AFM for Nanomechanical Assessment of Polymer Overcoatings on Nanoparticle-Decorated Biomaterials
Abstract
1. Introduction
2. Methods and Materials
2.1. Sample Preparation
2.2. Atomic Force Microscopy (AFM)
2.3. LFM Mode
2.4. Statistical Analysis
3. Results and Discussion
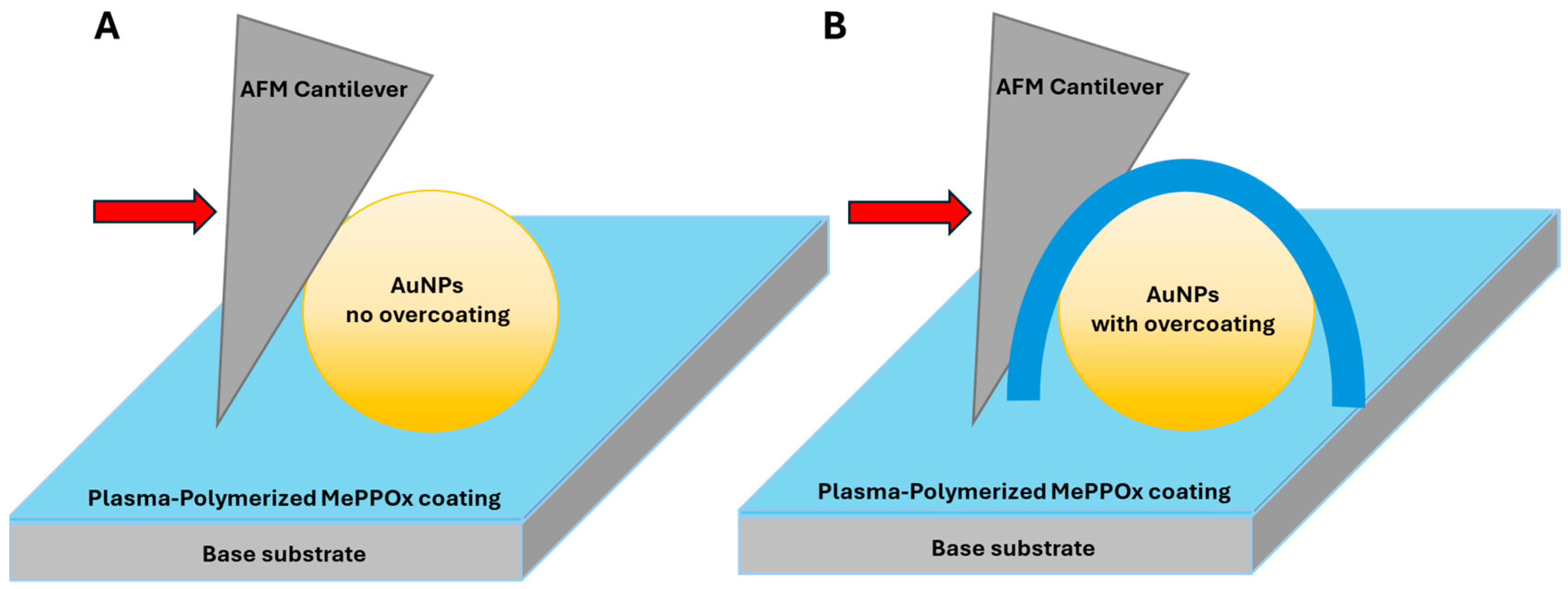
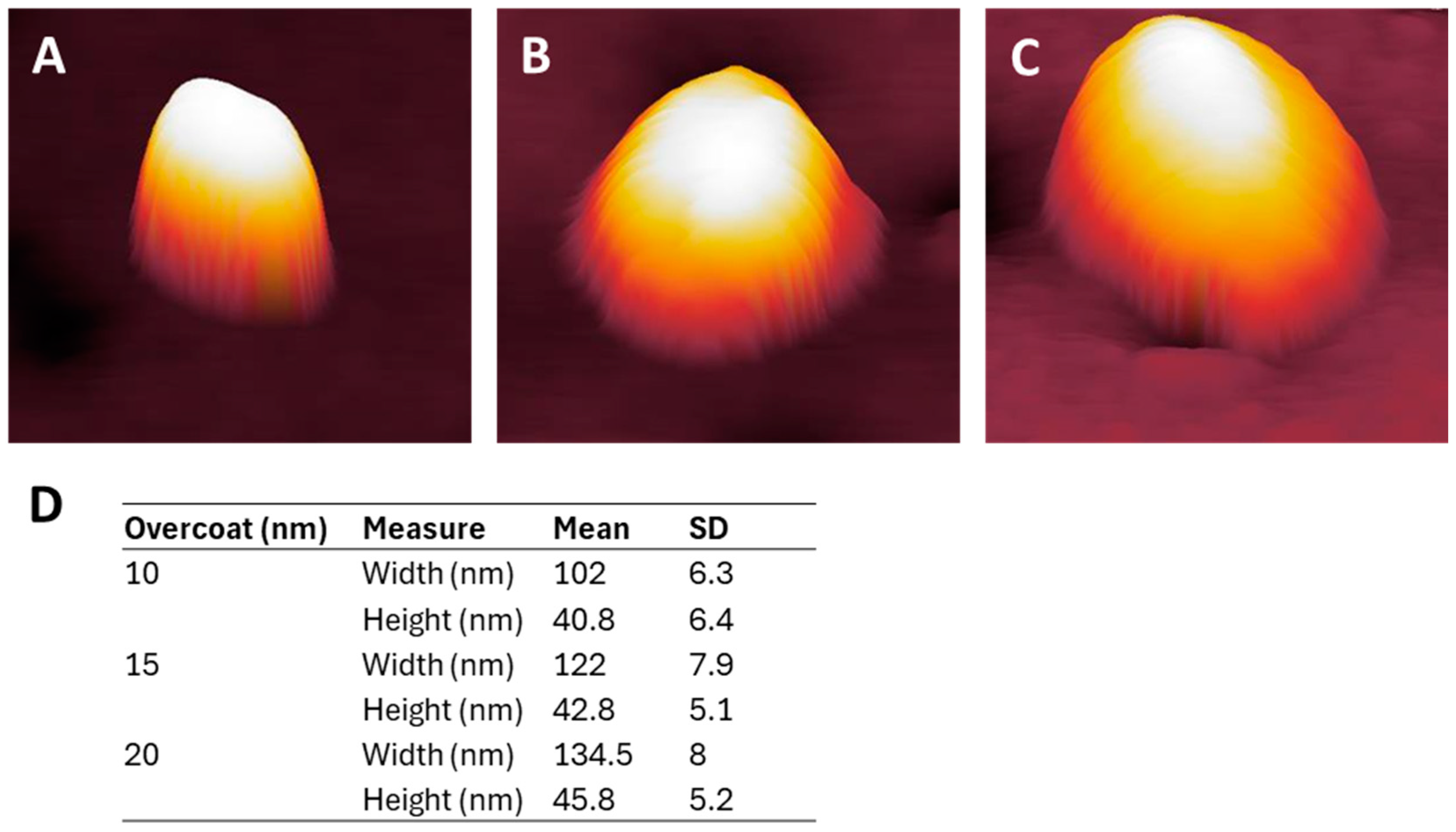
3.1. NP Resolution of Overcoating
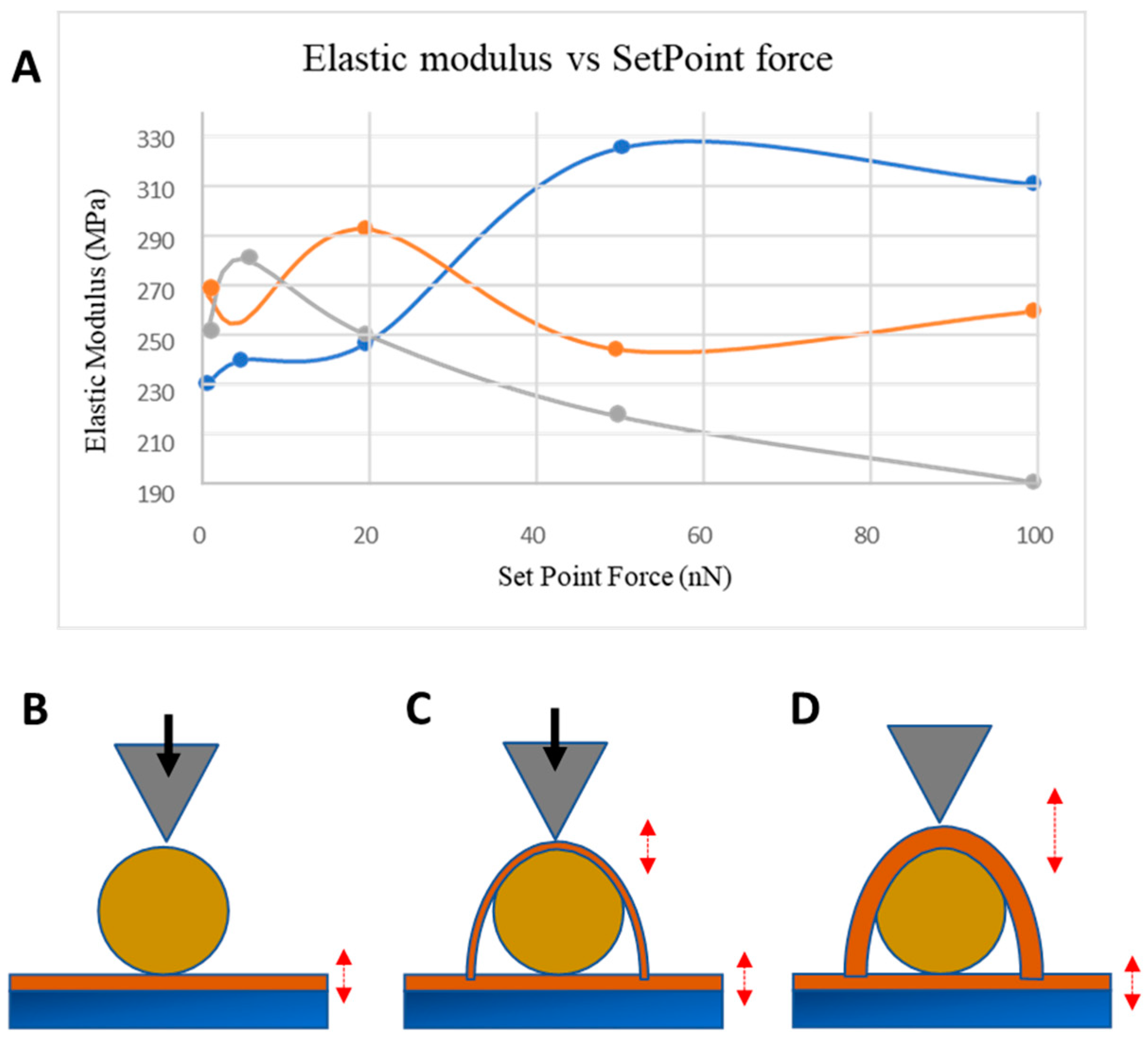
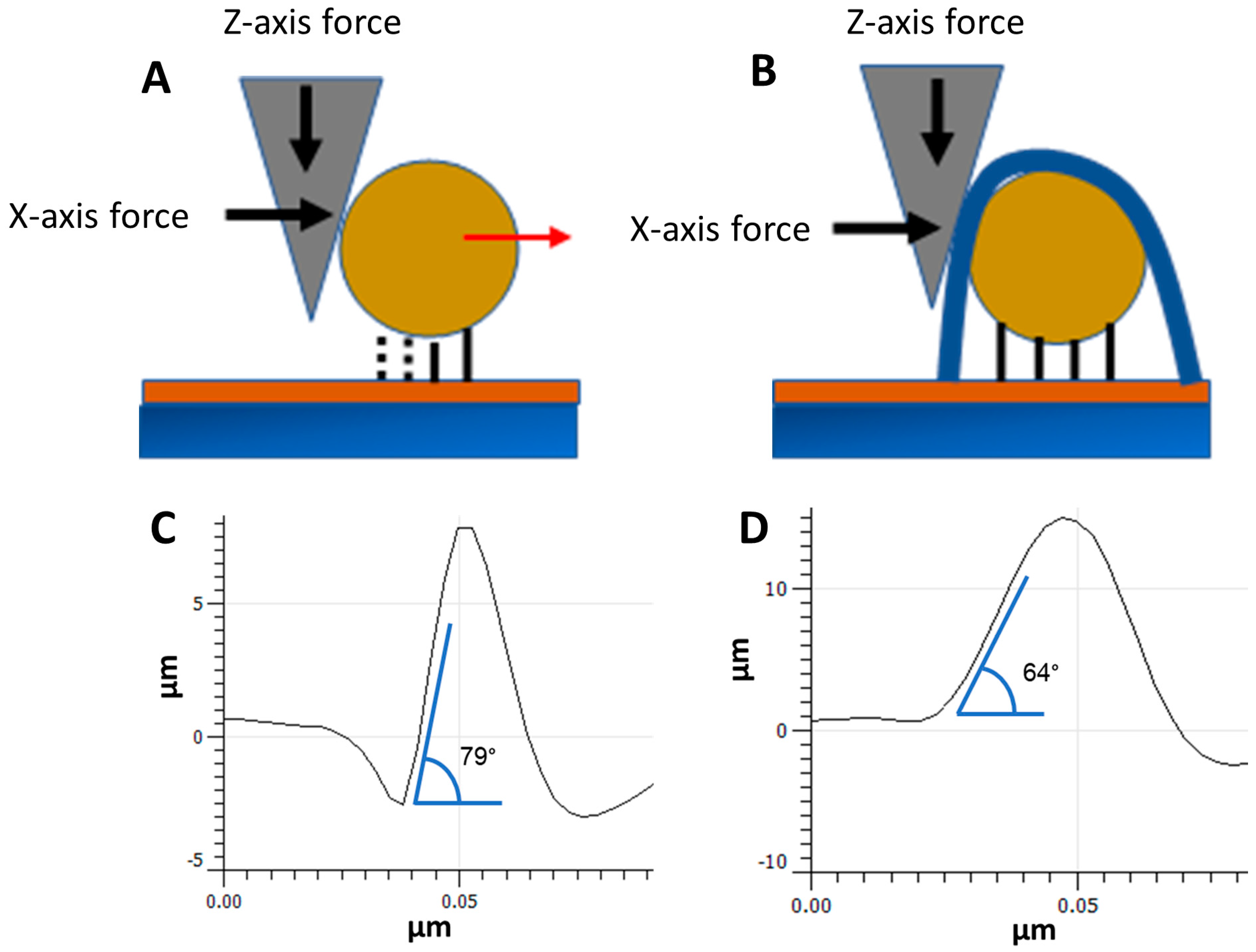
3.2. NP Adhesion
3.3. NP Stability with an Overcoating
3.4. NP Adhesion to Overcoating Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Garcia, L.E.; MacGregor-Ramiasa, M.; Visalakshan, R.M.; Vasilev, K. Protein Interactions with Nanoengineered Polyoxazoline Surfaces Generated via Plasma Deposition. Langmuir 2017, 33, 7322–7331. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Schoenenberger, M.; Gnecco, E.; Glatzel, T.; Meyer, E.; Brändlin, D.; Scandella, L. Characterization of nanoparticles using atomic force microscopy. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2007. [Google Scholar]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Tarnawski, R.; Ulbricht, M. Amphiphilic gold nanoparticles: Synthesis, characterization and adsorption to PEGylated polymer surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 13–21. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; MacGregor, M.N.; Cavallaro, A.A.; Sasidharan, S.; Bachhuka, A.; Mierczynska-Vasilev, A.M.; Hayball, J.D.; Vasilev, K. Creating Nano-engineered Biomaterials with Well-Defined Surface Descriptors. ACS Appl. Nano Mater. 2018, 1, 2796–2807. [Google Scholar] [CrossRef]
- Akhavan, B.; Bakhshandeh, S.; Najafi-Ashtiani, H.; Fluit, A.C.; Boel, E.; Vogely, C.; van der Wal, B.C.H.; Zadpoor, A.A.; Weinans, H.; Hennink, W.E.; et al. Direct covalent attachment of silver nanoparticles on radical-rich plasma polymer films for antibacterial applications. J. Mater. Chem. B 2018, 6, 5845–5853. [Google Scholar] [CrossRef]
- Körner, E.; Aguirre, M.H.; Fortunato, G.; Ritter, A.; Rühe, J.; Hegemann, D. Formation and Distribution of Silver Nanoparticles in a Functional Plasma Polymer Matrix and Related Ag+ Release Properties. Plasma Process. Polym. 2010, 7, 619–625. [Google Scholar] [CrossRef]
