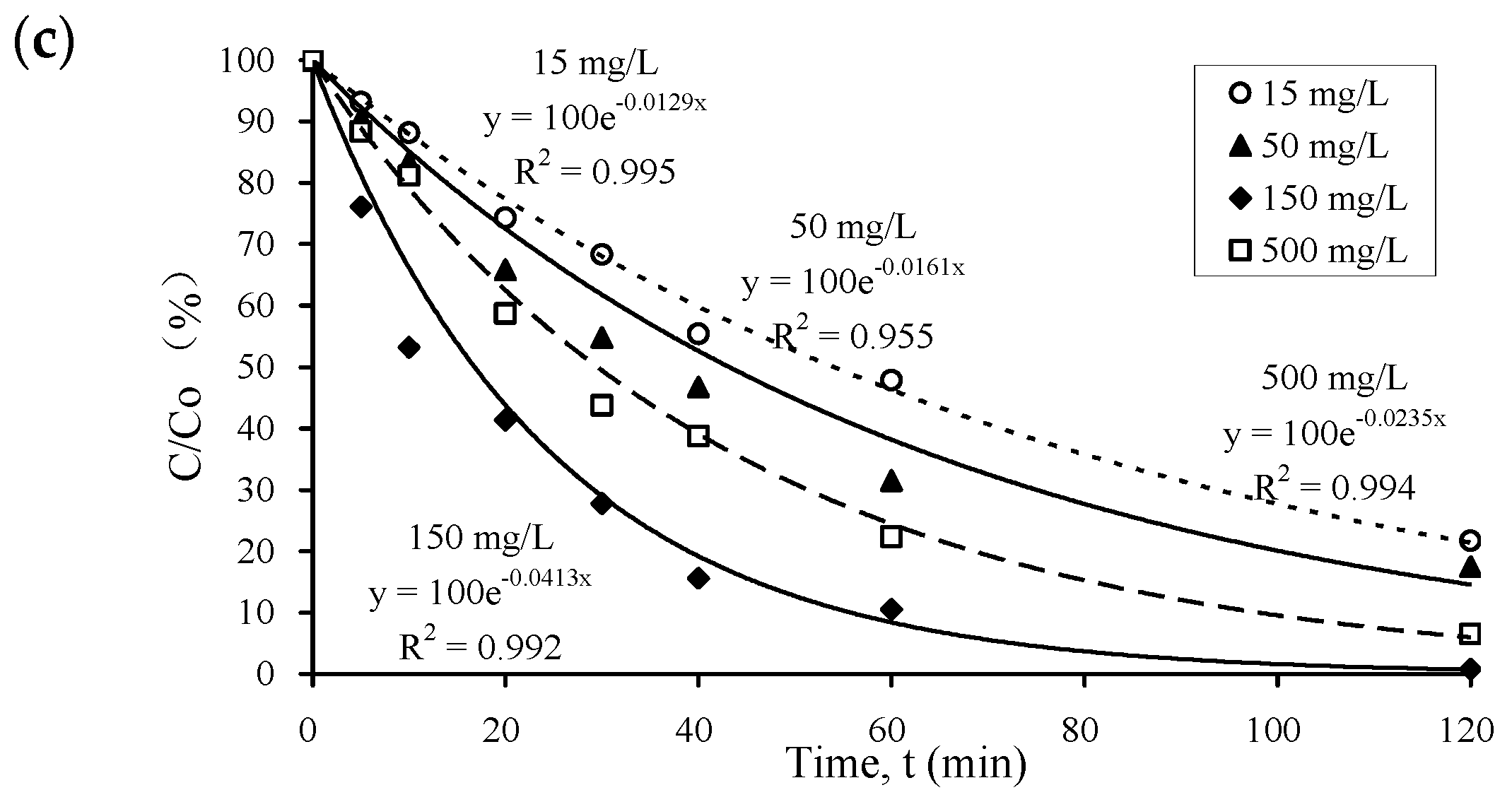
3.1. Kinetics of Pharmaceutical Photocatalysis in Water
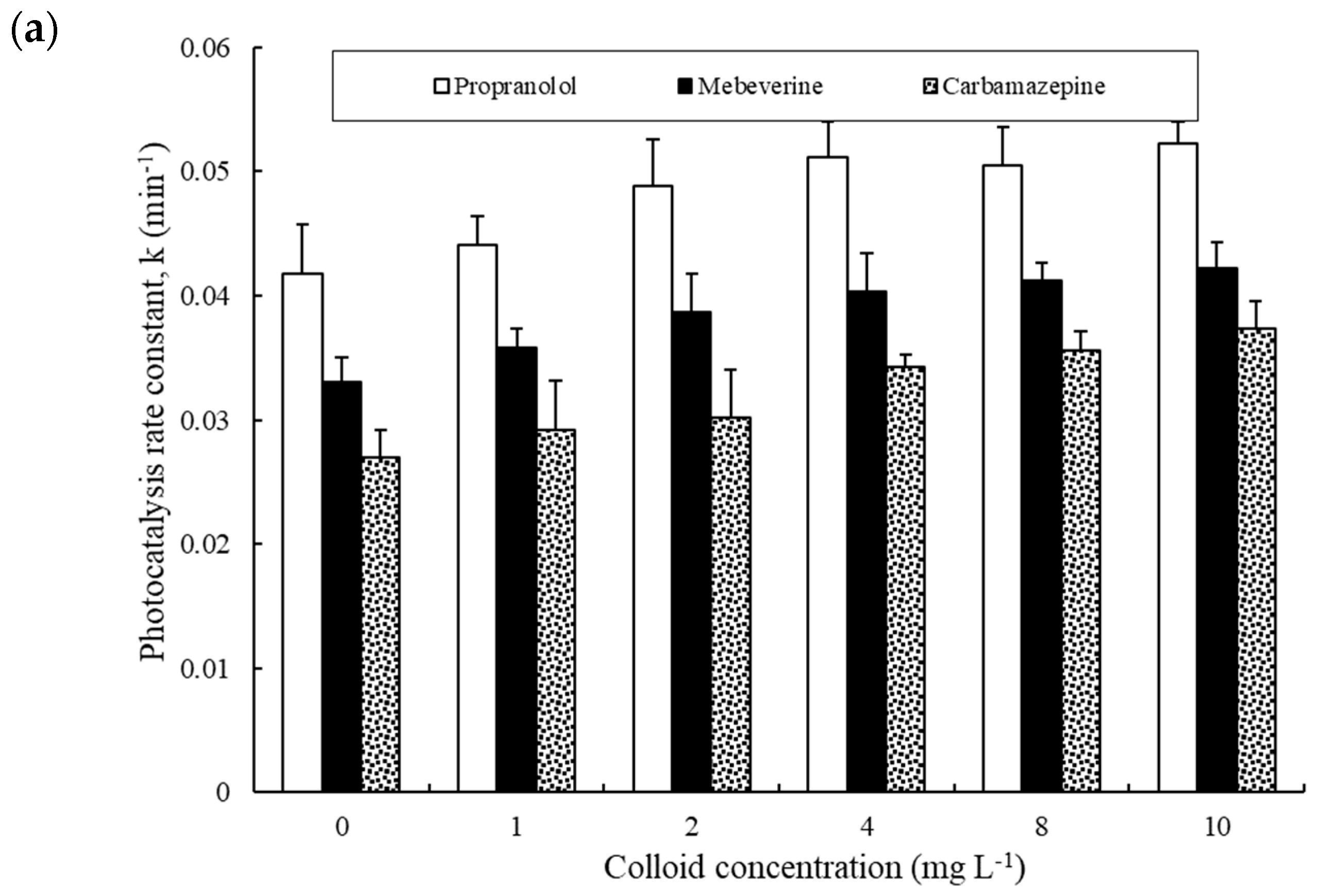
3.2. Performance of Different Photocatalysts at Different Dosages
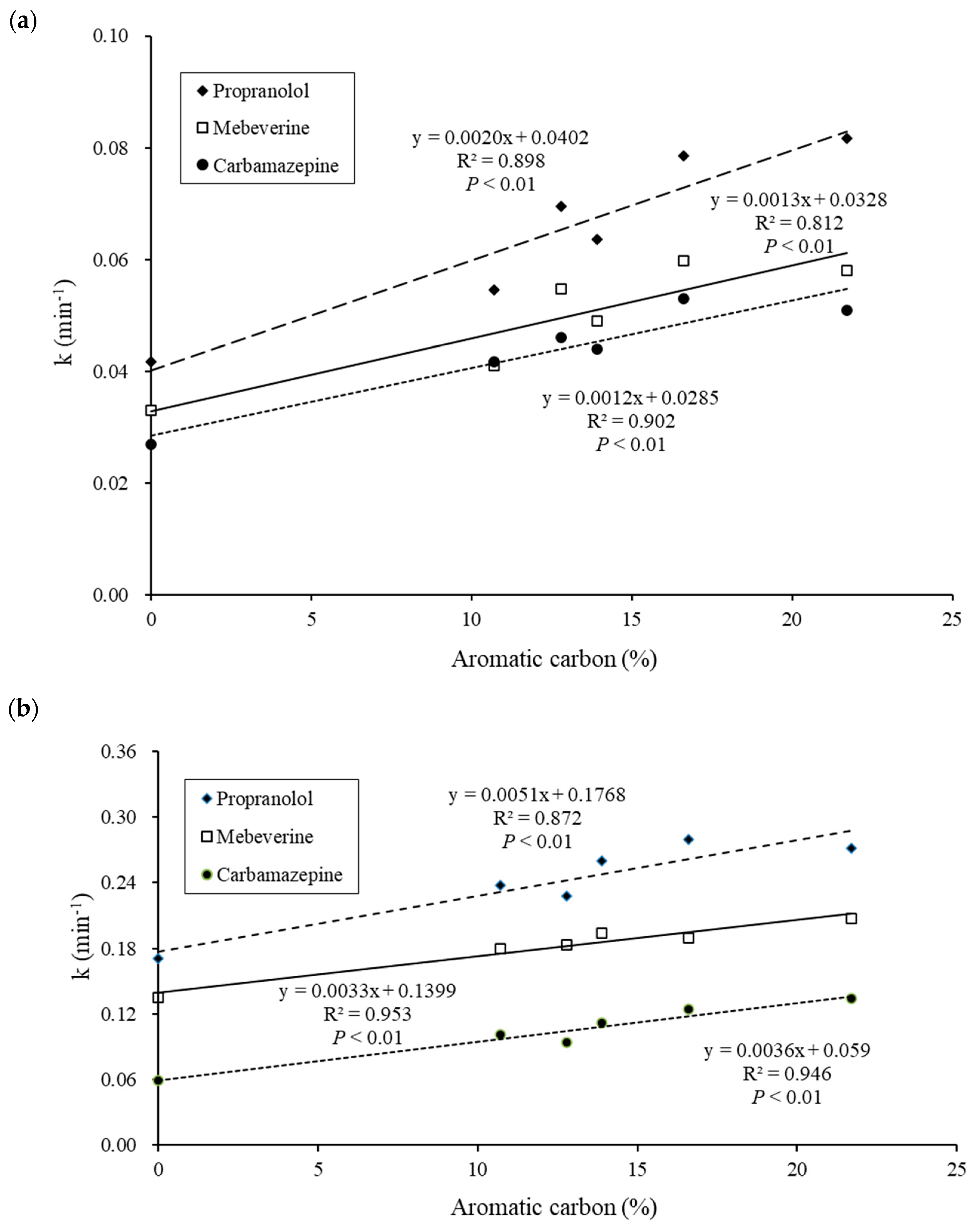
3.3. Effect of DOM on the Photocatalytic Degradation Rate
3.4. Probing of Radicals Involved in Pharmaceutical Photocatalysis
3.5. Photocatalytic Removal of Pharmaceuticals in Sewage Effluent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Madikizela, L.M.; Ncube, S. Health Effects and Risks Associated with the Occurrence of Pharmaceuticals and Their Metabolites in Marine Organisms and Seafood. Sci. Total Environ. 2022, 837, 155780. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Rezania, S.; Nazari, V.M. Pharmaceuticals and Personal Care Products in Aquatic Environments and Their Removal by Algae-Based Systems. Chemosphere 2022, 288, 132580. [Google Scholar] [CrossRef]
- Papac, J.; Ballesteros, S.G.; Tonkovic, S.; Kovacic, M.; Tomic, A.; Cvetnić, M.; Kusic, H.; Senta, I.; Terzić, S.; Ahel, M.; et al. Degradation of Pharmaceutical Memantine by Photo-Based Advanced Oxidation Processes: Kinetics, Pathways and Environmental Aspects. J. Environ. Chem. Eng. 2023, 11, 109334. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.L. Occurrence and Behavior of Antibiotics in Water and Sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef]
- Haroune, L.; Salaun, M.; Ménard, A.; Legault, C.Y.; Bellenger, J.-P. Photocatalytic Degradation of Carbamazepine and Three Derivatives Using TiO2 and ZnO: Effect of pH, Ionic Strength, and Natural Organic Matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef]
- Maskaoui, K.; Zhou, J.L. Colloids as a Sink for Certain Pharmaceuticals in the Aquatic Environment. Environ. Sci. Pollut. Res. 2010, 17, 898–907. [Google Scholar] [CrossRef]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved Removal of Estrogenic and Pharmaceutical Compounds in Sewage