Phase-Field Simulation of Precipitation and Grain Boundary Segregation in Fe-Cr-Al Alloys under Irradiation
Abstract
1. Introduction
2. Method
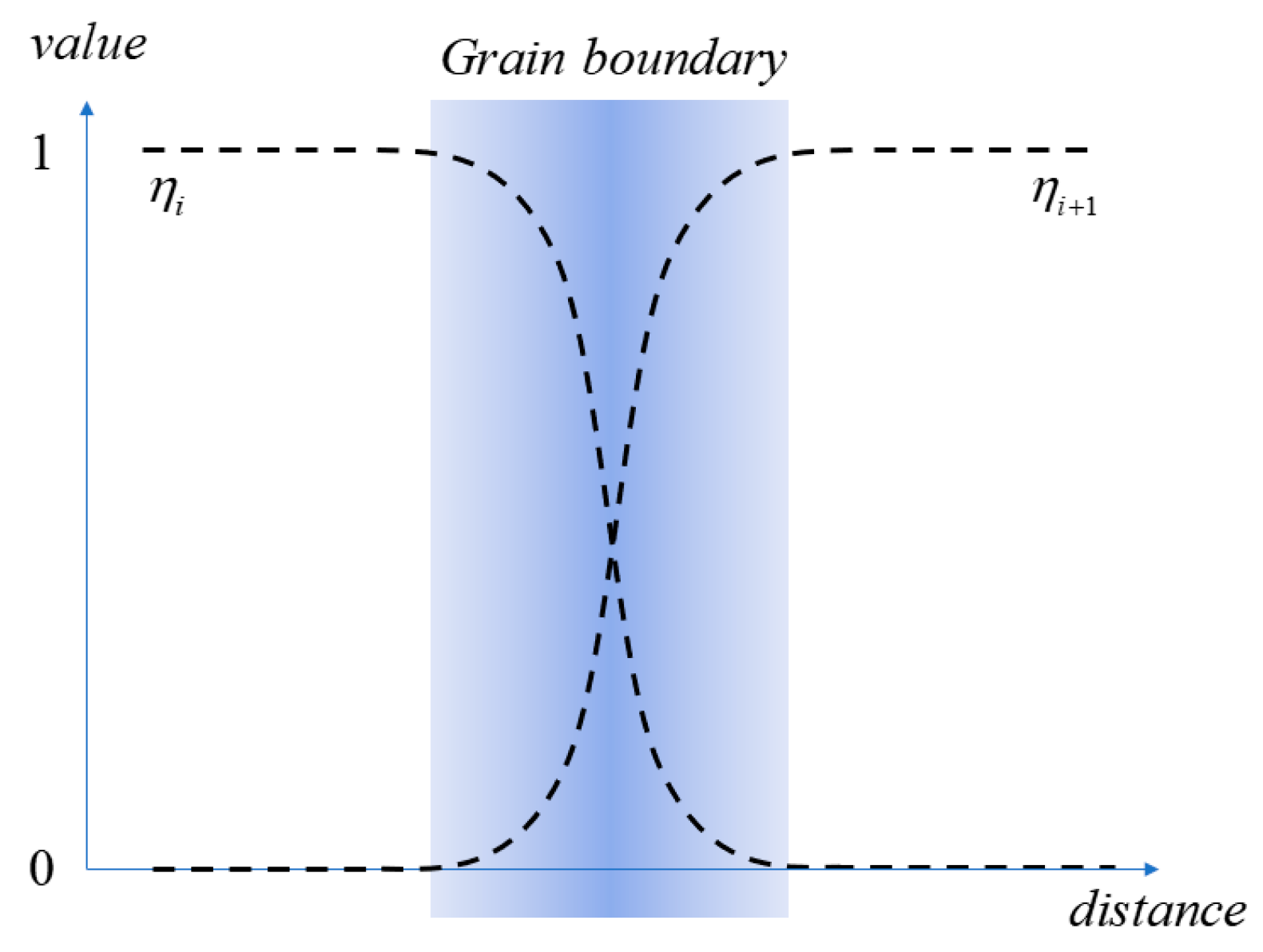
2.1. Phase-Field Parameters
2.2. Total Free Energy Function
2.3. Evolution Governing Equation
