Metal–Organic Frameworks for Overcoming the Blood–Brain Barrier in the Treatment of Brain Diseases: A Review
Abstract
1. Introduction
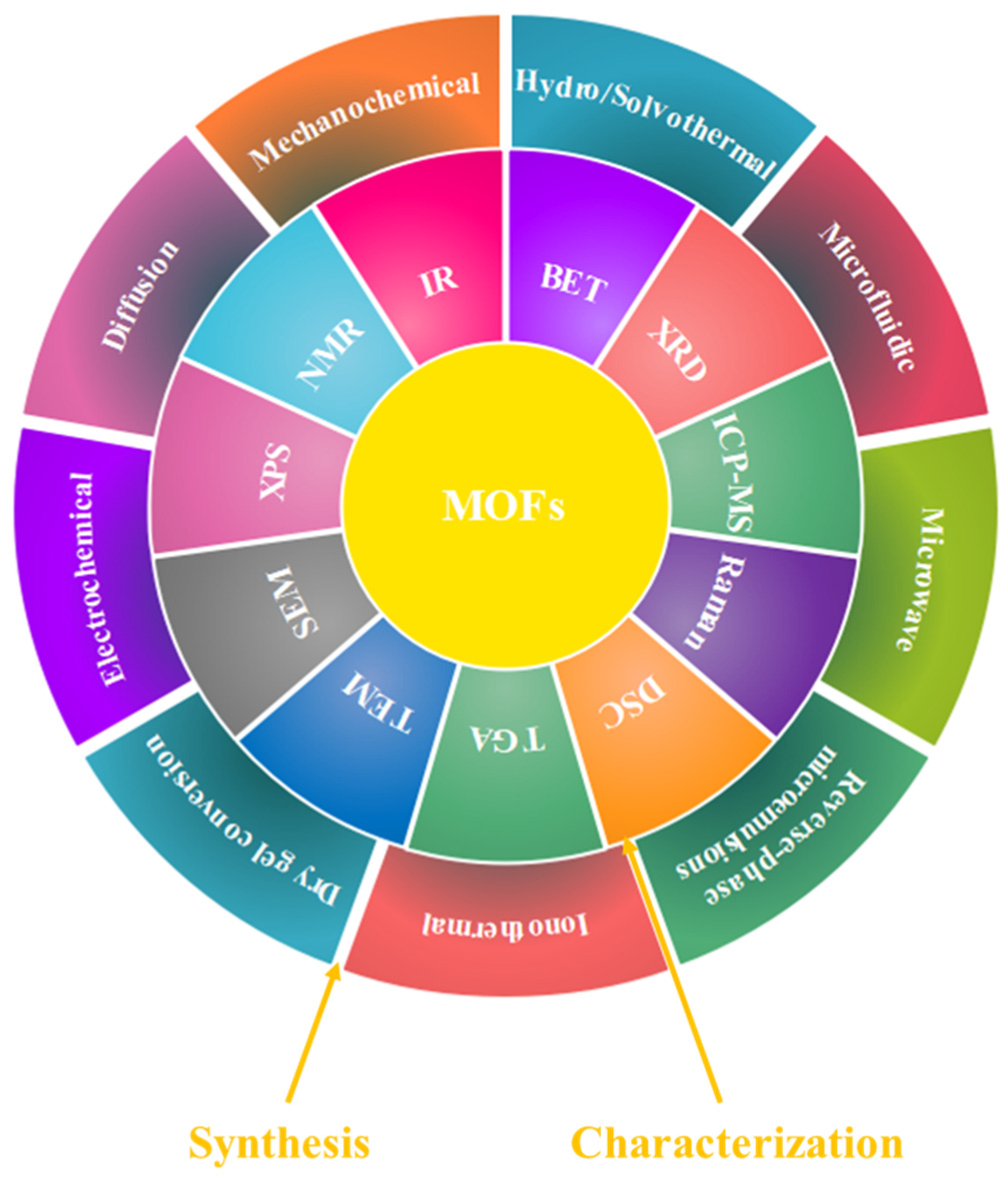
2. Synthesis and Characterization of MOFs
3. Drug Loading and Drug Release in MOFs
4. MOFs in the Treatment of Brain Diseases
5. MOFs in the Treatment of Glioblastoma Multiforme
6. MOFs in the Treatment of Parkinson
7. MOFs in the Treatment of Alzheimer’s
8. MOFs in the Treatment of Stroke
9. Challenges in Developing MOFs for Treating Brain Diseases
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| Aβ | Amyloid β |
| AIC | 5-Aminoimidazole-4-carboxamide |
| AD | Alzheimer’s disease |
| AFM | Atomic force microscopy |
| ALS | Amyotrophic lateral sclerosis |
| AuNPs | Gold nanoparticles |
| BCMD | BSO-CAT@MOF-199@DDM |
| BET | Brunauer–Emmett–Teller |
| BSO | Buthionine-sulfoximine |
| CBD | Cannabidiol |
| Ca-MOFs | Calcium-based MOFs |
| CD | Circular dichroism |
| CD-MOFs | Cyclodextrin-based MOFs |
| CeO2 | Cerium dioxide |
| CCM | Curcumin |
| DSC | Differential scanning calorimetry |
| DOX | Doxorubicin |
| Defluorinated MK6240, DMK6240 | 5-Amino-3-(pyrrolo [2,3-c]pyridin-1-yl)isoquinoline |
| FeCl3 | Fe3+ ions |
| Fe-TCPP | Fe-5,10,15,20-tetra (4-carboxyphenyl) porphyrin |
| FBF | Fenbufen |
| GFP-PSD95 | PSD95 interacted with green fluorescent protein |
| GA | L-glutamic acid |
| GA | Gallic acid |
| GBM | Glioblastoma multiforme |
| ICD | Immunogenic cell death |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| IR | Infrared |
| MDA | Malondialdehyde |
| MB | Methylene blue |
| MNPs | Magnetic nanoparticles |
| MOFs | Metal–organic frameworks |
| MMOF | Multifunctional MOF |
| MTIC | 5-(3-methyltriazen-1-yl)imidazole-4-carboxamide |
| MTX | Methotrexate |
| NIR | Near-infrared |
| NO | Nitric oxide |
| NPs | Nanoparticles |
| NMDARs | N-methyl-D-aspartate receptors |
| NOTA | 1,4,7-triazacyclononane-1,4,7-triacetic acid |
| OA | Okadaic acid |
| OXA | Oxaliplatin |
| PB | Prussian blue |
| PCN | Porous coordination network |
| PCN−222@ICG | Porphyrinic MOF and indocyanine green nanoprobe |
| PBS | Phosphate-buffered saline |
| Ptzymes | Platinum nanozymes |
| QCT | Quercetin |
| RB | Rose bengal |
| ROS | Reactive oxygen species |
| RVG | Rabies virus glycoprotein |
| SCXRD | Single crystal X-ray diffraction |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| TCPP | Tetra-kis(4-carboxyphenyl)porphyrin |
| ThT | Thioflavin T |
| TMZ | Temozolomide |
| XPS | X-ray photoelectron spectroscopy |
| XP | X-ray photoelectron spectroscopy |
References
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.L.; Manickam, D.S. Lipid nanoparticle-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Ma, Z.; Yang, G.Z. Micro/nanosystems for controllable drug delivery to the brain. Innovation 2024, 5, 100548. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the true global burden of mental illness. Lancet Psychiatr. 2016, 3, 171–178. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef]
- Torchilin, V.P. Liposomes as targetable drug carriers. Crit. Rev. Ther. Drug Carr. Syst. 1985, 2, 65–115. [Google Scholar]