Effluent by Full Scale Granular Activated Carbon: Impact on Receiving River Water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Berk, D.; Yargeau, V. Photocatalytic Removal of 17α-Ethinylestradiol (EE2) and Levonorgestrel (LNG) from Contraceptive Pill Manufacturing Plant Wastewater under UVC Radiation. Chem. Eng. J. 2012, 185–186, 52–60. [Google Scholar] [CrossRef]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The Spread of Antibiotic Resistance to Humans and Potential Protection Strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, B.; Qu, X.; Fu, H.; Zhu, D. Dissolved Black Carbon as an Efficient Sensitizer in the Photochemical Transformation of 17β-Estradiol in Aqueous Solution. Environ. Sci. Technol. 2018, 52, 10391–10399. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-Assisted Photocatalytic Degradation of Emerging Contaminants in a Municipal WWTP Effluent Using a Solar Pilot Plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Akatsuka, M.; Kawaguchi, Y.; Itoh, R.; Ozawa, A.; Yamamoto, M.; Tanabe, T.; Yoshida, T. Preparation of Ga2O3 Photocatalyst Highly Active for CO2 Reduction with Water without Cocatalyst. Appl. Catal. B Environ. 2020, 262, 118247. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Jiang, L.; Xu, J.; Li, J.; Xia, Z.; Tan, C.; Zuo, J.; Wang, Y. Novel Photocatalyst Ag/ZnO/BC Nanofilms Degradation of Low Concentration Ammonia Nitrogen Wastewater. Coatings 2023, 13, 2043. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Safaei, J.; Zhang, G.; Mojiri, A.; Ni, B.-J.; Huang, Z.; Zhou, J.L. Photocatalytic and Photoelectrocatalytic Degradation of Perfluorooctanoic Acid by Immobilised ZnO Nanoparticles Using Electrophoretic Deposition. Environ. Sci. Nano 2023, 10, 1955–1965. [Google Scholar] [CrossRef]
- Malec, D.; Warszyńska, M.; Repetowski, P.; Siomchen, A.; Dąbrowski, J.M. Enhancing Visible-Light Photocatalysis with Pd(II) Porphyrin-Based TiO2 Hybrid Nanomaterials: Preparation, Characterization, ROS Generation, and Photocatalytic Activity. Molecules 2023, 28, 7819. [Google Scholar] [CrossRef]
