Colloidal Titanium Nitride Nanoparticles by Laser Ablation in Solvents for Plasmonic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth of Titanium Nitride Targets
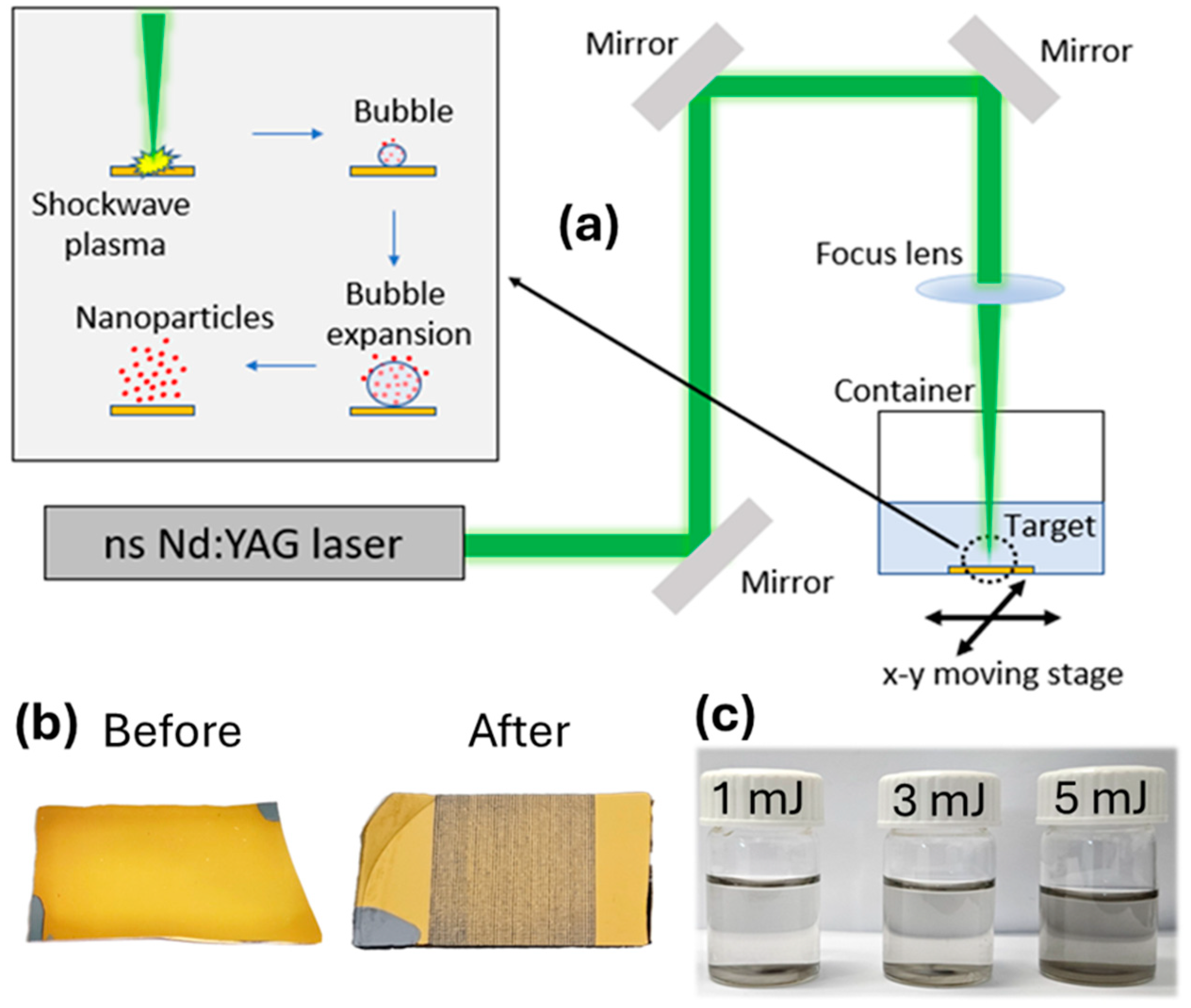
2.2. Synthesis of TiN Nanoparticles
2.3. Characterization
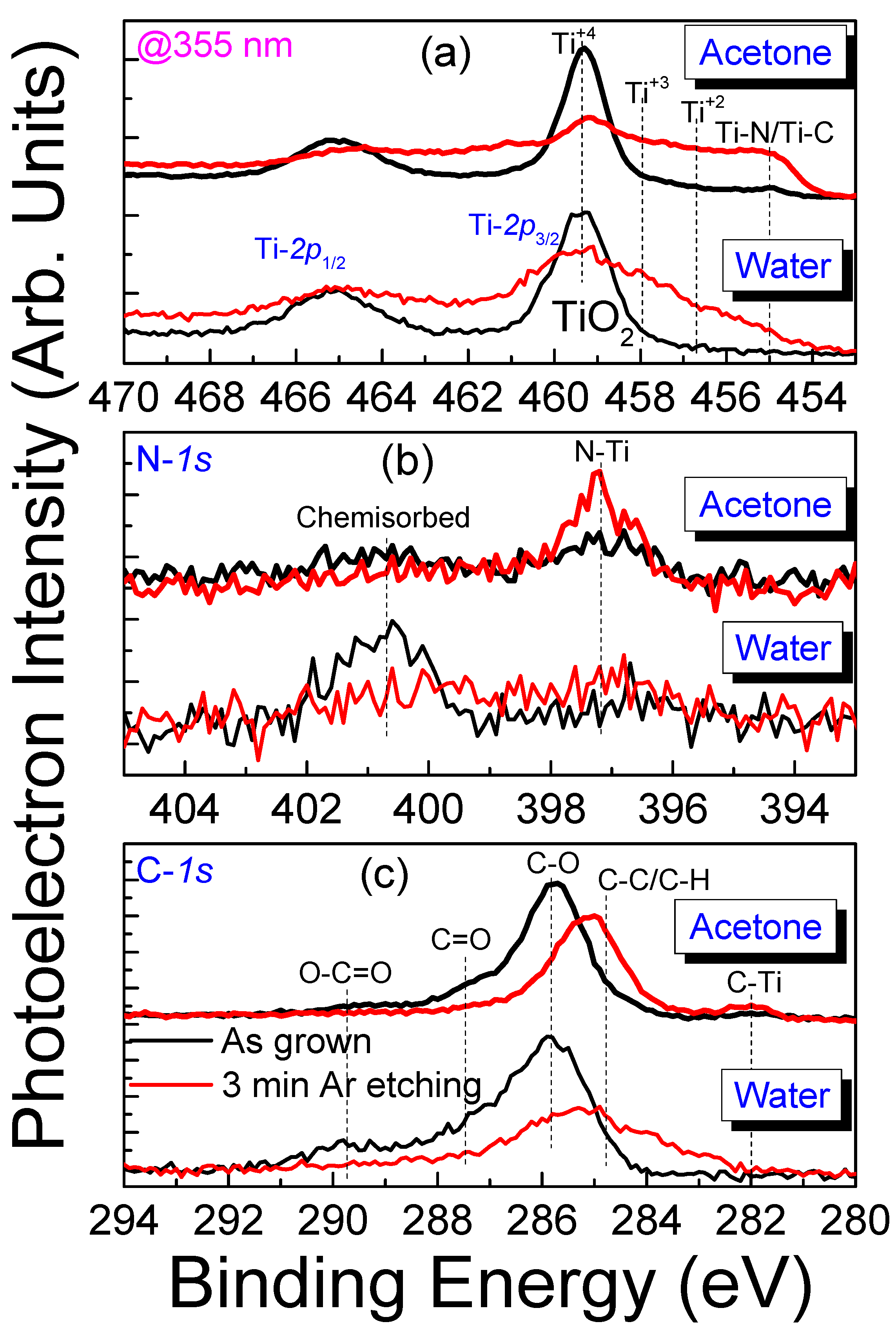
3. Results and Discussion
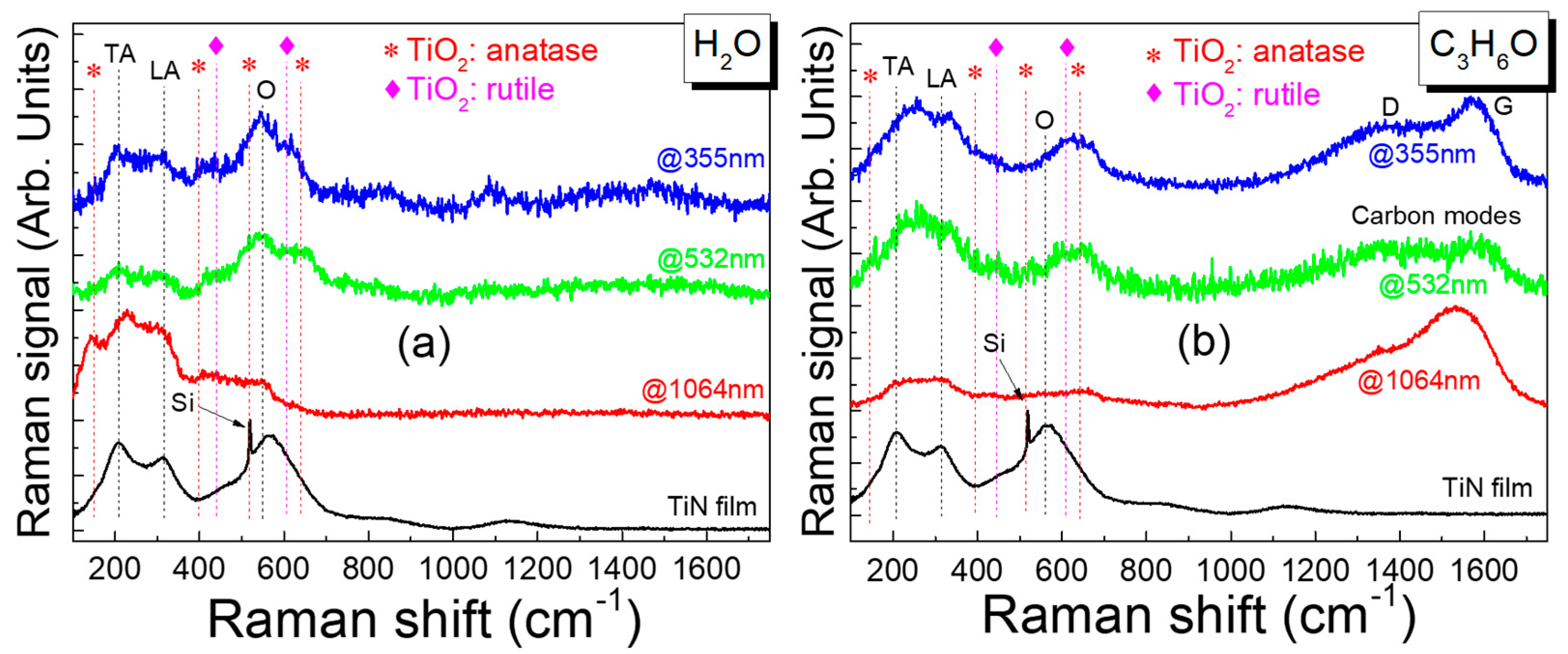
3.1. Characterization of TiN Films
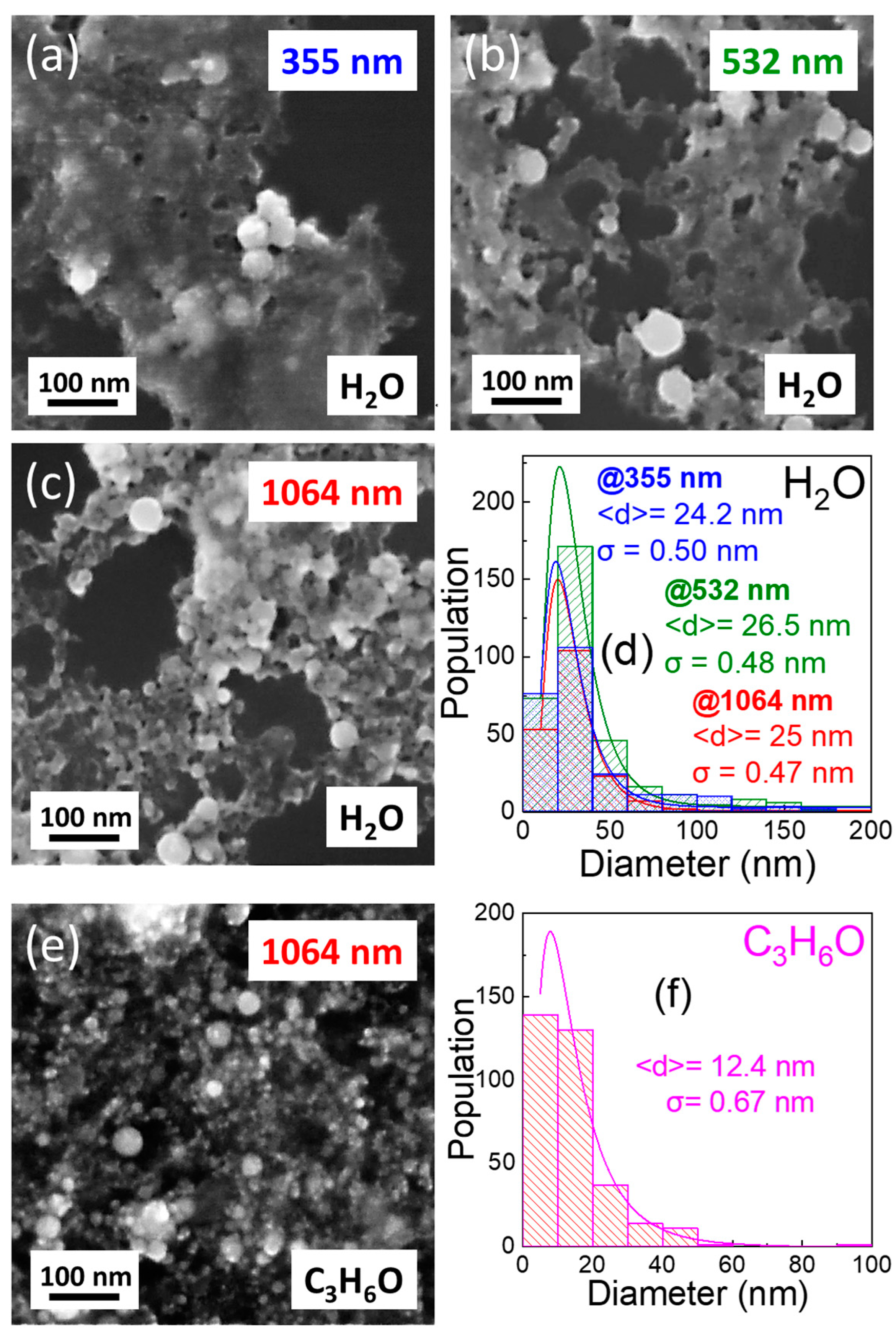
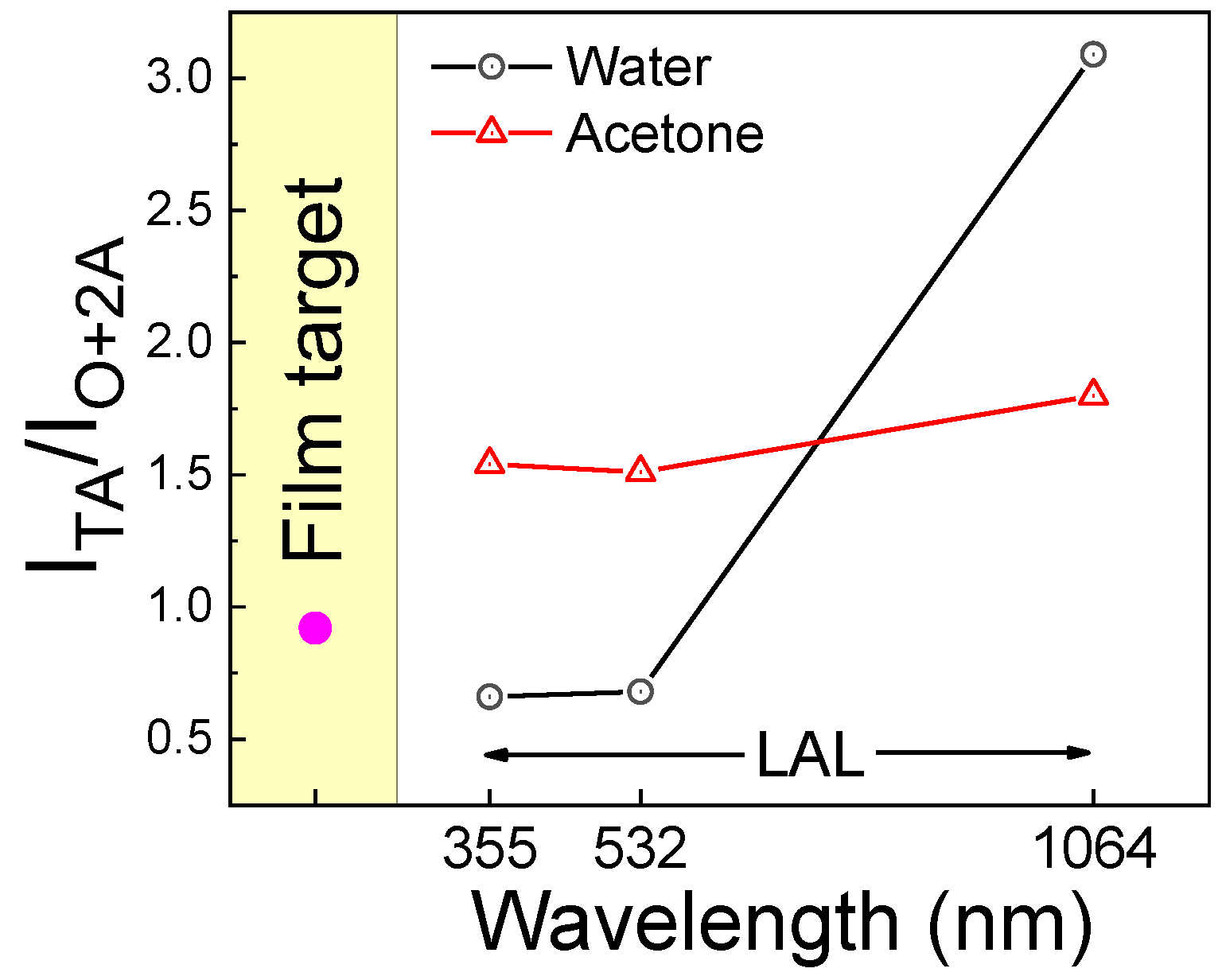
3.2. Fabrication and Characterization of Colloidal TiN Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dionne, J.A.; Diest, K.; Sweatlock, L.A.; Atwater, H.A. PlasMOStor: A Metal–Oxide–Si Field Effect Plasmonic Modulator. Nano Lett. 2009, 9, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, M.T.; Van De Groep, J.; Brown, A.M. Plasmoelectric potentials in metal nanostructures. Science 2014, 346, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, W.; Wang, J.; Wang, X.; Wang, S.; Chen, X.; Lu, W.; Wang, L.; Hu, W.; Wang, J.; et al. Plasmon resonant excitation in grating-gated AlN barrier transistors at terahertz frequency. Appl. Phys. Lett. 2012, 100, 123501. [Google Scholar] [CrossRef]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Imaging and Treatment of Cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Zhang, B.; Kumar, R.B.; Dai, H.; Feldman, B.J. A plasmonic chip for biomarker discovery and diagnosis of type 1 diabetes. Nat. Med. 2014, 20, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Deceglie, M.G.; Ferry, V.E.; Alivisatos, A.P.; Atwater, H.A. Design of Nanostructured Solar Cells Using Coupled Optical and Electrical Modeling. Nano Lett. 2012, 12, 2894–2900. [Google Scholar] [CrossRef]
