Improved Proton Conductivity of Chitosan-Based Composite Proton Exchange Membrane Reinforced by Modified GO Inorganic Nanofillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
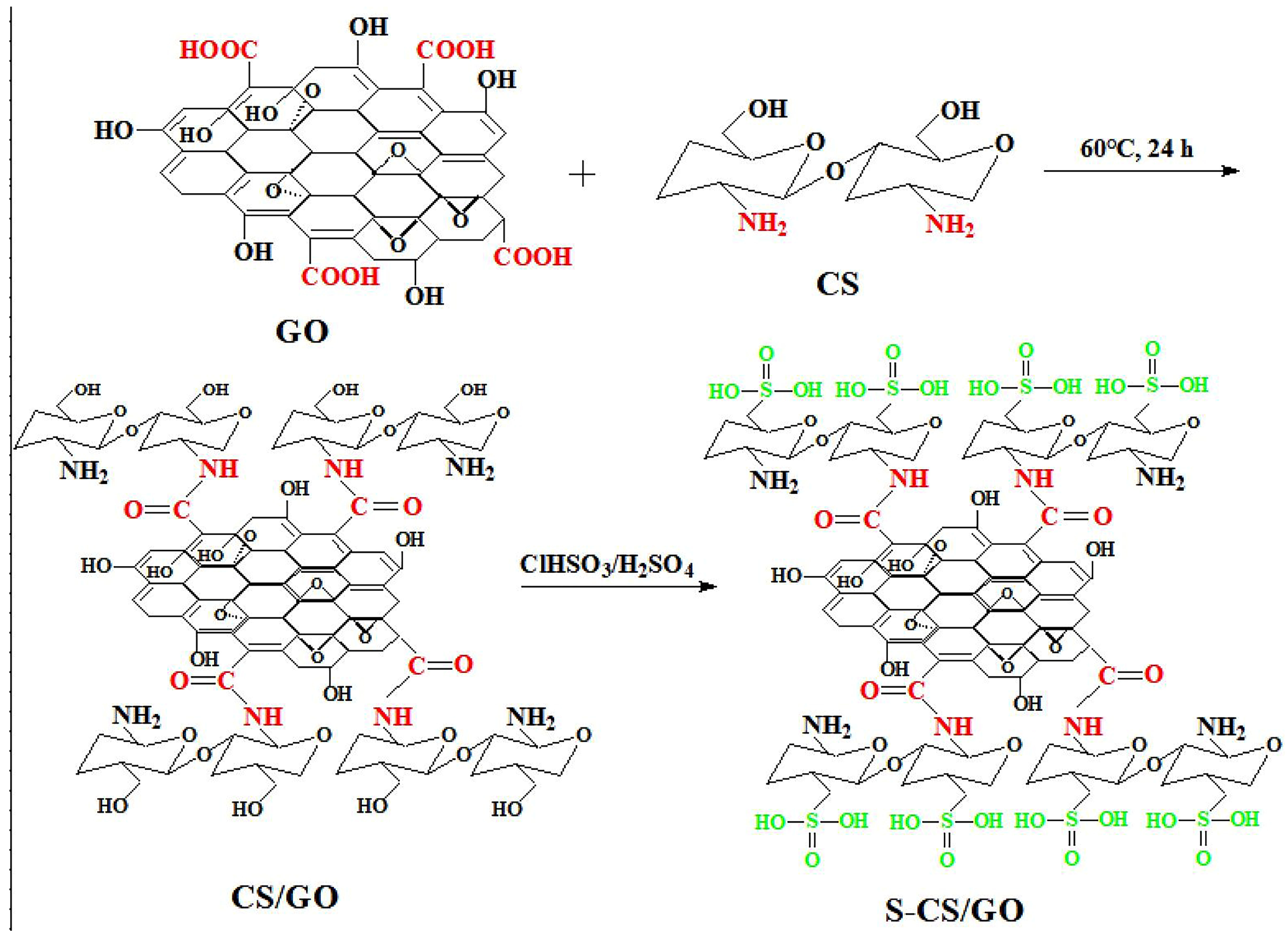
2.2. Preparation of CS/GO
2.3. Preparation of S-CS/GO Nanofillers
2.4. Preparation of S-CS/GO@CS Composite PEMs
2.5. Characterization
2.6. Performance Measurement
2.6.1. Measurement of Mechanical Properties
2.6.2. Measurement of Oxidation Stability
2.6.3. Measurement of Water Uptake (WU) and Swelling Ratio (SR)
2.6.4. Measurement of Ion Exchange Capacity (IEC), Proton Conductivity (σ), Selectivity and Methanol Permeability (P)
3. Results
3.1. Structure and Performance of S-CS/GO Nanofillers
3.1.1. Morphology Analysis
3.1.2. Chemical Structure Analysis
3.1.3. Zeta Potential Analysis
3.1.4. Thermal Stability Analysis
3.1.5. Analysis of Organic Elements
3.2. Performance of the S-CS/GO@CS Composite PEMs
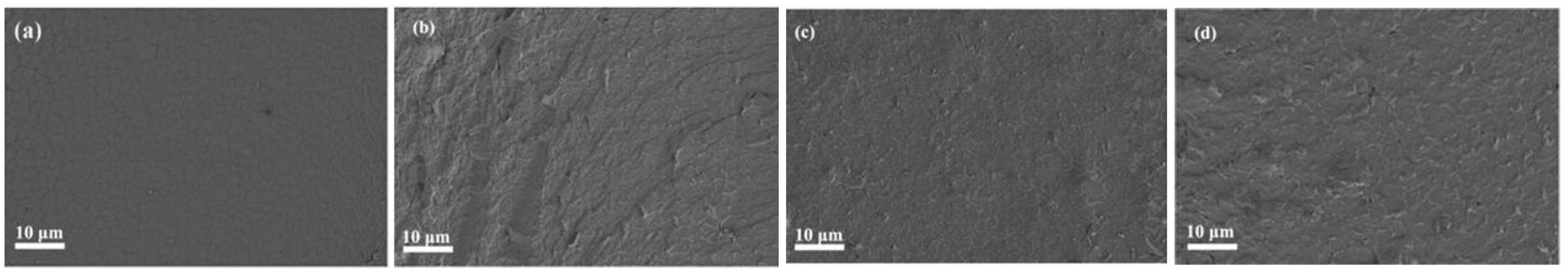
3.2.1. Surface Morphology
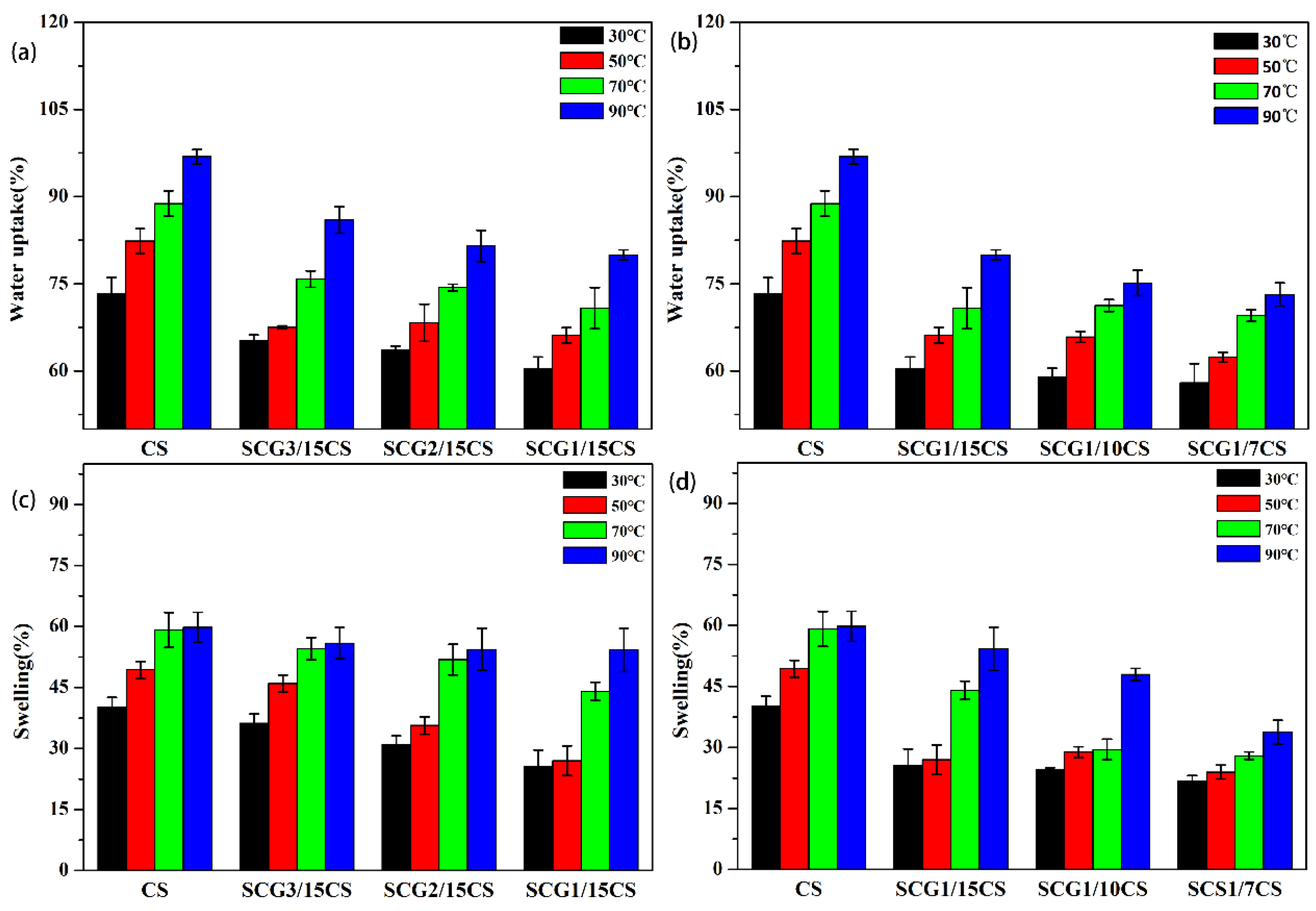
3.2.2. WU and SR
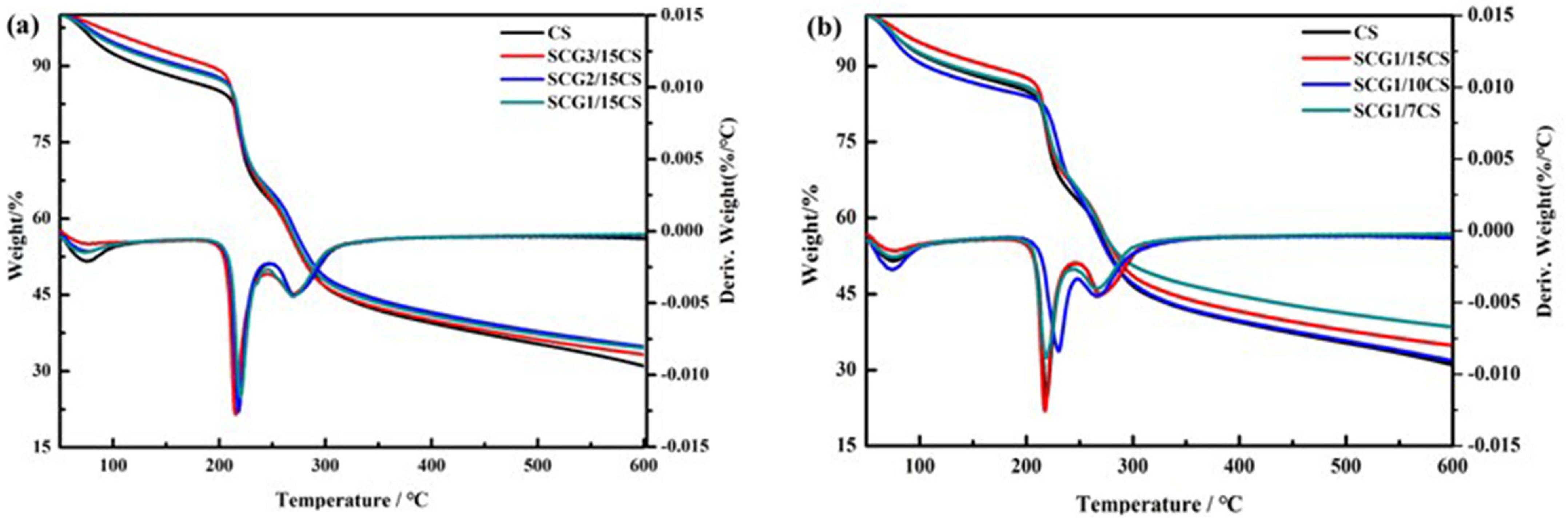
3.2.3. Thermal Stability Performance
3.2.4. Mechanical Properties
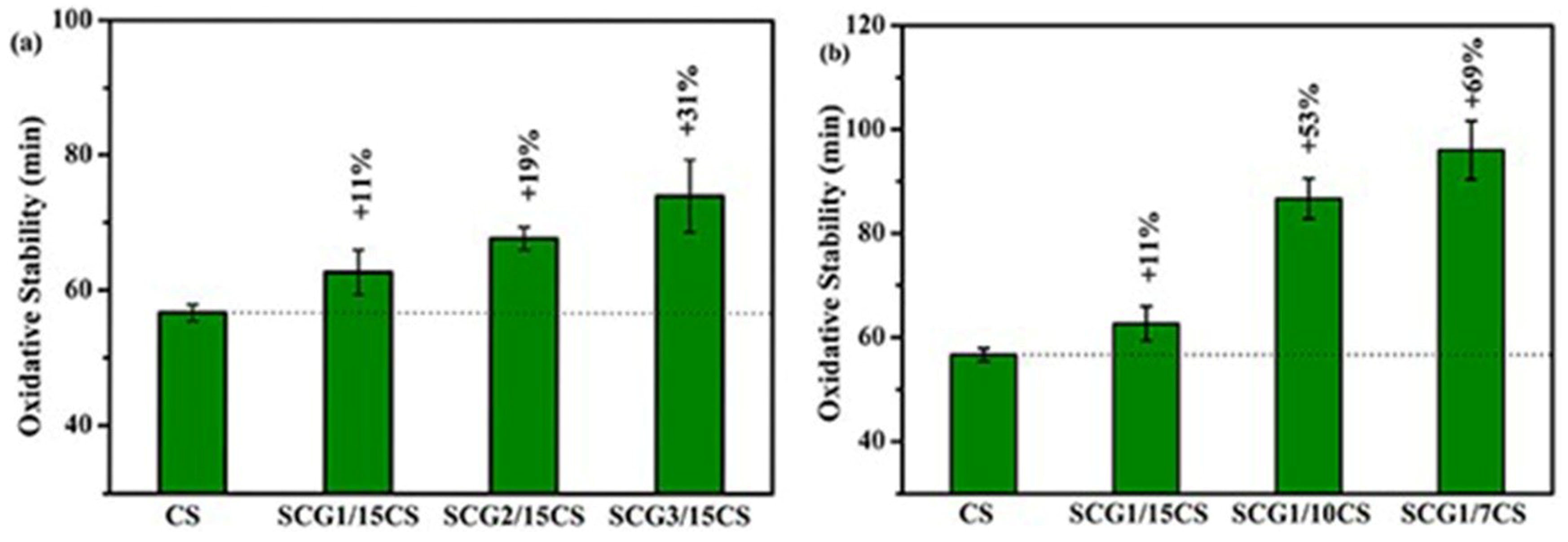
3.2.5. Oxidation Stability
3.2.6. IEC
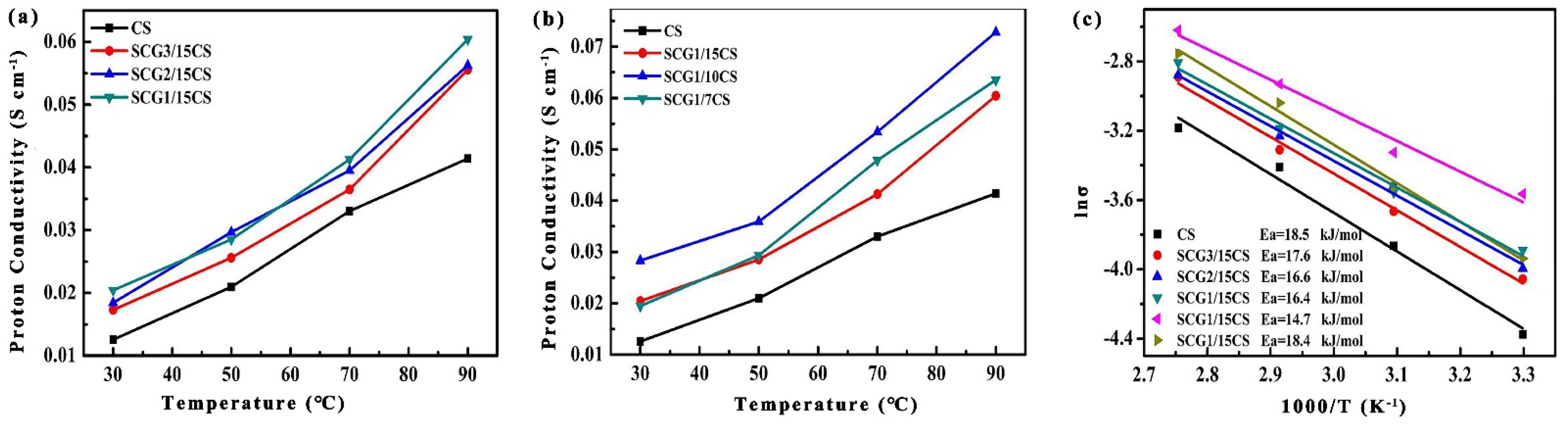
3.2.7. σ and Conduction Mechanism