- Mancuso, A.; Mottola, S.; Sacco, O.; Vaiano, V.; De Marco, I. Photocatalytic Degradation of Ceftriaxone Using TiO2 Coupled with ZnO Micronized by Supercritical Antisolvent Route. Nanomaterials 2023, 13, 3130. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, S.; Jiang, X.; Kang, Z.; Gao, S.; Liu, W.; Shen, Y. Visible-Light-Driven BiOBr-TiO2-Attapulgite Photocatalyst with Excellent Photocatalytic Activity for Multiple Xanthates. Catalysts 2023, 13, 1504. [Google Scholar] [CrossRef]
- Xiang, J.; Shang, J.; Wan, Z. Enhanced Photocatalytic Dehalogenation Performance of RuDoped In2O3 Nanoparticles Induced by Oxygen Vacancy. Photochem 2023, 3, 360–372. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, W.; Lin, C.; Xu, L.; Zhu, T.; Ai, X. CdS Deposited In Situ on g-C3N4 via a Modified Chemical Bath Deposition Method to Improve Photocatalytic Hydrogen Production. Molecules 2023, 28, 7846. [Google Scholar] [CrossRef]
- Nguyen Xuan, T.; Nguyen Thi, D.; Tran Thuong, Q.; Nguyen Ngoc, T.; Dang Quoc, K.; Molnár, Z.; Mukhtar, S.; Szabó-Bárdos, E.; Horváth, O. Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols. Molecules 2023, 28, 7810. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Hao, D.; Li, X.; Li, D.; Huang, Z.; Zhou, J.L. Key Factors in Improving the Synthesis and Properties of Visible-Light Activated g-C3N4 for Photocatalytic Hydrogen Production and Organic Pollutant Decomposition. Catal. Rev. 2023; in press. [Google Scholar] [CrossRef]
- Du, C.; Xu, J.; Ding, G.; He, D.; Zhang, H.; Qiu, W.; Li, C.; Liao, G. Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal. Nanomaterials 2023, 13, 3066. [Google Scholar] [CrossRef] [PubMed]
- Gaffar, S.; Kumar, A.; Alam, J.; Riaz, U. Efficient Visible Light–Induced Photocatalytic Degradation of Tetracycline Hydrochloride Using CuFe2O4 and PANI/CuFe2O4 Nanohybrids. Environ. Sci. Pollut. Res. 2023, 30, 108878–108888. [Google Scholar] [CrossRef] [PubMed]