- Gan, Q.; Bartoli, F.J.; Kafafi, Z.H. Plasmonic-Enhanced Organic Photovoltaics: Breaking the 10% Efficiency Barrier. Adv. Mater. 2013, 25, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.A.; Liu, J.S.Q.; Barnard, E.S.; Askarov, D.; Garnett, E.C.; Fan, S.; Brongersma, M.L. Optimization of non-periodic plasmonic light-trapping layers for thin-film solar cells. Nat. Commun. 2013, 4, 2095. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.M.; Langhammer, C.; Zorić, I.; Kasemo, B. Nanoplasmonic Probes of Catalytic Reactions. Science 2009, 326, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Stucky, G.D.; Moskovits, M.; Mubeen, S.; Lee, J.; Singh, N.; Kra, S. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef]
- Qian, K.; Sweeny, B.C.; Johnston-peck, A.C.; Niu, W.; Graham, J.O.; Duchene, J.S.; Qiu, J.; Wang, Y.; Engelhard, M.H.; Su, D.; et al. Surface Plasmon-Driven Water Reduction: Gold Nanoparticle Size Matters. J. Am. Chem. Soc. 2014, 136, 9842–9845. [Google Scholar] [CrossRef] [PubMed]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic efficiencies of nanoparticles made of metal nitrides (TiN, ZrN) compared with gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Ikuno, Y.; Fujii, M. Absolute Scattering Cross Sections of Titanium Nitride Nanoparticles Determined by Single-Particle Spectroscopy: Implications for Plasmonic Nanoantennas. ACS Appl. Nano Mater. 2019, 2, 6769–6773. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical Properties and Plasmonic Performance of Titanium Nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S.; Abadias, G.; Bellas, D.V.; Lekka, C.; Lidorikis, E. Conductive nitrides: Growth principles, optical and electronic properties, and their perspectives in photonics and plasmonics. Mater. Sci. Eng. R Rep. 2018, 123, 1–55. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S. Interface properties and structural evolution of TiN/Si and TiN/GaN heterostructures. J. Appl. Phys. 2003, 93, 989–998. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007. [Google Scholar]