- Švorčík, V.; Řezníčková, A.; Sajdl, P.; Kolská, Z.; Makajová, Z.; Slepička, P. Au nanoparticles grafted on plasma treated polymers. J. Mater. Sci. 2011, 46, 7917–7922. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Barton, M.; Whittle, J.D.; Majewski, P.; Smith, L.E.; Vasilev, K. Antibacterial Efficacy and Cytotoxicity of Silver Nanoparticle Based Coatings Facilitated by a Plasma Polymer Interlayer. Plasma Med. 2014, 4, 101–115. [Google Scholar] [CrossRef]
- Cihan, E.; İpek, S.; Durgun, E.; Baykara, M.Z. Structural lubricity under ambient conditions. Nat. Commun. 2016, 7, 12055. [Google Scholar] [CrossRef]
- Dietzel, D.; Mönninghoff, T.; Jansen, L.; Fuchs, H.; Ritter, C.; Schwarz, U.D.; Schirmeisen, A. Interfacial friction obtained by lateral manipulation of nanoparticles using atomic force microscopy techniques. J. Appl. Phys. 2007, 102, 084306. [Google Scholar] [CrossRef]
- Paolicelli, G.; Mougin, K.; Vanossi, A.; Valeri, S. Adhesion detachment and movement of gold nanoclusters induced by dynamic atomic force microscopy. J. Phys. Condens. Matter 2008, 20, 354011. [Google Scholar] [CrossRef][Green Version]
- Wood, J.; Hayles, A.; Bright, R.; Palms, D.; Vasilev, K.; Hasan, J. Nanomechanical tribological characterisation of nanostructured titanium alloy surfaces using AFM: A friction vs velocity study. Colloids Surf. B Biointerfaces 2022, 217, 112600. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Bright, R.; Palms, D.; Barker, D.; Vasilev, K. Damage Behavior with Atomic Force Microscopy on Anti-Bacterial Nanostructure Arrays. Nanomaterials 2024, 14, 253. [Google Scholar] [CrossRef]
- Pyrgiotakis, G.; Blattmann, C.O.; Pratsinis, S.; Demokritou, P. Nanoparticle-nanoparticle interactions in biological media by atomic force microscopy. Langmuir 2013, 29, 11385–11395. [Google Scholar] [CrossRef]
- Klapetek, P.; Valtr, M.; Nečas, D.; Salyk, O.; Dzik, P. Atomic force microscopy analysis of nanoparticles in non-ideal conditions. Nanoscale Res. Lett. 2011, 6, 514. [Google Scholar] [CrossRef]
- Kim, S.; Shafiei, F.; Ratchford, D.; Li, X. Controlled AFM manipulation of small nanoparticles and assembly of hybrid nanostructures. Nanotechnology 2011, 22, 115301. [Google Scholar] [CrossRef]