- Suo, S.; Ma, W.; Zhang, S.; Han, Z.; Wang, Y.; Li, Y.; Xiong, Y.; Liu, Y.; He, C.; Fang, P. MOF-Derived Spindle-Shaped Z-Scheme ZnO/ZnFe2O4 Heterojunction: A Magnetic Recovery Catalyst for Efficient Photothermal Degradation of Tetracycline Hydrochloride. Materials 2023, 16, 6639. [Google Scholar] [CrossRef] [PubMed]
- Navidpour, A.H.; Abbasi, S.; Li, D.; Mojiri, A.; Zhou, J.L. Investigation of Advanced Oxidation Process in the Presence of TiO2 Semiconductor as Photocatalyst: Property, Principle, Kinetic Analysis, and Photocatalytic Activity. Catalysts 2023, 13, 232. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Q.; Wu, H.; Zhou, Y.; Zhu, M.; Lu, Z.-H. Carbon-Doped Mesoporous TiO2-Immobilized Ni Nanoparticles: Oxygen Defect Engineering Enhances Hydrogen Production. Appl. Catal. B Environ. 2023, 339, 123153. [Google Scholar] [CrossRef]
- Lan, K.; Liu, Y.; Zhang, W.; Liu, Y.; Elzatahry, A.; Wang, R.; Xia, Y.; Al-Dhayan, D.; Zheng, N.; Zhao, D. Uniform Ordered Two-Dimensional Mesoporous TiO2 Nanosheets from Hydrothermal-Induced Solvent-Confined Monomicelle Assembly. J. Am. Chem. Soc. 2018, 140, 4135–4143. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.; Mithun Prakash, R.; Do, T.-O. Insights into the TiO2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Álvarez-Conde, J.; Bedia, J.; García-Frutos, E.M.; Belver, C. Azaindole Grafted Titanium Dioxide for the Photodegradation of Pharmaceuticals Under Solar Irradiation. J. Colloid Interface Sci. 2023, 629, 593–603. [Google Scholar] [CrossRef]
- Fauzi, A.A.; Jalil, A.A.; Hitam, C.N.C.; Aziz, F.F.A.; Chanlek, N. Superior Sulfate Radicals-Induced Visible-Light-Driven Photodegradation of Pharmaceuticals by Appropriate Ce Loading on Fibrous Silica Ceria. J. Environ. Chem. Eng. 2020, 8, 104484. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Maldonado, M.I.; Gimenez, J.; Esplugas, S.; Malato, S. Abatement of Ibuprofen by Solar Photocatalysis Process: Enhancement and Scale Up. Catal. Today 2009, 144, 112–116. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of Diclofenac by TiO2 Photocatalysis: UV Absorbance Kinetics and Process Evaluation Through a Set of Toxicity Bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of Paracetamol in Aqueous Solutions by TiO2 Photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. Factors Affecting Diclofenac Decomposition in Water by UV-A/TiO2 Photocatalysis. Chem. Eng. J. 2010, 161, 53–59. [Google Scholar] [CrossRef]
- Mohapatra, S.; Snow, D.; Shea, P.; Gálvez-Rodríguez, A.; Kumar, M.; Padhye, L.P.; Mukherji, S. Photodegradation of a Mixture of Five Pharmaceuticals Commonly Found in Wastewater: Experimental and Computational Analysis. Environ. Res. 2023, 216, 114659. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, Q.; Wang, J.; Chen, X.; Niu, J.; Chen, J. Effects of Dissolved Organic Matter Derived from Freshwater and Seawater on Photodegradation of Three Antiviral Drugs. Environ. Pollut. 2020, 258, 113700. [Google Scholar] [CrossRef]
- Wenk, J.; Aeschbacher, M.; Sander, M.; Gunten, U.v.; Canonica, S. Photosensitizing and Inhibitory Effects of Ozonated Dissolved Organic Matter on Triplet-Induced Contaminant Transformation. Environ. Sci. Technol. 2015, 49, 8541–8549. [Google Scholar] [CrossRef]
- Wenk, J.; Eustis, S.N.; McNeill, K.; Canonica, S. Quenching of Excited Triplet States by Dissolved Natural Organic Matter. Environ. Sci. Technol. 2013, 47, 12802–12810. [Google Scholar] [CrossRef]
- Zhou, J.L.; Liu, R.; Wilding, A.; Hibberd, A. Sorption of Selected Endocrine Disrupting Chemicals to Different Aquatic Colloids. Environ. Sci. Technol. 2007, 41, 206–213. [Google Scholar] [CrossRef]
- Zhou, J.L.; Maskaoui, K.; Lufadeju, A. Optimization of Antibiotic Analysis in Water by Solid-Phase Extraction and High Performance Liquid Chromatography–Mass Spectrometry/Mass Spectrometry. Anal. Chim. Acta 2012, 731, 32–39. [Google Scholar] [CrossRef]