- Torchilin, V. Liposomes as delivery agents for medical imaging. Mol. Med. Today 1996, 2, 242–249. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Abdou, E.M.; Kandil, S.M.; Miniawy, H.M.F.E. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Microemulsion-based drug delivery system for transnasal delivery of Carbamazepine: Preliminary brain-targeting study. Drug Deliv. 2016, 23, 207–213. [Google Scholar] [CrossRef]
- Khunt, D.; Polaka, S.; Shrivas, M.; Misra, M. Biodistribution and amyloid beta induced cell line toxicity study of intranasal Rivastigmine microemulsion enriched with Fish Oil and Butter oil. J. Drug Deliv. Sci. Technol. 2020, 57, 101661. [Google Scholar] [CrossRef]
- Ahmad, E.; Lv, Y.; Zhu, Q.; Qi, J.; Dong, X.; Zhao, W.; Chen, Z.; Wu, W.; Lu, Y. TAT modification facilitates nose-to-brain transport of intact mPEG-PDLLA micelles: Evidence from aggregation-caused quenching probes. Appl. Mater. Today 2020, 19, 100556. [Google Scholar] [CrossRef]
- Pokharkar, V.; Suryawanshi, S.; Dhapte-Pawar, V. Exploring micellar-based polymeric systems for effective nose-to-brain drug delivery as potential neurotherapeutics. Drug Deliv. Transl. Res. 2019, 10, 1019–1031. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Contr. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Pailla, S.R.; Talluri, S.; Rangaraj, N.; Ramavath, R.; Challa, V.S.; Doijad, N.; Sampathi, S. Intranasal Zotepine Nanosuspension: Intended for improved brain distribution in rats, DARU. J. Pharm. Sci. 2019, 27, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Al Jayoush, A.R.; Hassan, H.A.F.M.; Asiri, H.; Jafar, M.; Saeed, R.; Harati, R.; Haider, M. Niosomes for nose-to-brain delivery: A non-invasive versatile carrier system for drug delivery in neurodegenerative diseases. J. Drug Deliv. Sci. Technol. 2023, 89, 105007. [Google Scholar] [CrossRef]
- Nerli, G.; Robla, S.; Bartalesi, M.; Luceri, C.; D’Ambrosio, M.; Csaba, N.; Maestrelli, F. Chitosan coated niosomes for nose-to-brain delivery of clonazepam: Formulation, stability and permeability studies. Carbohydr. Polym. Technol. Appl. 2023, 6, 100332. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-brain delivery of antipsychotics using nanotechnology: A review. Expet Opin. Drug Deliv. 2020, 17, 839–853. [Google Scholar] [CrossRef]
- Abbas, H.; Sayed, N.S.E.; Youssef, N.A.H.A.; MEGaafar, P.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel luteolin-loaded chitosan decorated nanoparticles for brain-targeting delivery in a sporadic Alzheimer’s disease mouse model: Focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef]
- Nabipour, H.; Qiu, S.; Wang, X.; Song, L.; Hu, Y. Adenine as an efficient adsorbent for zinc ions removal from wastewater to in situ form bio-based metal–organic frameworks: A novel approach to preparing fire-safe polymers. Compos. A Appl. Sci. Manuf. 2022, 161, 107099. [Google Scholar] [CrossRef]
- Nabipour, H.; Hu, Y. Development of fully bio-based pectin/curcumin@bio-MOF-11 for colon specific drug delivery. Chem. Pap. 2022, 76, 2969–2979. [Google Scholar] [CrossRef]