3.2.8. Methanol Permeability and Selectivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.Y.; Zhang, Z.X.; Xie, Z.; Guo, X.R.; Yang, T.J.; Li, Z.L.; Tu, M.; Rao, H.X. High performance and self-humidifying of novel cross-linked and nanocomposite proton exchange membranes based on sulfonated polysulfone. Nanomaterials 2022, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Ge, X.L.; Zhang, F.; Wu, L.; Yang, Z.J.; Xu, T.W. Current challenges and perspectives of polymer electrolyte membranes. Macromolecules 2022, 55, 3773–3787. [Google Scholar] [CrossRef]
- Hou, H.Y.; Di Vona, M.L.; Knauth, P. Building bridges: Crosslinking of sulfonated aromatic polymers—A review. J. Membr. Sci. 2012, 423–424, 113–127. [Google Scholar] [CrossRef]
- You, P.Y.; Kamarudin, S.K.; Masdar, M.S. Improved performance of sulfonated polyimide composite membranes with rice husk ash as a bio-filler for application in direct methanol fuel cells. Int. J. Hydrogen Energy 2019, 44, 1857–1866. [Google Scholar] [CrossRef]
- Yang, T.J.; Li, Z.L.; Lyu, H.L.; Zheng, J.J.; Liu, J.L.; Liu, F.N.; Zhang, Z.Y.; Rao, H.X. A graphene oxide polymer brush based crosslinked nanocomposite proton exchange membrane for direct methanol fuel cells. RSC Adv. 2018, 8, 15740–15753. [Google Scholar] [CrossRef]
- Shirdast, A.; Sharif, A.; Abdollahi, M. Effect of the incorporation of sulfonated chitosan/sulfonated graphene oxide on the proton conductivity of chitosan membranes. J. Power Sources 2016, 306, 541–551. [Google Scholar] [CrossRef]