- Ngo, H.-S.; Nguyen, T.-L.; Tran, N.-T.; Le, H.-C. Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst. Catalysts 2023, 13, 225. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, L.M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Kinetics and Mechanism of Aqueous Degradation of Carbamazepine by Heterogeneous Photocatalysis Using Nanocrystalline TiO2, ZnO and Multi-Walled Carbon Nanotubes–Anatase Composites. Appl. Catal. B Environ. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, L.M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Aqueous Degradation of Diclofenac by Heterogeneous Photocatalysis Using Nanostructured Materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- So, C.M.; Cheng, M.Y.; Yu, J.C.; Wong, P.K. Degradation of Azo Dye Procion Red MX-5B by Photocatalytic Oxidation. Chemosphere 2002, 46, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Shaya, J.; Tesana, S.; Golovko, V.B.; Wang, S.-Y.; Liao, Y.-Y.; Lu, C.-S.; Chen, C.-C. Visible-Light Driven Photocatalytic Degradation of Pirimicarb by Pt-Doped AgInS2 Nanoparticles. Catalysts 2020, 10, 857. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the Photoelectronic and Photocatalytic Activities of Various Anatase and Rutile Forms of Titania in Pure Liquid Organic Phases and in Aqueous Solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Kaneko, M.; Okura, I. Photocatalysis: Science and Technology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar/UV-Induced Photocatalytic Degradation of Three Commercial Textile Dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Garbin, J.R.; Milori, D.M.B.P.; Simões, M.L.; da Silva, W.T.L.; Neto, L.M. Influence of Humic Substances on the Photolysis of Aqueous Pesticide Residues. Chemosphere 2007, 66, 1692–1698. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Miller, P.L.; Zeng, L.; Cawley, K.; Weavers, L.K. Photosensitized Degradation of Bisphenol A by Dissolved Organic Matter. Environ. Sci. Technol. 2004, 38, 5888–5894. [Google Scholar] [CrossRef]