- Nabipour, H.; Aliakbari, F.; Volkening, K.; Strong, M.J.; Rohani, S. New metal-organic framework coated sodium alginate for the delivery of curcumin as a sustainable drug delivery and cancer therapy system. Int. J. Biol. Macromol. 2024, 259, 128875. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, H.; Aliakbari, F.; Volkening, K.; Strong, M.J.; Rohani, S. The development of a bio-based metal-organic framework coated with carboxymethyl cellulose with the ability to deliver curcumin with anticancer properties. Mater. Today Chem. 2024, 37, 101976. [Google Scholar] [CrossRef]
- Connolly, B.M.; Madden, D.G.; Wheatley, A.E.; Fairen-Jimenez, D. Shaping the future of fuel: Monolithic metal–organic frameworks for high-density gas storage. J. Am. Chem. Soc. 2020, 142, 8541–8549. [Google Scholar] [CrossRef]
- Altass, H.M.; Ahmed, S.A.; Salama, R.S.; Moussa, Z.; Jassas, R.S.; Alsantali, R.I.; Al-Rooqi, M.M.; Ibrahim, A.A.; Khder, M.A.; Morad, M. Low temperature CO oxidation over highly active gold nanoparticles supported on reduced graphene oxide@ Mg-BTC nanocomposite. Catal. Lett. 2023, 153, 876–886. [Google Scholar] [CrossRef]
- Hanikel, N.; Pei, X.K.; Chheda, S.; Lyu, H.; Jeong, W.; Sauer, J.; Gagliardi, L.; Yaghi, O.M. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting. Science 2021, 374, 454–459. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, H.; Liu, W.; Li, Y.; Zhang, J.; Hou, H.; Yang, J. Ultrathin NiCo-MOF nanosheets for high-performance supercapacitor electrodes. ACS Appl. Energy Mater. 2019, 2, 2063–2071. [Google Scholar] [CrossRef]
- Xu, R.; Jian, M.; Ji, Q.; Hu, C.; Tang, C.; Liu, R.; Zhang, X.; Qu, J. 2D water-stable zinc-benzimidazole framework nanosheets for ultrafast and selective removal of heavy metals. Chem. Eng. J. 2020, 382, 122658. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Farimani, A.B. Water desalination with two-dimensional metal–organic framework membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- de Lima, L.S.; Mortari, M.R. Therapeutic nanoparticles in the brain: A review of types, physicochemical properties and challenges. Int. J. Pharm. 2022, 612, 121367. [Google Scholar] [CrossRef]
- Ahamed, J.; Gowda, B.J.; Almalki, W.H.; Gupta, N.; Sahebkar, A.; Kesharwani, P. Recent advances in nanoparticle-based approaches for the treatment of brain tumors: Opportunities and challenges. Eur. Polym. J. 2023, 193, 112111. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.S.; Meng, H.; Li, Z. Zinc-based metal-organic frameworks in drug delivery, cell imaging, and sensing. Molecules 2021, 27, 100. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal–organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Naimi-Jamal, M.R.; Panahi, L. Mechanochemical Solvent-Free in Situ Synthesis of Drug-Loaded {Cu2(1,4-Bdc)2(Dabco)}n MOFs for Controlled Drug Delivery. J. Solid State Chem. 2018, 259, 35–42. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 6, 7004–7020. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal–Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Liu, J.; Bao, T.Y.; Yang, X.Y.; Zhu, P.P.; Wu, L.H.; Sha, J.Q.; Lan, Y.Q. Controllable porosity conversion of metal-organic frameworks composed of natural ingredients for drug delivery. Chem. Commun. 