- Terbish, N.; Lee, C.H.; Popuri, S.R.; Nalluri, L.P. An investigation into polymer blending, plasticization and cross-linking effect on the performance of chitosan-based composite proton exchange membranes for microbial fuel cell applications. J. Polym. Res. 2020, 27, 280. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Wu, H.; Zheng, B.; Jiang, Z.; Hao, X.; Wang, B. Effect of zeolites on chitosan/zeolite hybrid membranes for direct methanol fuel cell. J. Power Sources 2008, 178, 9–19. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, H.; He, Y.; Liu, J.; Zhang, B.; Wang, J. Enhanced proton conduction of chitosan membrane enabled by halloysite nanotubes bearing sulfonate polyelectrolyte brushes. J. Membr. Sci. 2014, 454, 220–232. [Google Scholar] [CrossRef]
- Palanisamy, G.; Muhammed, A.P.; Thangarasu, S.; Oh, T.H. Investigating the sulfonated chitosan/polyvinylidene fluoride-based proton exchange membrane with fSiO2 as filler in microbial fuel cells. Membranes 2023, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yang, M.; Guo, Z.B.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Holder, S.L.; Lee, C.H.; Popuri, S.R.; Zhuang, M.X. Enhanced surface functionality and microbial fuel cell performance of chitosan membranes through phosphorylation. Carbohydr. Polym. 2016, 149, 251–262. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Hashem, A.E.; Tamer, T.M.; Omer, A.M.; Yossuf, M.E.; Sabet, M.M. Development of cross linked chitosan/alginate polyelectrolyte proton exchanger membranes for fuel cell applications. Int. J. Electrochem. Sci. 2017, 12, 3840–3858. [Google Scholar] [CrossRef]