- Caupos, E.; Mazellier, P.; Croue, J.-P. Photodegradation of Estrone Enhanced by Dissolved Organic Matter Under Simulated Sunlight. Water Res. 2011, 45, 3341–3350. [Google Scholar] [CrossRef]
- Leech, D.M.; Snyder, M.T.; Wetzel, R.G. Natural Organic Matter and Sunlight Accelerate the Degradation of 17ß-Estradiol in Water. Sci. Total Environ. 2009, 407, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, W.; Han, L.; Chen, L.; Xu, E.G.; Xing, B.; Yang, Z. Unraveling the Role of Natural and Pyrogenic Dissolved Organic Matter in Photodegradation of Biodegradable Microplastics in Freshwater. Carbon Res. 2023, 2, 18. [Google Scholar] [CrossRef]
- Vinge, S.L.; Shaheen, S.W.; Sharpless, C.M.; Linden, K.G. Nitrate with Benefits: Optimizing Radical Production During UV Water Treatment. Environ. Sci. Water Res. Technol. 2020, 6, 1163–1175. [Google Scholar] [CrossRef]
- Zhan, M.; Yang, X.; Xian, Q.; Kong, L. Photosensitized Degradation of Bisphenol A Involving Reactive Oxygen Species in the Presence of Humic Substances. Chemosphere 2006, 63, 378–386. [Google Scholar] [CrossRef] [PubMed]






| Photocatalyst | Degussa P25 | Aldrich | Hombikat UV100 |
| Manufacturer | Degussa AG | Aldrich | Sachtleben Chemie GmBH |
| Density (g mL−1) at 20 °C | 3.8 | 4.17 | 3.9 |
| Average diameter of primary particles (nm) | 21 | 72 | 5 |
| BET surface area (m2 g−1) | 50 | 152 | 270 |
| Anatase by XRD (%) | 70 | 5 | 100 |
| Rutile by XRD (%) | 30 | 95 | 0 |
| Photocatalyst | Photocatalyst Dose (mg L−1) | Reactor | Propranolol | Mebeverine | Carbamazepine | |||
|---|---|---|---|---|---|---|---|---|
| k (min−1) | t½ (min) | k (min−1) | t½ (min) | k (min−1) | t½ (min) | |||
| Degussa P25 | 50 | TQ 150 | 0.032 ± 0.005 | 21.7 | 0.028 ± 0.006 | 24.8 | 0.019 ± 0.004 | 36.5 |
| 150 | 0.042 ± 0.004 | 16.5 | 0.033 ± 0.002 | 21.0 | 0.027 ± 0.002 | 25.7 | ||