2017, 53, 7804–7807. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Han, L.; Singh, V.; Xu, X.; Guo, T.; Meng, F.; Xu, X.; York, P.; Liu, Z.; et al. Microwave-assisted rapid synthesis of γ-cyclodextrin metal–organic frameworks for size control and efficient drug loading. Cryst. Growth Des. 2017, 17, 1654–1660. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal–organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Teplensky, M.H.; Fantham, M.; Li, P.; Wang, T.C.; Mehta, J.P.; Young, L.J.; Moghadam, P.Z.; Hupp, J.T.; Farha, O.K.; Kaminski, C.F.; et al. Temperature treatment of highly porous zirconium-containing metal–organic frameworks extends drug delivery release. J. Am. Chem. Soc. 2017, 139, 7522–7532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-Organic Frameworks for Advanced Drug Delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Morris, W.; Briley, W.E.; Auyeung, E.; Cabezas, M.D.; Mirkin, C.A. Nucleic Acid-Metal Organic Framework (MOF) Nanoparticle Conjugates. J. Am. Chem. Soc. 2014, 20, 7261–7264. [Google Scholar] [CrossRef]
- Christodoulou, I.; Serre, C.; Gref, R. Metal-organic frameworks for drug delivery: Degradation mechanism and in vivo fate. Met. Fram. Biomed. Appl. 2020, 467–489. [Google Scholar] [CrossRef]
- Mhettar, P.; Kale, N.; Pantwalawalkar, J.; Nangare, S.; Jadhav, N. Metal-organic frameworks: Drug delivery applications and future prospects. ADMET DMPK 2021, 12, 27–62. [Google Scholar] [CrossRef]
- Lin, W.; Cui, Y.; Yang, Y.; Hu, Q.; Qian, G. A biocompatible metal-organic framework as a pH and temperature dual-responsive drug carrier. Dalton Trans. 2018, 47, 15882–15887. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Hou, Y.K.; Chen, M.W.; Yu, X.Z.; Chen, S.Y.; Yue, Y.R.; Guo, X.T.; Chen, J.X.; Zhou, Q.A. pH-responsive metal-organic framework for the co-delivery of HIF-2α SiRNA and curcumin for enhanced therapy of osteoarthritis. J. Nanobiotechnol. 2021, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, M.; Parisse, V.; Guglielmi, P.; Giannini, G.; Secci, D. Metal-organic frameworks (MOFs) as biomolecules drug delivery systems for anticancer purposes. Eur. J. Med. Chem. 2022, 244, 114801. [Google Scholar] [CrossRef] [PubMed]
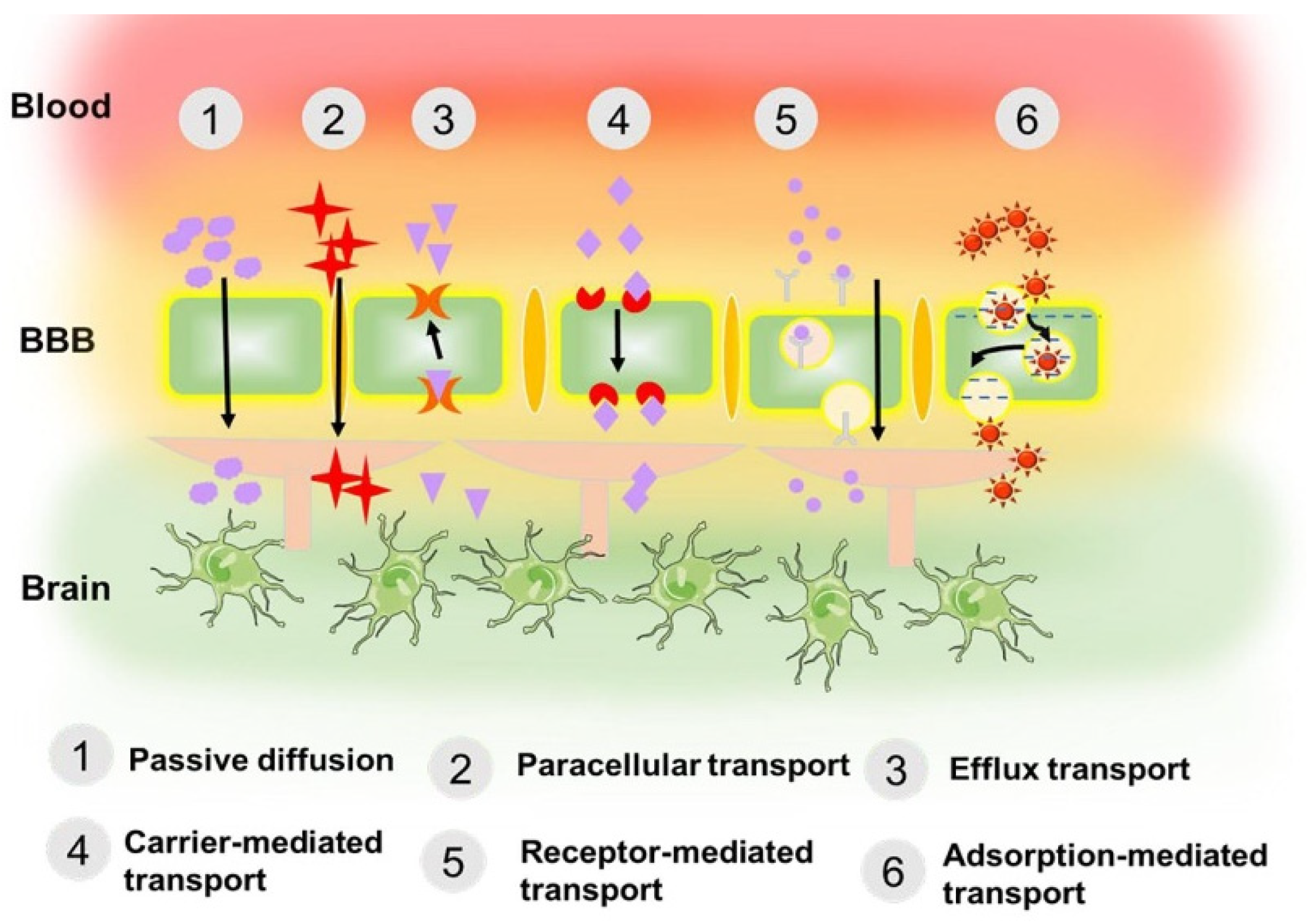
- Odom, T.L.; LeBroc, H.D.; Callmann, C.E. Biomacromolecule-tagged nanoscale constructs for crossing the blood–brain barrier. Nanoscale 2024, 16, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhao, S.; Xu, T.; Wang, Q.; Lan, M.; Yan, L.; Chen, X. Getting drugs to the brain: Advances and prospects of organic nanoparticle delivery systems for assisting drugs to cross the blood–brain barrier. J. Mater. Chem. B 2022, 10, 9314–9333. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Tatiparti, K.; Sau, S.; Iyer, A.K. Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov. Today 2021, 26, 1944–1952. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, S.; Xu, T.; Wang, Q.; Zhang, M.; Yan, L.; Chen, X.; Lan, M. Inorganic nano-drug delivery systems for crossing the blood–brain barrier: Advances and challenges. Coord. Chem. Rev. 2023, 494, 215344. [Google Scholar] [CrossRef]