- Enoch, S.; Bonod, N. Plasmonics: From Basics to Advanced Topics; Springer Science and Business Media LLC: New York, NY, USA, 2007; pp. 105–148, 151–176. [Google Scholar]
- Guler, U.; Suslov, S.; Kildishev, A.V.; Boltasseva, A.; Shalaev, V.M. Colloidal Plasmonic Titanium Nitride Nanoparticles: Properties and Applications. Nanophotonics 2015, 4, 269–276. [Google Scholar] [CrossRef]
- Bora, B.; Aomoa, N.; Kakati, M.; Bhuyan, H. Studies on a supersonic thermal plasma expansion process for synthesis of titanium nitride nanoparticles. Powder Technol. 2013, 246, 413–418. [Google Scholar] [CrossRef]
- Fu, Q.; Kokalj, D.; Stangier, D.; Kruis, F.E.; Tillmann, W. Aerosol synthesis of titanium nitride nanoparticles by direct current arc discharge method. Adv. Powder Technol. 2020, 31, 4119–4128. [Google Scholar] [CrossRef]
- Howell, I.R.; Giroire, B.; Garcia, A.; Li, S.; Aymonier, C.; Watkins, J.J. Fabrication of plasmonic TiN nanostructures by nitridation of nanoimprinted TiO2 nanoparticles. J. Mater. Chem. C Mater. 2018, 6, 1399–1406. [Google Scholar] [CrossRef]
- Kaskel, S.; Schlichte, K.; Chaplais, G.; Khanna, M. Synthesis and characterisation of titanium nitride based nanoparticles. J. Mater. Chem. 2003, 13, 1496–1499. [Google Scholar] [CrossRef]
- Bayon, N.N.; Gunasekaran, N.K.; Tumkur, P.P.; Lamani, B.R.; Koehne, J.E.; Arasho, W.D.; Prabhakaran, K.; Hall, J.C.; Ramesh, G.T. Synthesis and characterization of titanium nitride nanoparticles. Mater. Express 2022, 12, 1211–1215. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Dizajghorbani-Aghdam, H.; Malekfar, R. Surface-Enhanced Raman scattering of methylene blue on titanium nitride nanoparticles synthesized by laser ablation in organic solvents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 257, 119721. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C 2021, 120, 111717. [Google Scholar] [CrossRef]