- Wang, W.; Shan, B.; Zhu, L.; Xie, C.; Liu, C.; Cui, F. Anatase titania coated CNTs and sodium lignin sulfonate doped chitosan proton exchange membrane for DMFC application. Carbohydr. Polym. 2018, 187, 35–42. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, M.; Cai, Y.B.; Lu, Y.H.; Ahmad, Z.; Khannal, S.; Xu, S. Novel sulfonated multi-walled carbon nanotubes filled chitosan composite membrane for fuel-cell applications. J. Appl. Polym. Sci. 2019, 136, 47603. [Google Scholar] [CrossRef]
- Anu Karthi, A.K.S.; Cindrella, L. Self-humidifying novel chitosan-geopolymer hybrid membrane for fuel cell applications. Carbohydr. Polym. 2019, 223, 115073. [Google Scholar]
- Wang, J.; Gong, C.L.; Wen, S.; Liu, H.; Qin, C.Q.; Xiong, C.X.; Dong, L.J. Proton exchange membrane based on chitosan and solvent-free carbon nanotube fluids for fuel cells applications. Carbohydr. Polym. 2018, 186, 200–207. [Google Scholar] [CrossRef]
- Wang, J.; Han, Z. The combustion behavior of polyacrylate ester/graphite oxide composites. Polym. Advan. Technol. 2006, 17, 335–340. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Zhang, W.D.; Tong, Y.J. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xiao, M.; Meng, Y.; Hay, A.S. Direct synthesis of poly (arylenedisulfide)/carbon nanosheet composites via the oxidation with graphite oxide. Carbon 2005, 43, 195–197. [Google Scholar] [CrossRef]
- Yang, X.M.; Tu, Y.F.; Li, L.; Shang, S.M.; Tao, X.M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Han, D.L.; Yan, L.F.; Chen, W.F.; Li, W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 2011, 83, 653–658. [Google Scholar] [CrossRef]