- Ramachandran, T.R.; Baur, C.; Bugacov, A.; Madhukar, A.; Koel, B.E.; Requicha, A.; Gazen, C. Direct and controlled manipulation of nanometer-sized particles using the non-contact atomic force microscope. Nanotechnology 1998, 9, 237. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.H.; Zheng, Y.; Dhuey, S.; Cabrini, S.; Bach, U. DNA-Directed Self-Assembly of Gold Nanoparticles onto Nanopatterned Surfaces: Controlled Placement of Individual Nanoparticles into Regular Arrays. ACS Nano 2010, 4, 6153–6161. [Google Scholar] [CrossRef] [PubMed]
- Subramani, C.; Saha, K.; Creran, B.; Bajaj, A.; Moyano, D.F.; Wang, H.; Rotello, V.M. Cell alignment using patterned biocompatible gold nanoparticle templates. Small 2012, 8, 1126, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Chapter 7—Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 85–98. [Google Scholar]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: Alive and Kicking. Macromol. Chem. Phys. 2007, 208, 18–25. [Google Scholar] [CrossRef]
- Eaton, P.; West, P. Atomic Force Microscopy; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Canet-Ferrer, J.; Coronado, E.; Forment-Aliaga, A.; Pinilla-Cienfuegos, E. Correction of the tip convolution effects in the imaging of nanostructures studied through scanning force microscopy. Nanotechnology 2014, 25, 395703. [Google Scholar] [CrossRef]
- Dong, R.; Yu, L.E. Investigation of Surface Changes of Nanoparticles Using TM-AFM Phase Imaging. Environ. Sci. Technol. 2003, 37, 2813–2819. [Google Scholar] [CrossRef]
- Magonov, S.N.; Elings, V.; Whangbo, M.H. Phase imaging and stiffness in tapping-mode atomic force microscopy. Surf. Sci. 1997, 375, L385–L391. [Google Scholar] [CrossRef]
- Tamayo, J.; García, R. Deformation, Contact Time, and Phase Contrast in Tapping Mode Scanning Force Microscopy. Langmuir 1996, 12, 4430–4435. [Google Scholar] [CrossRef]
- Carpick, R.W.; Salmeron, M. Scratching the Surface: Fundamental Investigations of Tribology with Atomic Force Microscopy. Chem. Rev. 1997, 97, 1163–1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J. Friction Determination by Atomic Force Microscopy in Field of Biochemical Science. Micromachines 2018, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Green, N.H.; Allen, S.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M. Force sensing and mapping by atomic force microscopy. TrAC Trends Anal. Chem. 2002, 21, 65–74. [Google Scholar] [CrossRef]
- Gwyddion. Available online: http://gwyddion.net/download.php (accessed on 22 February 2024).