- Popov, A.A.; Tikhonowski, G.V.; Shakhov, P.V.; Popova-Kuznetsova, E.A.; Tselikov, G.I.; Romanov, R.I.; Markeev, A.M.; Klimentov, S.M.; Kabashin, A.V. Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions. Nanomaterials 2022, 12, 1672. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser-synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef]
- Through, N. Large-Scale Synthesis of Uniform Silver. Adv. Mater. 2002, 14, 833–837. [Google Scholar]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Laser Ablation Synthesis of Silver Nanoparticles Embedded in Graphitic Carbon Matrix. Sci. Adv. Mater. 2012, 4, 497–500. [Google Scholar] [CrossRef]
- Dizajghorbani Aghdam, H.; Azadi, H.; Esmaeilzadeh, M.; Moemen Bellah, S.; Malekfar, R. Ablation time and laser fluence impacts on the composition, morphology and optical properties of copper oxide nanoparticles. Opt. Mater. 2019, 91, 433–438. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Sun, J.; Zhang, Q.; Guo, J.; Yan, D. Synthesis of Nanocrystalline Titanium Nitride Powders by Direct Nitridation of Titanium Oxide. J. Am. Ceram. Soc. 2001, 47, 3045–3047. [Google Scholar] [CrossRef]
- Dolgaev, S.I.; Simakin, A.V.; Voronov, V.V.; Shafeev, G.A.; Bozon-verduraz, F. Nanoparticles produced by laser ablation of solids in liquid environment. Appl. Surf. Sci. 2002, 186, 546–551. [Google Scholar] [CrossRef]
- Tilaki, R.M.; Zad, A.I.; Mahdavi, S.M. Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. Appl. Phys. A Mater. Sci. Process. 2007, 419, 415–419. [Google Scholar] [CrossRef]