- Crea, J.; DiGiusto, R.; Lincoln, S.F.; Willioms, E.H. A nuclear magnetic resonance study of ligand exchange on dioxopentakis (trimethyl phosphate) uranium (VI) ion and its triethyl phosphate analog. Inorg. Chem. 1977, 16, 2825–2829. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, R. Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. J. Colloid Interf. Sci. 2003, 264, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.Q.; Pan, Y.Z.; Ping, Y.; Sahoo, N.G.; Wu, T.F.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.Y.; Liu, H.L.; Cheng, F.; Wang, G.J.; Zheng, G.W.; Qin, C.Q.; Wen, S. Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, T.; Wang, J.; Pei, F.; He, Y.; Liu, J. Enhanced proton conductivity of sulfonated poly (ether ether ketone) membrane embedded by dopamine-modified nanotubes for proton exchange membrane fuel cell. Fuel Cells 2013, 13, 1155–1165. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, S.K.; Wang, C.H.; Huang, H.C.; Du, H.Y.; Chen, K.H. Highly proton-selective biopolymer layer-coated ion-exchange membrane for direct methanol fuel cells. Chem. Sus. Chem. 2012, 5, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Zhang, Y.; Wang, L. Sulfonated polyimide/chitosan composite membrane for vanadium redox flow battery: Membrane preparation, characterization, and single cell performance. J. Appl. Polym. Sci. 2013, 127, 4150–4159. [Google