- GraphPad Software, Inc. Available online: www.graphpad.com (accessed on 29 May 2024).
- Ramirez-Aguilar, K.A.; Rowlen, K.L. Tip Characterization from AFM Images of Nanometric Spherical Particles. Langmuir 1998, 14, 2562–2566. [Google Scholar] [CrossRef]
- Ricci, D.; Braga, P.C. Recognizing and avoiding artifacts in AFM imaging. Methods Mol. Biol. 2004, 242, 25–37. [Google Scholar] [CrossRef]
- Kylián, O.; Choukourov, A.; Biederman, H. Nanostructured plasma polymers. Thin Solid Films 2013, 548, 1–17. [Google Scholar] [CrossRef]
- Wong, C.; West, P.E.; Olson, K.S.; Mecartney, M.L.; Starostina, N. Tip dilation and AFM capabilities in the characterization of nanoparticles. JOM 2007, 59, 12–16. [Google Scholar] [CrossRef]
- Jaskiewicz, K.; Makowski, M.; Kappl, M.; Landfester, K.; Kroeger, A. Mechanical Properties of Poly(dimethylsiloxane)-block-poly(2-methyloxazoline) Polymersomes Probed by Atomic Force Microscopy. Langmuir 2012, 28, 12629–12636. [Google Scholar] [CrossRef]