- Amendola, V.; Rizzi, G.A.; Polizzi, S.; Meneghetti, M. Synthesis of Gold Nanoparticles by Laser Ablation in Toluene: Quenching and Recovery of the Surface Plasmon Absorption. J. Phys. Chem. B 2005, 109, 23125–23128. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Li, H.; Ye, R.; Zhang, X.; Lyu, J.; Cai, Y. Low-threshold random lasers enhanced by titanium nitride nanoparticles suspended randomly in gain solutions. Opt. Express 2022, 30, 8222. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S. Optical, electronic, and transport properties of nanocrystalline titanium nitride thin films. J. Appl. Phys. 2001, 90, 4725–4734. [Google Scholar] [CrossRef]
- Sugavaneshwar, R.P.; Ishii, S.; Dao, T.D.; Ohi, A.; Nabatame, T.; Nagao, T. Fabrication of Highly Metallic TiN Films by Pulsed Laser Deposition Method for Plasmonic Applications. ACS Photonics 2018, 5, 814–819. [Google Scholar] [CrossRef]
- Chang, C.C.; Nogan, J.; Yang, Z.P.; Kort-Kamp, W.J.M.; Ross, W.; Luk, T.S.; Dalvit, D.A.R.; Azad, A.K.; Chen, H.T. Highly Plasmonic Titanium Nitride by Room-Temperature Sputtering. Sci. Rep. 2019, 9, 15287. [Google Scholar] [CrossRef]
- Patsalas, P.; Charitidis, C.; Logothetidis, S. The effect of substrate temperature and biasing on the mechanical properties and structure of sputtered titanium nitride thin films. Surf. Coat. Technol. 2000, 125, 335–340. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Hendry, E.; Chang, H.; Wears, M.L. Laser ablation of a bulk Cr target in liquids for nanoparticle synthesis. RSC Adv. 2014, 4, 50406–50411. [Google Scholar] [CrossRef]
- Subhan, A.; Mourad, A.-H.I.; Al-Douri, Y. Influence of Laser Process Parameters, Liquid Medium, and External Field on the Synthesis of Colloidal Metal Nanoparticles Using Pulsed Laser Ablation in Liquid: A Review. Nanomaterials 2022, 12, 2144. [Google Scholar] [CrossRef]