Scholar] [CrossRef]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. Chitosan–graphene oxide nanocomposites: Effect of graphene oxide nanosheets and glycerol plasticizer on thermal and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 45092. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Chitosan/partially sulfonated poly (vinylidene fluoride) blends as polymer electrolyte membranes for direct methanol fuel cell applications. Cellulose 2018, 25, 661–681. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Yao, H.; Tong, C.; Lei, G.; Liu, L.; Lv, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on sulfonated poly (ether ether ketone) membrane for direct methanol fuel cells. J. Membr. Sci. 2013, 425, 11–22. [Google Scholar] [CrossRef]
- Du, J.; Bai, Y.; Chu, W.Y.; Qiao, L.J. The structure and electric characters of proton-conducting chitosan membranes with various ammonium salts as complexant. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 880–885. [Google Scholar] [CrossRef]
- Yang, H.; Wu, H.; Shen, X.; Zhen, L.; Jiang, Z. Enhanced proton conductivity of proton exchange membrane at low humidity based on poly (methacrylic acid)-loaded imidazole microcapsules. RSC Adv. 2015, 5, 9079–9088. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Shi, Z.; Holdcroft, S. Transport properties of composite membranes containing silicon dioxide and Nafion®. J. Membr. Sci. 2008, 325, 346–356. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, J.T.; Zhang, H.Q.; Ma, C.M.; Liu, J.D.; Cao, S.K.; Zhang, X. Enhancement of proton conductivity of chitosan membrane enabled by sulfonated graphene oxide under both hydrated and anhydrous conditions. J. Power Sources 2014, 269, 898–911. [Google Scholar] [CrossRef]


| Samples | GO and CS Mass Ratio | GO Content (%) | CS Content (%) | N Content (%) | C Content (%) | S Content (%) | H Content (%) |
|---|---|---|---|---|---|---|---|
| CS | 0 | 100.00 | 7.57 | 40.39 | 0 | 7.15 | |
| S-CS/GO-1 | 1:15 | 6.25 | 93.75 | 1.97 | 46.36 | 3.81 | 3.48 |
| S-CS/GO-2 | 1:10 | 9.09 | 90.91 | 1.55 | 49.43 | 3.45 | 3.26 |
| S-CS/GO-3 | 1:5 | 16.70 | 83.30 | 1.32 | 48.90 | 3.29 | 2.90 |
| Membrane Samples | SCG/CS (wt/wt) | Initial Decomposition Temperature (℃) | Final Residual Carbon Content (%) |
|---|---|---|---|
| CS | 213.35 | 31.03 | |
| SCG-3/15CS | 3/15 | 209.57 | 33.29 |
| SCG-2/15CS | 2/15 | 211.84 | 34.85 |
| SCG-1/15CS | 1/15 | 212.91 | 34.54 |
| SCG-1/15CS | 1/15 | 212.97 | 34.54 |
| SCG-1/10CS | 1/10 | 217.47 | 31.80 |
| SCG-1/7CS | 1/7 | 210.92 | 38.50 |
| Membrane Samples | SCG/CS (wt/wt) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| CS | 21.5 ± 2.2 | 17.4 ± 2.5 | |
| SCG-1/15CS | 1/15 | 23.8 ± 1.5 | 12.8 ± 1.7 |
| SCG-2/15CS | 1/15 | 26.9 ± 2.8 | 8.9 ± 1.5 |
| SCG-3/15CS | 2/15 | 35.3 ± 3.7 | 9.1 ± 2.5 |
| SCG-1/10CS | 1/10 | 42.6 ± 3.3 | 17.8 ± 1.4 |
| SCG-1/7CS | 1/7 | 36.6 ± 2.4 | 14.5 ± 2.8 |
| Membrane Samples | IEC (mmol/g) | Methanol Permeability (p, × 10−7 cm2/s) | Selectivity (SP, ×104 S·s·cm−3) |