- Levent Demirel, A.; Yurteri, S.; Cianga, I.; Yagci, Y. Synthesis and morphological characterization of poly(ϵ-caprolactone) and poly(2-methyloxazoline) substituted phenyl rings and phenylene oligomers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2091–2104. [Google Scholar] [CrossRef]
- Wood, J.; Palms, D.; Dabare, R.; Vasilev, K.; Bright, R. Exploring the Challenges of Characterising Surface Topography of Polymer–Nanoparticle Composites. Nanomaterials 2024, 14, 1275. [Google Scholar] [CrossRef]
- Campbell, C.T. The Energetics of Supported Metal Nanoparticles: Relationships to Sintering Rates and Catalytic Activity. Acc. Chem. Res. 2013, 46, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.E.; Matheson, L.A.; Bradford, E.B. Sintering of synthetic latex particle. J. Colloid Sci. 1951, 6, 108–117. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Huwiler, C.; Drobek, T.; Vörös, J.; Spencer, N.D. Systematic study of osteoblast response to nanotopography by means of nanoparticle-density gradients. Biomaterials 2007, 28, 5000–5006. [Google Scholar] [CrossRef] [PubMed]
- Van Caeter, P.; Goethals, E.J.; Gancheva, V.; Velichkova, R. Synthesis and bulk properties of poly(tetrahydrofuran)-poly(2-methyl-2-oxazoline) ABA triblock copolymers. Polym. Bull. 1997, 39, 589–596. [Google Scholar] [CrossRef]
- Bai, L.; Tan, L.; Chen, L.; Liu, S.; Wang, Y. Preparation and characterizations of poly(2-methyl-2-oxazoline) based antifouling coating by thermally induced immobilization. J. Mater. Chem. B 2014, 2, 7785–7794. [Google Scholar] [CrossRef]
- Alarifi, H.; Hu, A.; Yavuz, M.; Zhou, Y.N. Silver Nanoparticle Paste for Low-Temperature Bonding of Copper. J. Electron. Mater. 2011, 40, 1394–1402. [Google Scholar] [CrossRef]