| 15 | TNN 15–32 | 0.079 ± 0.006 | 8.8 | 0.062 ± 0.006 | 11.2 | 0.030 ± 0.006 | 23.1 | |
| 50 | 0.102 ± 0.014 | 6.8 | 0.089 ± 0.016 | 7.8 | 0.040 ± 0.008 | 17.3 | ||
| 150 | 0.171 ± 0.035 | 4.1 | 0.136 ± 0.009 | 5.1 | 0.059 ± 0.004 | 11.7 | ||
| Aldrich | 15 | TNN 15–32 | 0.018 ± 0.002 | 38.5 | 0.014 ± 0.003 | 49.5 | 0.013 ± 0.002 | 53.3 |
| 50 | 0.023 ± 0.002 | 30.1 | 0.018 ± 0.003 | 38.5 | 0.016 ± 0.001 | 43.3 | ||
| 150 | 0.051 ± 0.003 | 13.6 | 0.045 ± 0.005 | 15.4 | 0.041 ± 0.003 | 16.9 | ||
| 500 | 0.032 ± 0.004 | 21.7 | 0.027 ± 0.002 | 25.7 | 0.024 ± 0.002 | 28.9 | ||
| Hombikat UV100 | 15 | TNN 15–32 | 0.027 ± 0.003 | 25.7 | 0.020 ± 0.001 | 34.7 | 0.018 ± 0.002 | 38.5 |
| 50 | 0.030 ± 0.004 | 23.1 | 0.028 ± 0.003 | 24.8 | 0.022 ± 0.003 | 31.5 | ||
| 150 | 0.058 ± 0.004 | 11.9 | 0.048 ± 0.004 | 14.4 | 0.042 ± 0.004 | 16.5 | ||
| 500 | 0.039 ± 0.002 | 17.8 | 0.032 ± 0.004 | 21.7 | 0.031 ± 0.005 | 22.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navidpour, A.H.; Ahmed, M.B.; Zhou, J.L. Photocatalytic Degradation of Pharmaceutical Residues from Water and Sewage Effluent Using Different TiO2 Nanomaterials. Nanomaterials 2024, 14, 135. https://doi.org/10.3390/nano14020135
Navidpour AH, Ahmed MB, Zhou JL. Photocatalytic Degradation of Pharmaceutical Residues from Water and Sewage Effluent Using Different TiO2 Nanomaterials. Nanomaterials. 2024; 14(2):135. https://doi.org/10.3390/nano14020135
Chicago/Turabian StyleNavidpour, Amir Hossein, Mohammad Boshir Ahmed, and John L. Zhou. 2024. "Photocatalytic Degradation of Pharmaceutical Residues from Water and Sewage Effluent Using Different TiO2 Nanomaterials" Nanomaterials 14, no. 2: 135. https://doi.org/10.3390/nano14020135
APA StyleNavidpour, A. H., Ahmed, M. B., & Zhou, J. L. (2024). Photocatalytic Degradation of Pharmaceutical Residues from Water and Sewage Effluent Using Different TiO2 Nanomaterials. Nanomaterials, 14(2), 135. https://doi.org/10.3390/nano14020135