- Albert Irudayaraj, A.; Srinivasan, R.; Kuppusami, P.; Mohandas, E.; Kalainathan, S.; Ramachandran, K. Photoacoustic measurement of thermal properties of TiN thin films. J. Mater. Sci. 2008, 43, 1114–1120. [Google Scholar] [CrossRef]
- Nolte, S.; Momma, C.; Jacobs, H.; Tü, A.; Chichkov, B.N.; Wellegehausen, B.; Welling, H. Ablation of metals by ultrashort laser pulses. J. Opt. Soc. Am. B 1997, 14, 2716–2722. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Nikov, R.G.; Nedyalkov, N.N.; Atanasov, P.A.; Karashanova, D.B. Laser-assisted fabrication and size distribution modification of colloidal gold nanostructures by nanosecond laser ablation in different liquids. Appl. Phys. A 2017, 123, 490. [Google Scholar] [CrossRef]
- Stoehr, M.; Shin, C.S.; Petrov, I.; Greene, J.E. Raman scattering from TiNx (0.67 ≤ x ≤ 1.00) single crystals grown on MgO(001). J. Appl. Phys. 2011, 110, 083503. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Tay, B.K.; Lau, S.P.; Kupfer, H.; Richter, F. Substrate bias dependence of Raman spectra for TiN films deposited by filtered cathodic vacuum arc. J. Appl. Phys. 2002, 92, 1845–1849. [Google Scholar] [CrossRef]
- Gioti, M.; Arvanitidis, J.; Christofilos, D.; Chaudhuri, K.; Zorba, T.; Abadias, G.; Gall, D.; Shalaev, V.M.; Boltasseva, A.; Patsalas, P. Plasmonic and phononic properties of epitaxial conductive transition metal nitrides. J. Opt. 2020, 22, 084001. [Google Scholar] [CrossRef]
- Spengler, W.; Kaiser, R.; Christensen, A.N.; Müller-Vogt, G. Raman scattering, superconductivity, and phonon density of states of stoichiometric and nonstoichiometric TiN. Phys. Rev. B 1978, 17, 1095–1101. [Google Scholar] [CrossRef]
- Jagannadham, K.; Chowdhury, R.; Biunno, N.; Narayan, J. Raman spectroscopy of TiN films deposited on silicon (001) substrate by laser physical vapor deposition. Mater. Res. Soc. Symp. Proc. 1994, 317, 193–198. [Google Scholar] [CrossRef]