|---|---|---|---|
| CS | 0.38 ± 0.02 | 3.38 ± 0.58 | 3.72 ± 0.16 |
| SCG-3/15CS | 0.44 ± 0.05 | 2.33 ± 0.06 | 7.42 ± 0.28 |
| SCG-2/15CS | 0.54 ± 0.06 | 2.09 ± 0.26 | 8.82 ± 0.34 |
| SCG-1/15CS | 0.61 ± 0.03 | 1.86 ± 0.23 | 10.99 ± 0.51 |
| SCG-1/10CS | 0.74 ± 0.05 | 1.66 ± 0.36 | 17.01 ± 0.68 |
| SCG-1/7CS | 0.64 ± 0.04 | 1.19 ± 0.17 | 16.39 ± 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhang, Z.; Liu, Z.; Huang, H.; Zhang, C.; Rao, H. Improved Proton Conductivity of Chitosan-Based Composite Proton Exchange Membrane Reinforced by Modified GO Inorganic Nanofillers. Nanomaterials 2024, 14, 1217. https://doi.org/10.3390/nano14141217
Guo X, Zhang Z, Liu Z, Huang H, Zhang C, Rao H. Improved Proton Conductivity of Chitosan-Based Composite Proton Exchange Membrane Reinforced by Modified GO Inorganic Nanofillers. Nanomaterials. 2024; 14(14):1217. https://doi.org/10.3390/nano14141217
Chicago/Turabian StyleGuo, Xinrui, Zhongxin Zhang, Zhanyan Liu, Hui Huang, Chunlei Zhang, and Huaxin Rao. 2024. "Improved Proton Conductivity of Chitosan-Based Composite Proton Exchange Membrane Reinforced by Modified GO Inorganic Nanofillers" Nanomaterials 14, no. 14: 1217. https://doi.org/10.3390/nano14141217
APA StyleGuo, X., Zhang, Z., Liu, Z., Huang, H., Zhang, C., & Rao, H. (2024). Improved Proton Conductivity of Chitosan-Based Composite Proton Exchange Membrane Reinforced by Modified GO Inorganic Nanofillers. Nanomaterials, 14(14), 1217. https://doi.org/10.3390/nano14141217