- Alahri, M.B.; Arshadizadeh, R.; Raeisi, M.; Khatami, M.; Sajadi, M.S.; Abdelbasset, W.K.; Akhmadeev, R.; Iravani, S. Theranostic applications of metal–organic frameworks (MOFs)-based materials in brain disorders: Recent advances and challenges. Inorg. Chem. Commun. 2021, 134, 108997. [Google Scholar] [CrossRef]
- Han, Z.; Yuan, M.; Nguyen, N.; Zhou, H.; Hubbard, J.E.; Wang, Y. Brain-specific targeted delivery of therapeutic agents using metal–organic framework-based nanomedicine. Coord. Chem. Rev. 2024, 514, 215926. [Google Scholar] [CrossRef]
- Qiao, C.Q.; Zhang, R.L.; Wang, Y.D.; Jia, Q.; Wang, X.F.; Yang, Z.; Xue, T.F.; Ji, R.C.; Cui, X.F.; Wang, Z.L. Rabies virus-inspired metal-organic frameworks (MOFs) for targeted imaging and chemotherapy of glioma. Angew. Chem.-Int. Edit. 2020, 59, 16982–16988. [Google Scholar] [CrossRef]
- Fu, D.-Y.; Liu, X.; Zheng, X.; Zhou, M.; Wang, W.; Su, G.; Liu, T.; Wang, L.; Xie, Z. Polymer-metal-organic framework hybrids for bioimaging and cancer therapy. Coord. Chem. Rev. 2022, 456, 214393. [Google Scholar] [CrossRef]
- Huang, Q.-X.; Liang, J.-L.; Chen, Q.-W.; Jin, X.-K.; Niu, M.-T.; Dong, C.-Y.; Zhang, X.-Z. Metal-organic framework nanoagent induces cuproptosis for effective immunotherapy of malignant glioblastoma. Nano Today 2023, 51, 101911. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Monforte, F.; Presti, F.L.; Volti, G.L.; Carota, G.; Sinatra, F.; Bongiorno, C.; Mannino, G.; Cambria, M.T.; Condorelli, G.G. Synthesis of MIL-modified Fe3O4 magnetic nanoparticles for enhancing uptake and efficiency of temozolomide in glioblastoma treatment. Int. J. Mol. Sci. 2022, 23, 2874. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Li, C.; Hazari, P.P.; Mahajan, S.D.; Aalinkeel, R.; Sharma, R.K.; Swihart, M.T. A cannabidiol-loaded Mg-gallate metal–organic framework-based potential therapeutic for glioblastomas. J. Mater. Chem. B 2021, 9, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, H.; Xue, J.; Luo, J.; Liu, Q.; Chen, X.; Pan, Y.; Zhou, J.; Liu, Z.; Chen, T. Near-infrared radiation-assisted drug delivery nanoplatform to realize blood–brain barrier crossing and protection for parkinsonian therapy. ACS Appl. Mater. Interfaces 2021, 13, 37746–37760. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Q.; Zhang, R.; Li, J.; Lin, Q.; Li, J.; Zhou, X.; Yan, X.; Fan, K. Chiral metal-organic frameworks incorporating nanozymes as neuroinflammation inhibitors for managing Parkinson’s disease. Nat. Commun. 2023, 14, 8137. [Google Scholar] [CrossRef]