- Saoula, N.; Djerourou, S.; Yahiaoui, K.; Henda, K.; Kesri, R.; Erasmus, R.M.; Comins, J.D. Study of the deposition of Ti/TiN multilayers by magnetron sputtering. Surf. Interface Anal. 2010, 42, 1176–1179. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014, 4, 027116. [Google Scholar] [CrossRef]
- Vanitha, M.; Camellia, P.; Balasubramanian, N. Augmentation of graphite purity from mineral resources and enhancing % graphitization using microwave irradiation: XRD and Raman studies. Diam. Relat. Mater. 2018, 88, 129–136. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Chan, M.H.; Lu, F.H. X-ray photoelectron spectroscopy analyses of titanium oxynitride films prepared by magnetron sputtering using air/Ar mixtures. Thin Solid Films 2009, 517, 5006–5009. [Google Scholar] [CrossRef]
- Chinh, V.D.; Broggi, A.; Di Palma, L.; Scarsella, M.; Speranza, G.; Vilardi, G.; Thang, P.N. XPS Spectra Analysis of Ti2+, Ti3+ Ions and Dye Photodegradation Evaluation of Titania-Silica Mixed Oxide Nanoparticles. J. Electron. Mater. 2018, 47, 2215–2224. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS spectra of TiN. J. Electron. Spectros. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.-H.; Navinšek, B.; Panjan, P. Titanium Nitride by XPS. Surf. Sci. Spectra 1998, 5, 145–151. [Google Scholar] [CrossRef]
- Alvarez Barragan, A.; Ilawe, N.V.; Zhong, L.; Wong, B.M.; Mangolini, L. A Non-Thermal Plasma Route to Plasmonic TiN Nanoparticles. J. Phys. Chem. C 2017, 121, 2316–2322. [Google Scholar] [CrossRef]
- Wu, H.Z.; Chou, T.C.; Mishra, A.; Anderson, D.R.; Lampert, J.K.; Gujrathi, S.C. Characterization of Titanium nitride thin films. Thin Solid Films 1990, 191, 55–67. [Google Scholar] [CrossRef]