- Hu, A.; Guo, J.; Alarifi, H.; Patane, G.; Zhou, Y.; Compagnini, G.; Xu, C. Low temperature sintering of Ag nanoparticles for flexible electronics packaging. Appl. Phys. Lett. 2010, 97, 153117. [Google Scholar] [CrossRef]
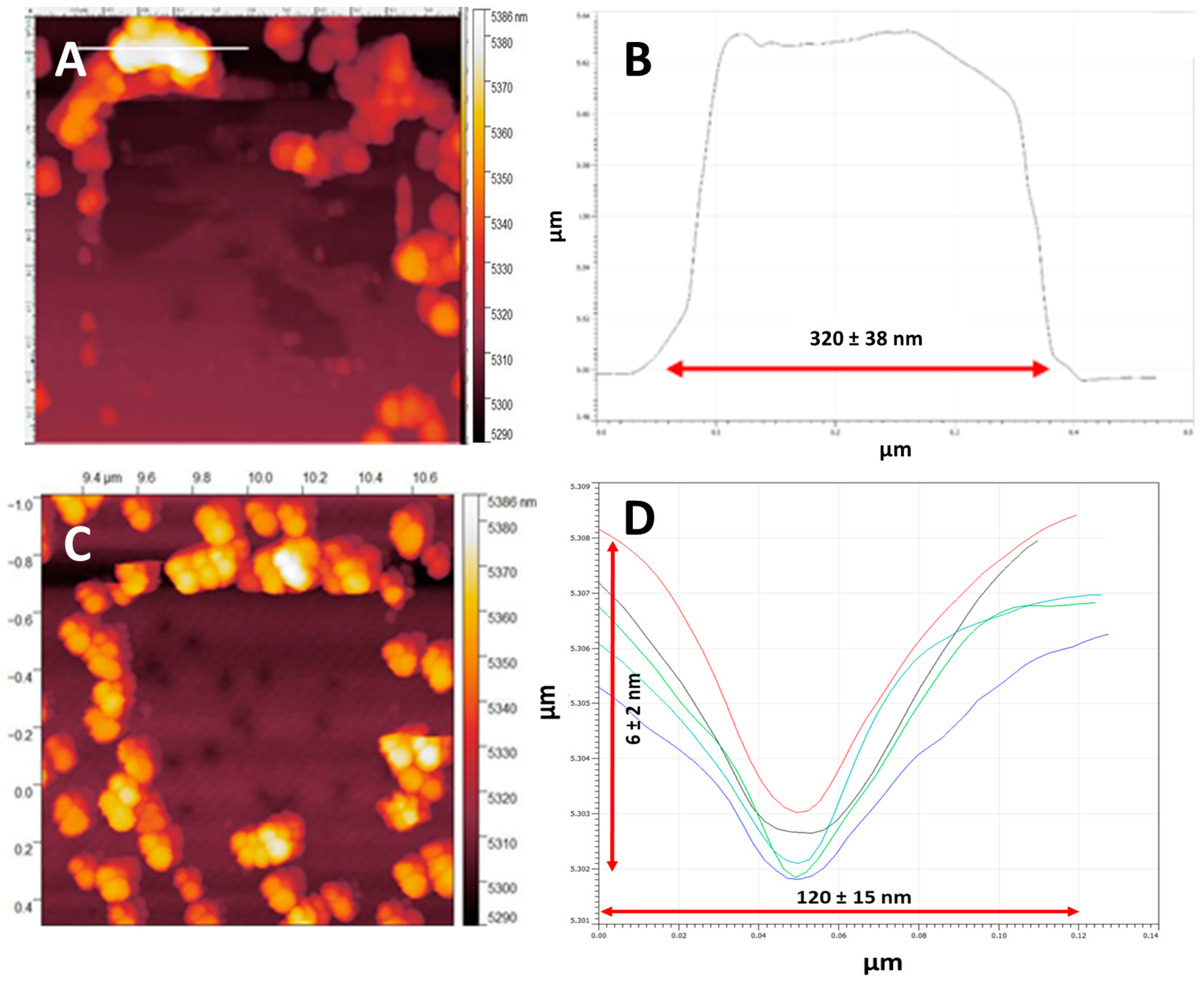
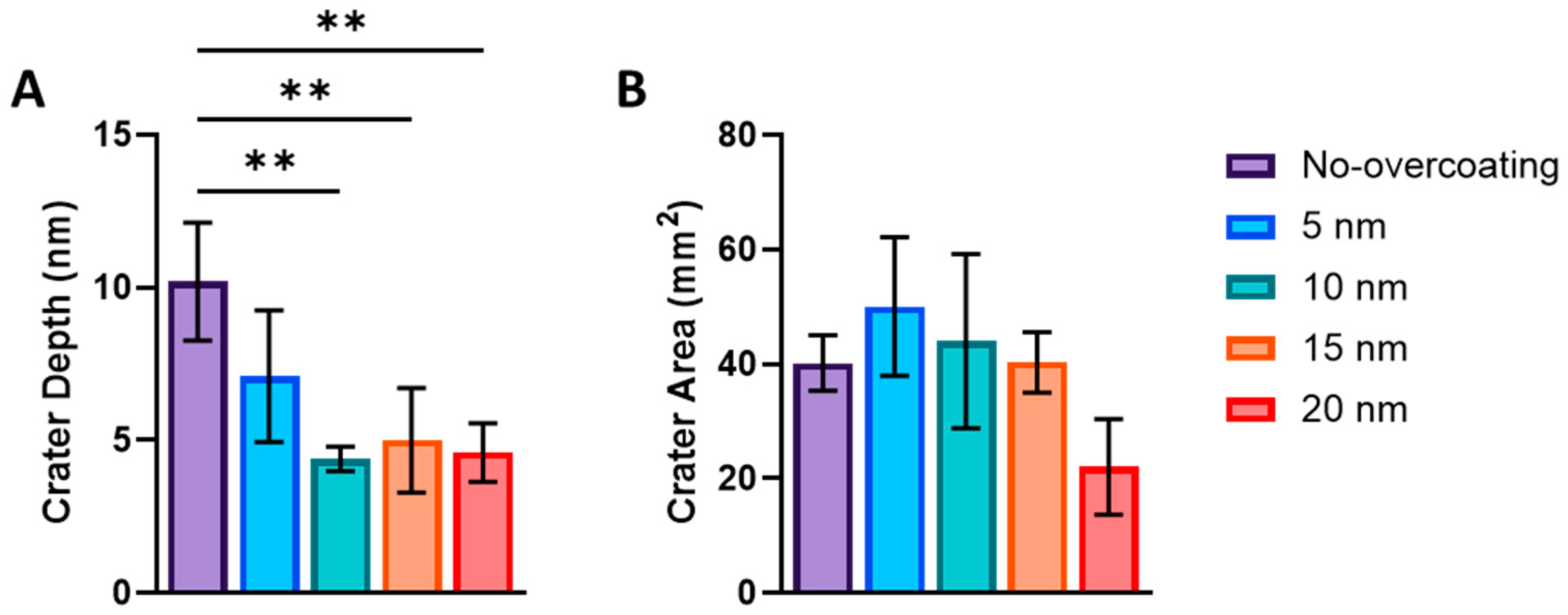
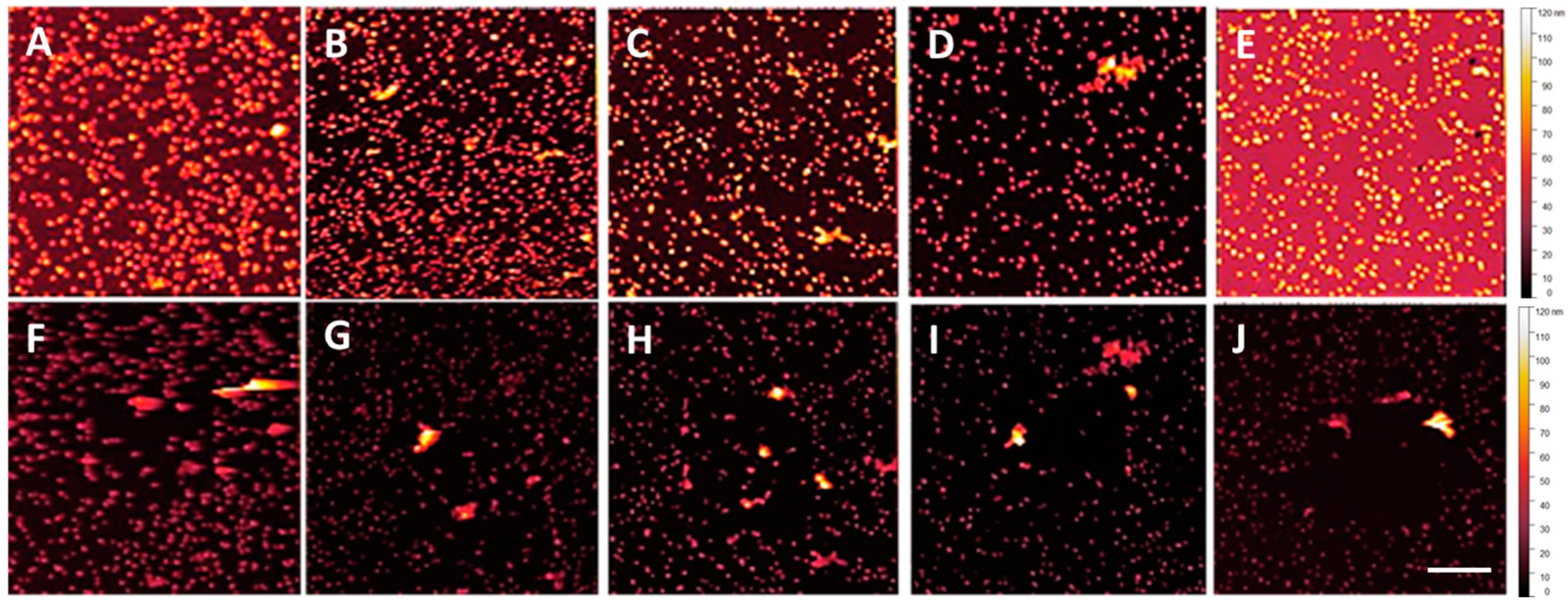


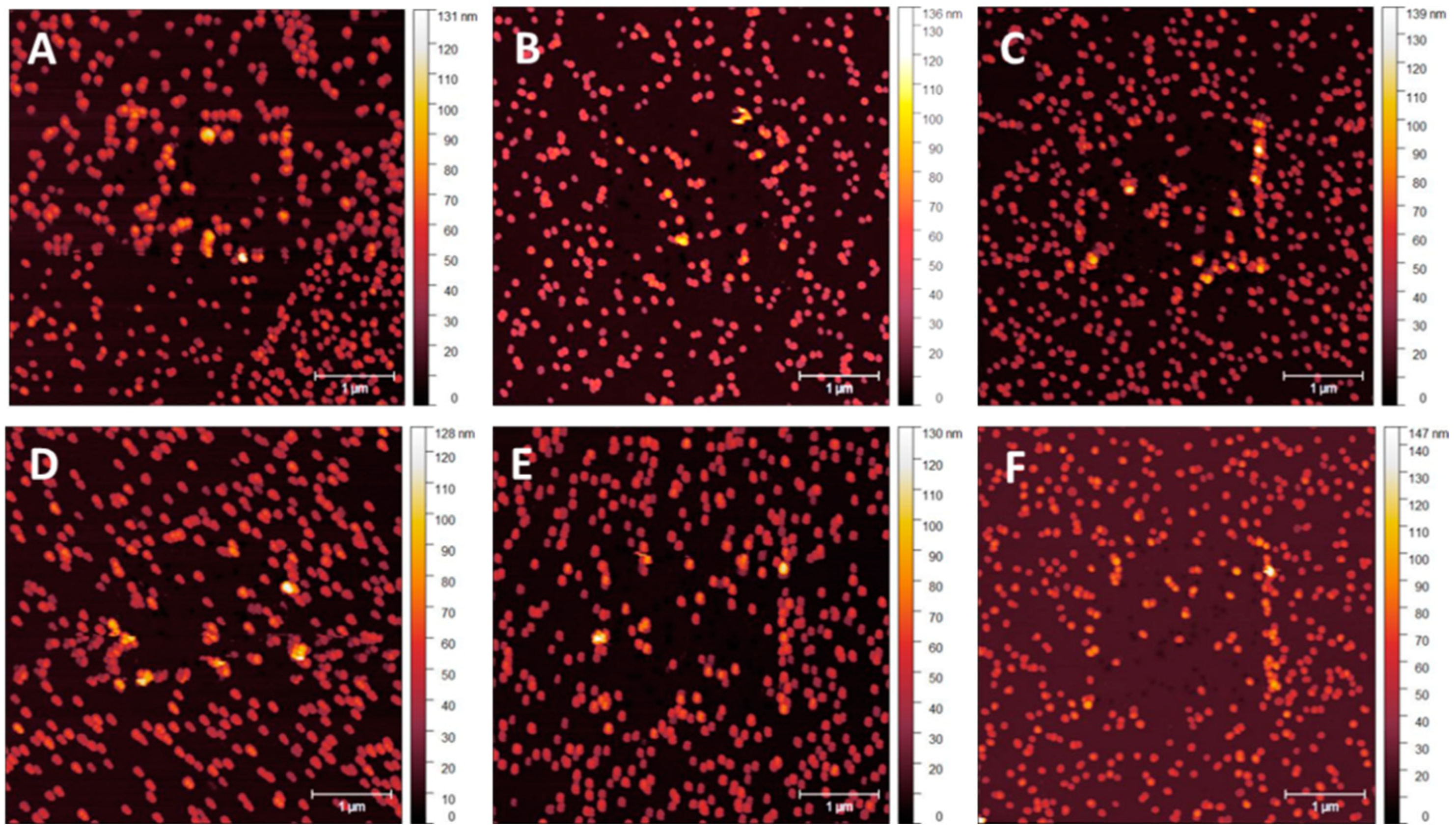
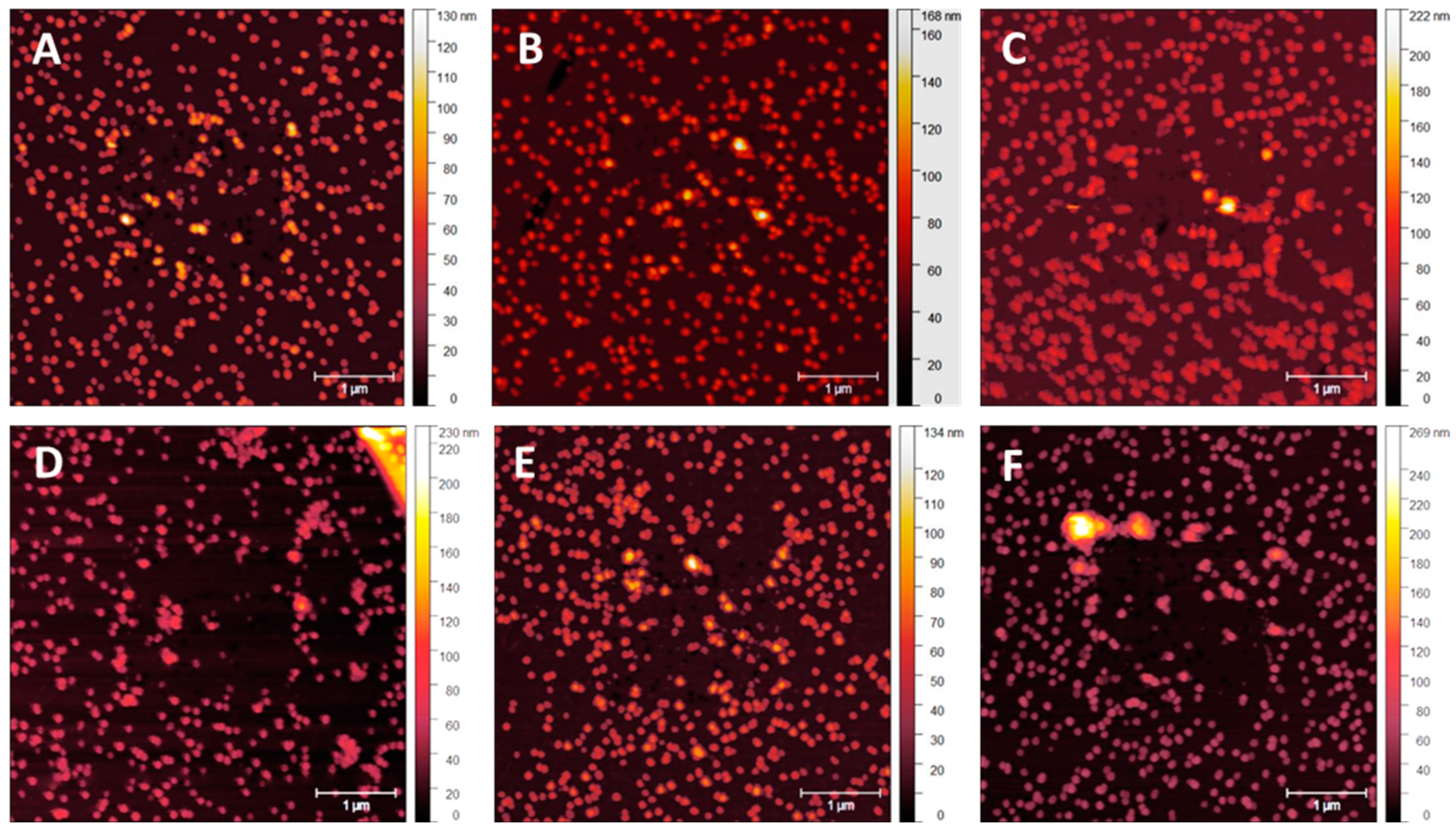
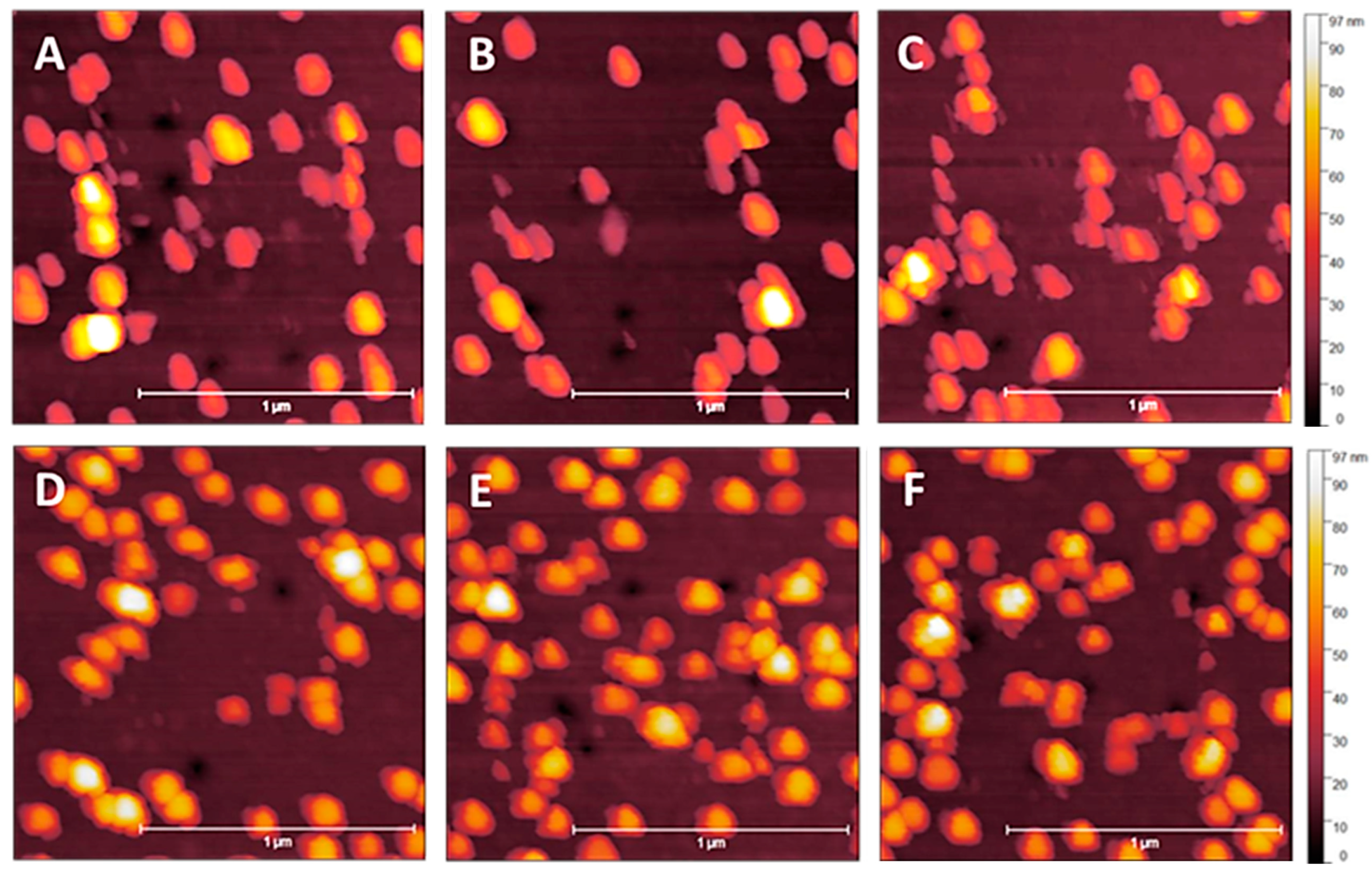
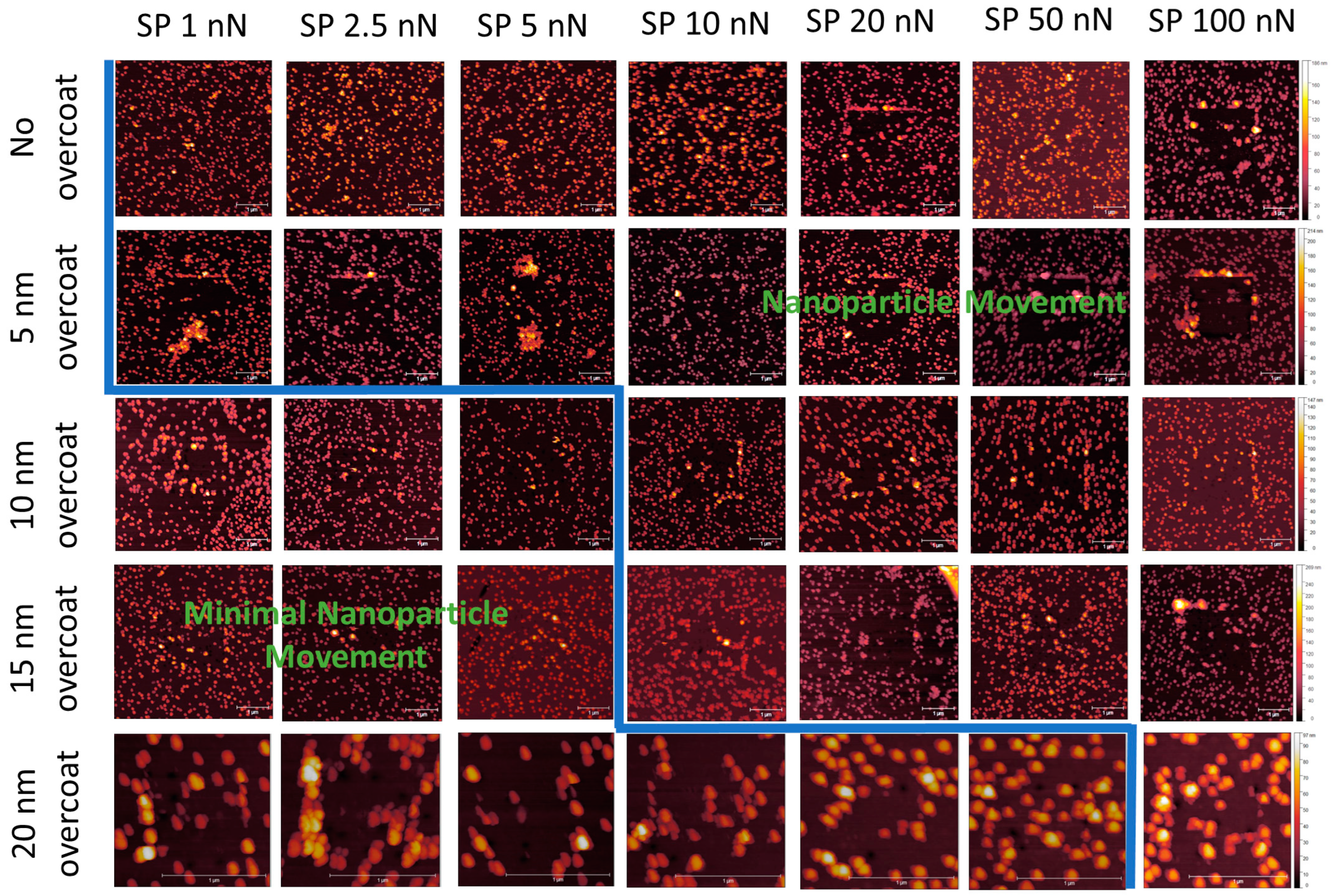
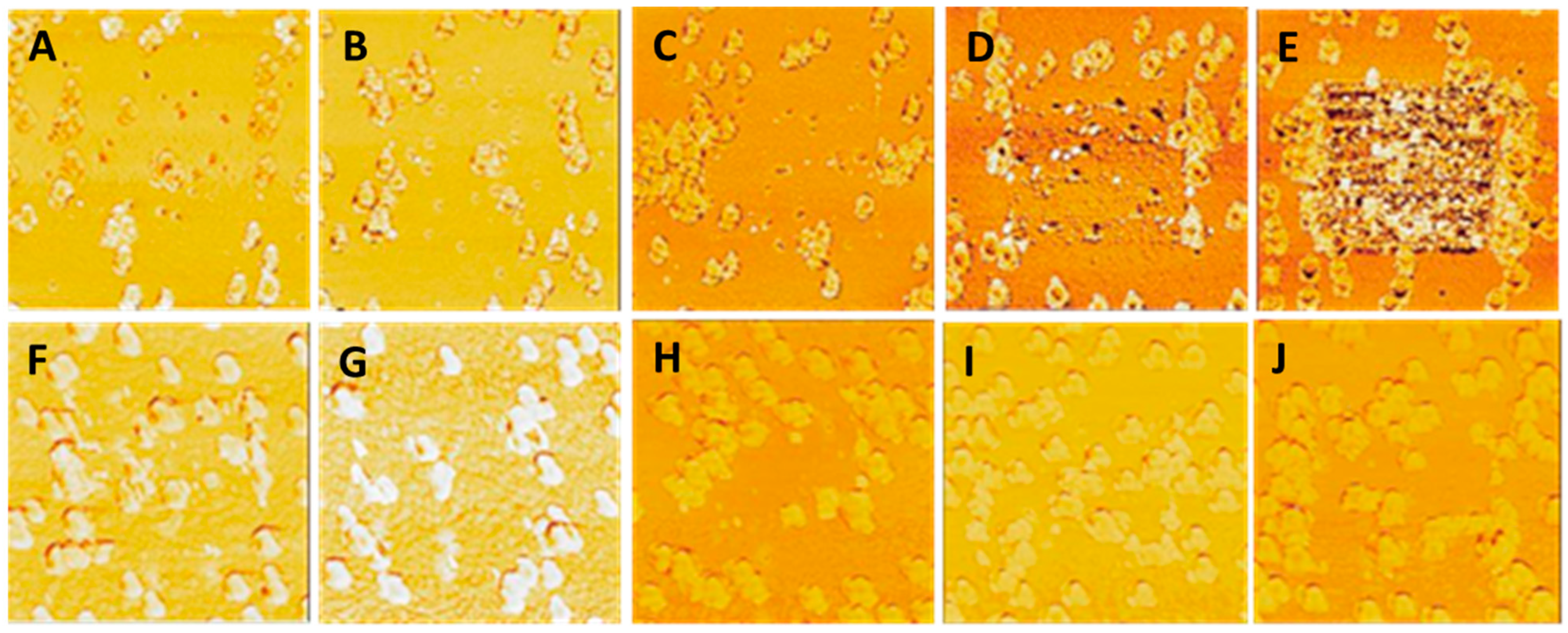
| Roughness | No Overcoat | 5 nm | 10 nm | 15 nm | 20 nm |
|---|---|---|---|---|---|
| RMS (nm) | 14.4 ± 6.6 | 14.8 ± 3.7 | 11.5 ± 2.9 | 13.0 ± 5.9 | 12.9 ± 7.0 |
| Ra (nm) | 10.9 ± 4.5 | 11.1 ± 8.4 | 7.0 ± 6.5 | 9.1 ± 3.3 | 8.5 ± 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, J.; Palms, D.; Dabare, R.; Vasilev, K.; Bright, R. AFM for Nanomechanical Assessment of Polymer Overcoatings on Nanoparticle-Decorated Biomaterials. Nanomaterials 2024, 14, 1475. https://doi.org/10.3390/nano14181475
Wood J, Palms D, Dabare R, Vasilev K, Bright R. AFM for Nanomechanical Assessment of Polymer Overcoatings on Nanoparticle-Decorated Biomaterials. Nanomaterials. 2024; 14(18):1475. https://doi.org/10.3390/nano14181475
Chicago/Turabian StyleWood, Jonathan, Dennis Palms, Ruvini Dabare, Krasimir Vasilev, and Richard Bright. 2024. "AFM for Nanomechanical Assessment of Polymer Overcoatings on Nanoparticle-Decorated Biomaterials" Nanomaterials 14, no. 18: 1475. https://doi.org/10.3390/nano14181475
APA StyleWood, J., Palms, D., Dabare, R., Vasilev, K., & Bright, R. (2024). AFM for Nanomechanical Assessment of Polymer Overcoatings on Nanoparticle-Decorated Biomaterials. Nanomaterials, 14(18), 1475. https://doi.org/10.3390/nano14181475