- Li, Q.; Ding, X.; Chang, Z.; Fan, X.; Pan, J.; Yang, Y.; Li, X.; Jiang, W.; Fan, K. Metal–organic framework based nanozyme system for NLRP3 inflammasome-mediated neuroinflammatory regulation in Parkinson’s disease. Adv. Healthc. Mater. 2023, 13, 2303454. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Jia, Z.; Yuan, X.; Liu, J. Electrostatic assembly of gold nanoparticle and metal-organic framework nanoparticles attenuates amyloid beta aggregate-mediated neurotoxicity. J. Mater. Chem. B 2023, 11, 4453–4463. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, F.; Ji, L.; Wang, C.; Shi, C.; Liu, X.; Yang, H.; Wang, X.; Kong, L. Development of a tau-targeted drug delivery system using a multifunctional nanoscale metal-organic framework for Alzheimer’s disease therapy. ACS Appl. Mater. Interfaces 2020, 12, 44447–44458. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Tan, Y.; Zhao, X.; Zhang, Y.; Cheng, C.; Yang, M. Porphyrinic metal–organic framework PCN-224 nanoparticles for near-infrared-induced attenuation of aggregation and neurotoxicity of Alzheimer’s amyloid-β peptide. ACS Appl. Mater. Interfaces 2018, 10, 36615. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, Y.; Kou, Q.; Lu, L.; Jiang, H.; Li, X.; Gui, R.; Huang, R.; Huang, X.; Ma, J.; et al. Study on the role of an erythrocyte membrane-coated nanotheranostic system in targeted immune regulation of Alzheimer’s disease. Adv. Sci. 2023, 10, e2301361. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y.; Liu, X.; Fan, Y.; Zhang, Y.; Yi, C.; Cheng, C.; Yang, M. Near-infrared photothermally enhanced photo-oxygenation for inhibition of amyloid-beta aggregation based on RVG-conjugated porphyrinic metal-organic framework and indocyanine green nanoplatform. Int. J. Mol. Sci. 2022, 23, 10885. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Han, M.; Li, J.; Ke, H.; Kong, Y.; Wang, W.; Wang, L.; Ma, W.; Qiu, J.; Wang, X.; et al. Delivery of miRNAs through metal–organic framework nanoparticles for assisting neural stem cell therapy for ischemic stroke. ACS Nano 2022, 16, 14503–14516. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis ofischaemic stroke. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- He, L.; Huang, G.; Liu, H.; Sang, C.; Liu, X.; Chen, T. Highly bioactive zeolitic imidazolate framework-8–capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci. Adv. 2020, 6, eaay9751. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, Y.; Peng, Y.; Mai, R.; Teng, H.; Qi, Z.; Mo, J. Advancing stroke therapy: The potential of MOF-based nanozymes in biomedical applications. Front. Bioeng. Biotechnol. 2024, 12, 1363227. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jin, Y.; Peng, T.; Gao, Y.; Zang, Y.; He, H.; Li, F.; Zhang, Y.; Zhang, H.; Chen, L. Fabrication of multifunctional metal–organic frameworks nanoparticles via layer-by-layer self-assembly to efficiently discover PSD95-nNOS uncouplers for stroke treatment. J. Nanobiotechnol. 2022, 20, 379. [Google Scholar] [CrossRef]
- Ren, F.; Yang, B.; Cai, J.; Jiang, Y.; Xu, J.; Wang, S. Toxic effect of zinc nanoscale metal-organic frameworks on rat pheochromocytoma (PC12) cells in vitro. J. Hazard. Mater. 2014, 271, 283–291. [Google Scholar] [CrossRef]
- Niu, H.; Chen, J.; Jin, J.; Qi, X.; Bai, K.; Shu, C.; Wu, A.; Xiao, Y.; Wu, C.; Bu, H.; et al. Engineering metalloporphyrin-integrated nanosystems for targeted sono-/chemo-dynamic therapy of leptomeningeal carcinomatosis through intrathecal administration. Chem. Eng. J. 2022, 437, 135373. [Google Scholar] [CrossRef]