- Baker, M.A.; Bacon, S.R.; Sweeney, S.J.; Hinder, S.J.; Bushell, A.; Nunney, T.S.; White, R.G. Femtosecond laser ablation (fs-LA) XPS—A novel XPS depth profiling technique for thin films, coatings and multi-layered structures. Appl. Surf. Sci. 2024, 654, 159405. [Google Scholar] [CrossRef]
- Persson, I.; Näslund, L.Å.; Halim, J.; Barsoum, M.W.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.Å. On the organization and thermal behavior of functional groups on Ti3C2 MXene surfaces in vacuum. 2D Mater. 2018, 5, 015002. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pliatsikas, N.; Panos, S.; Odutola, T.; Kassavetis, S.; Papoulia, C.; Fekas, I.; Arvanitidis, J.; Christofilos, D.; Pavlidou, E.; Gioti, M.; et al. Colloidal Titanium Nitride Nanoparticles by Laser Ablation in Solvents for Plasmonic Applications. Nanomaterials 2024, 14, 1214. https://doi.org/10.3390/nano14141214
Pliatsikas N, Panos S, Odutola T, Kassavetis S, Papoulia C, Fekas I, Arvanitidis J, Christofilos D, Pavlidou E, Gioti M, et al. Colloidal Titanium Nitride Nanoparticles by Laser Ablation in Solvents for Plasmonic Applications. Nanomaterials. 2024; 14(14):1214. https://doi.org/10.3390/nano14141214
Chicago/Turabian StylePliatsikas, Nikolaos, Stavros Panos, Tamara Odutola, Spyridon Kassavetis, Chrysanthi Papoulia, Ilias Fekas, John Arvanitidis, Dimitris Christofilos, Eleni Pavlidou, Maria Gioti, and et al. 2024. "Colloidal Titanium Nitride Nanoparticles by Laser Ablation in Solvents for Plasmonic Applications" Nanomaterials 14, no. 14: 1214. https://doi.org/10.3390/nano14141214
APA StylePliatsikas, N., Panos, S., Odutola, T., Kassavetis, S., Papoulia, C., Fekas, I., Arvanitidis, J., Christofilos, D., Pavlidou, E., Gioti, M., & Patsalas, P. (2024). Colloidal Titanium Nitride Nanoparticles by Laser Ablation in Solvents for Plasmonic Applications. Nanomaterials, 14(14), 1214. https://doi.org/10.3390/nano14141214