| Delivery System | Advantages | Disadvantages |
|---|---|---|
| MOFs | High surface area and porosity for high drug loading; tunable pore size; easy functionalization; potential for targeted delivery; high drug loading capacity; protective encapsulation; versatility; low density; structural diversity | Potential for premature drug release; challenges in scalability and cost-effectiveness in clinical settings; difficulty in targeting; stability issues under certain conditions; limited biocompatibility |
| Liposomes | Biocompatibility and biodegradability; versatility in drug encapsulation; ability to encapsulate both hydrophilic and hydrophobic drugs; reduced toxicity; flexibility in size and surface modification; enhanced permeability and retention effect; potential for targeted delivery; protection of drugs; relatively low immunogenicity | Instability; potential for drug leakage; relatively lower drug loading capacity; high production costs; limited penetration; complex preparation; difficulty in controlling/sustaining drug release |
| Micelles | Good for solubilizing hydrophobic drugs; good biocompatibility; ease of preparation; enhanced permeability and retention effect; ability to enhance drug solubility; long circulation time; potential for targeted delivery; low toxicity; stimulus responsiveness; minimal size; self-assembly | Lower drug loading capacity compared to other systems; potential for premature drug release; difficulty in controlling release rates; challenges in large-scale production; poor solubility; low loading capacity; undesirable physical stability |
| Nanoparticles | Good stability; controlled drug release; good; large surface area; biocompatibility; high drug loading capacity; versatility in drug delivery; enhanced drug solubility; targeted delivery; ability to cross blood–brain barrier; facile synthesis; easy surface decoration; ultra-small sizes | Burst release; rapid elimination; potential toxicity; processes; high production costs; potential for aggregation; poor degradability; low solubility; immunogenicity |
| MOF System | Drug Delivered | How to Overcome BBB | Disease Type | Therapeutic Efficiency | Ref. |
|---|---|---|---|---|---|
| MILB@LR | Oxaliplatin (OXA) | RABV-mimetic design with RVG29 peptide for BBB penetration and targeting GBM cells | GBM | Effective brain tumor penetration, significant tumor growth inhibition | [59] |
| BSO-CAT@MOF-199@DDM (BCMD) | Cu2+ Ions, Buthionine-Sulfoximine (BSO), Catalase (CAT) | Intranasal administration for direct brain tumor delivery | GBM | Induced cuproptosis, enhanced immune response, improved therapeutic efficacy with immune checkpoint blockade | [61] |
| MNPs@MIL-101(Fe) | Temozolomide (TMZ) | Integration of MIL frameworks with Fe3O4 magnetic nanoparticles for improved BBB penetration | GBM | Enhanced drug loading and release, increased cytotoxicity against GBM cells | [62] |
| CBD/Mg-GA MOF | Cannabidiol (CBD), Gallic Acid (GA) | Increased permeability (pH-sensitive degradation) | GBM | Significant increase in cell death and reduced colony formation, improved antioxidant activity | [63] |
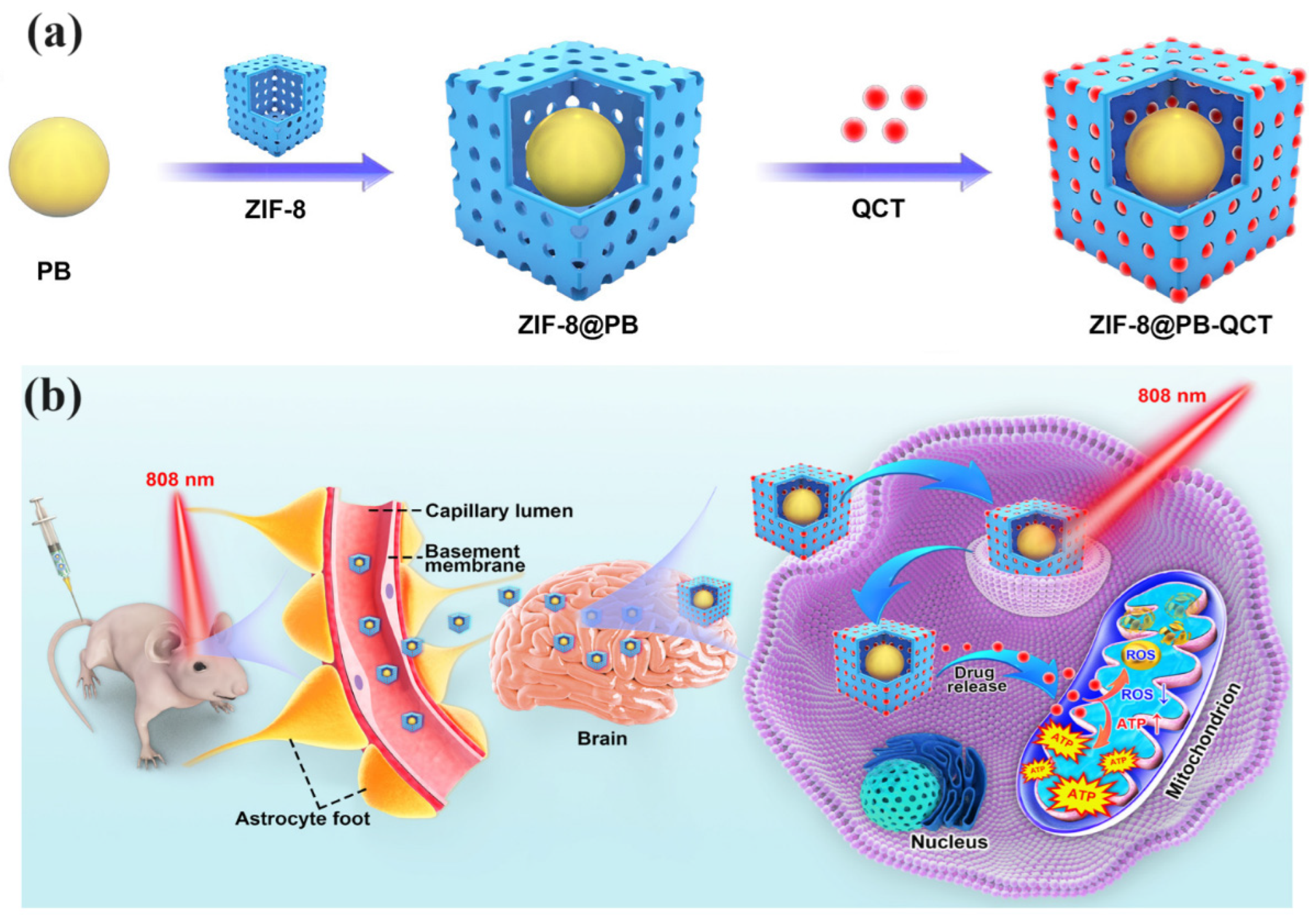
| ZIF-8@PB-QCT | Quercetin (QCT) | NIR light activation to enhance BBB permeability | PD | Improved mitochondrial function, reduced PD symptoms in mice, enhanced motor function | [64] |
| Ptzyme@D-ZIF | Nanozymes (Ptzymes) | Enhanced BBB accumulation through clathrin- and caveolae-mediated endocytosis | PD | Reduced neuroinflammation, improved behavioral and pathological outcomes, superior ROS scavenging | [65] |
| MOF@Man Liposome | Fe-TCPP, Zr6 Clusters | Mannitol-coated liposomes for BBB permeability | PD | Enhanced motor function, memory, and gene normalization in mice | [66] |
| AuNPs@PEG@MIL-101 | Amyloid β Binder | Direct interaction with Aβ monomers and fibrils | AD | Inhibited Aβ aggregation, protected PC12 cells from Aβ-induced damage, reduced ROS values | [67] |
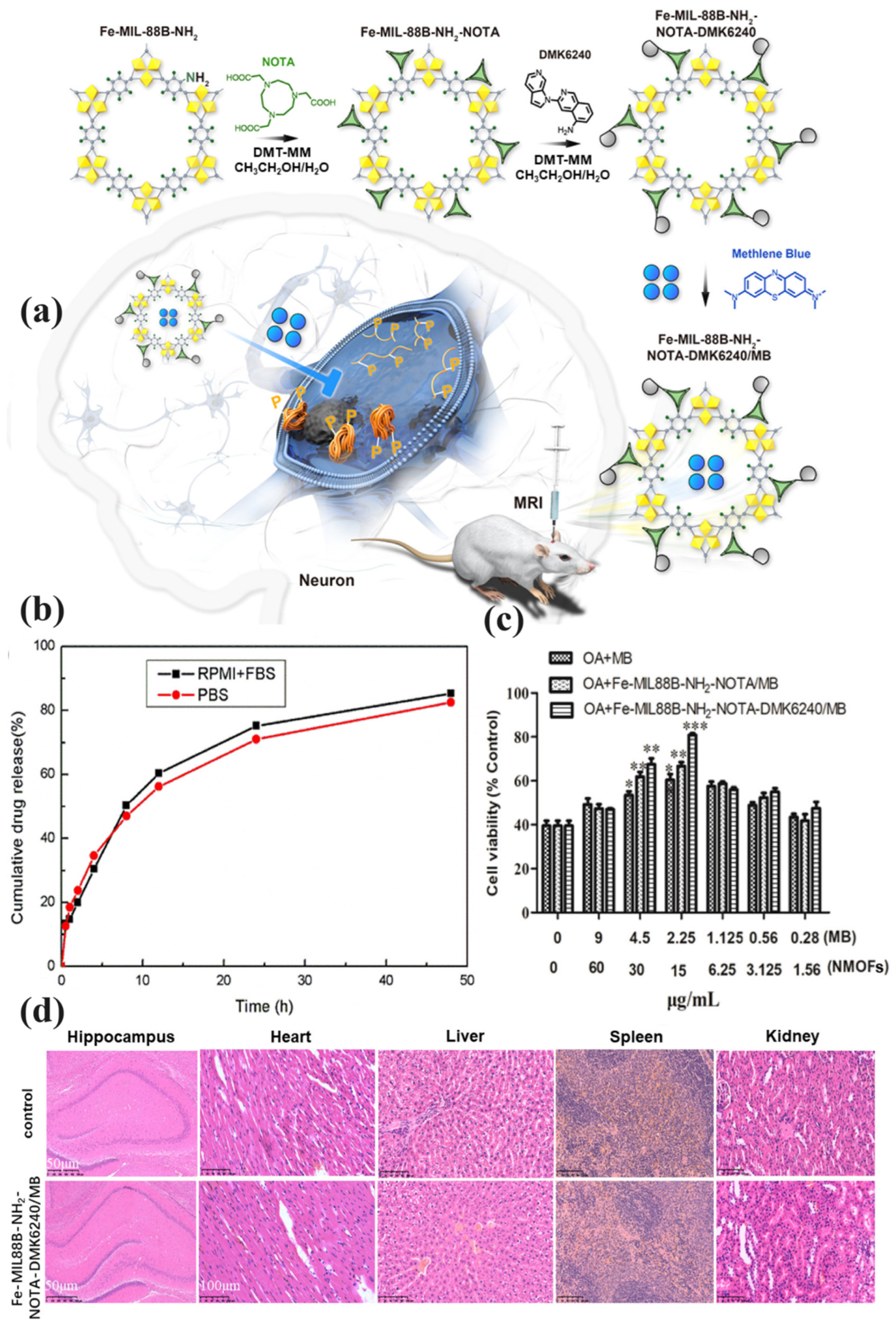
| Fe-MIL-88B-NH2-NOTA-DMK6240/MB | Methylene Blue | Functionalized with NOTA and DMK6240 for targeting tau proteins | AD | Inhibited tau protein aggregation, reduced neuronal death, improved cognitive function | [68] |
| PCN-224 | Singlet Oxygen | NIR light activation for photooxygenation | AD | Prevented Aβ aggregation, reduced Aβ-induced cytotoxicity, enhanced singlet oxygen production | [69] |
| TR-ZRA | CD22 shRNA, Aβ Aptamers, Chlorogenic Acid | Erythrocyte membrane camouflaging for BBB crossing and Aβ plaque monitoring | AD | Reduced Aβ burden, improved microglial phagocytosis, enhanced memory function | [70] |
| PCN−222@ICG | Indocyanine Green | RVG peptide modification for BBB penetration | AD | Reduced Aβ plaque density and size, enhanced therapeutic efficacy | [71] |
| Ca-MOF@miR-124 | MicroRNA-124 (miR-124) | Nano delivery system for improved stability and internalization | Ischemic Stroke | Increased neuronal differentiation, reduced infarct size in mice, improved neuronal survival | [72] |
| CeO2@ZIF-8 | CeO2 | Lysosome-mediated endocytosis | Ischemic Stroke | Reduced oxidative stress and infarct size, improved neurological outcomes | [74] |
| PSD95-nNOS/Fe-MOF | PSD95-nNOS Uncouplers | Magnetic Fe-MOFs for screening uncouplers | Ischemic Stroke | High-throughput screening for PSD95-nNOS uncouplers | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabipour, H.; Rohani, S. Metal–Organic Frameworks for Overcoming the Blood–Brain Barrier in the Treatment of Brain Diseases: A Review. Nanomaterials 2024, 14, 1379. https://doi.org/10.3390/nano14171379
Nabipour H, Rohani S. Metal–Organic Frameworks for Overcoming the Blood–Brain Barrier in the Treatment of Brain Diseases: A Review. Nanomaterials. 2024; 14(17):1379. https://doi.org/10.3390/nano14171379
Chicago/Turabian StyleNabipour, Hafezeh, and Sohrab Rohani. 2024. "Metal–Organic Frameworks for Overcoming the Blood–Brain Barrier in the Treatment of Brain Diseases: A Review" Nanomaterials 14, no. 17: 1379. https://doi.org/10.3390/nano14171379
APA StyleNabipour, H., & Rohani, S. (2024). Metal–Organic Frameworks for Overcoming the Blood–Brain Barrier in the Treatment of Brain Diseases: A Review. Nanomaterials, 14(17), 1379. https://doi.org/10.3390/nano14171379