2.4. Numerical Method and Parameters
3. Result and Discussion
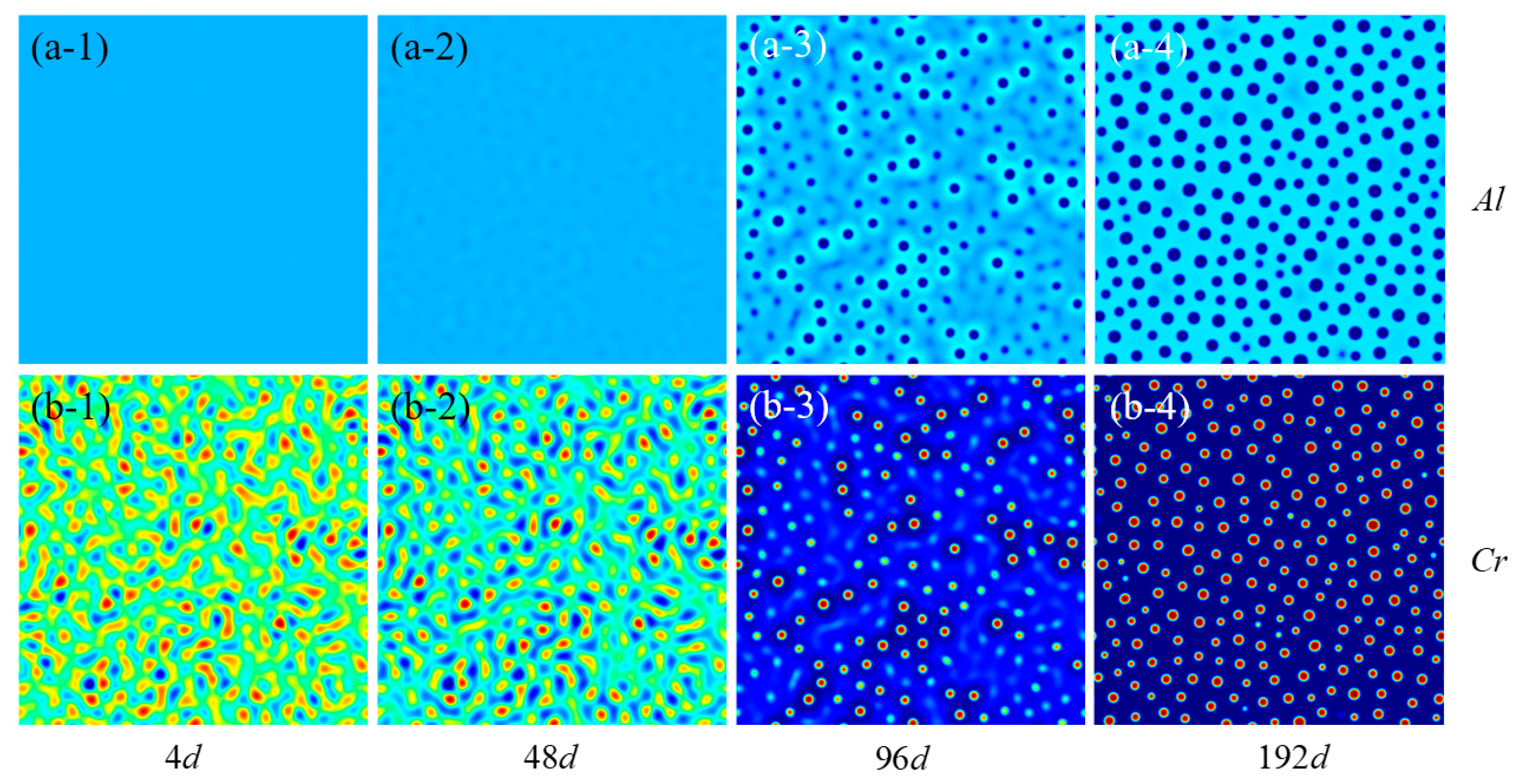
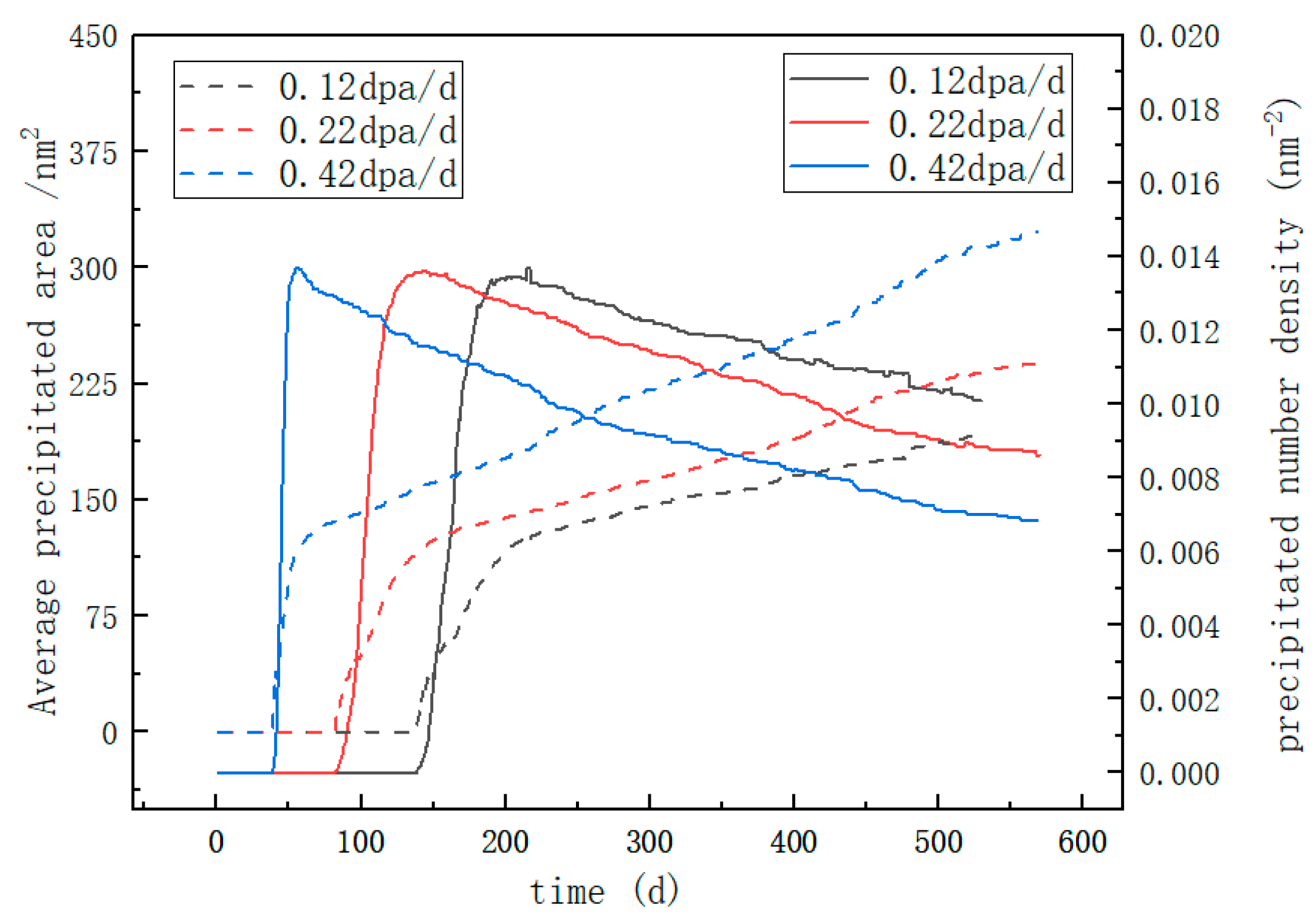
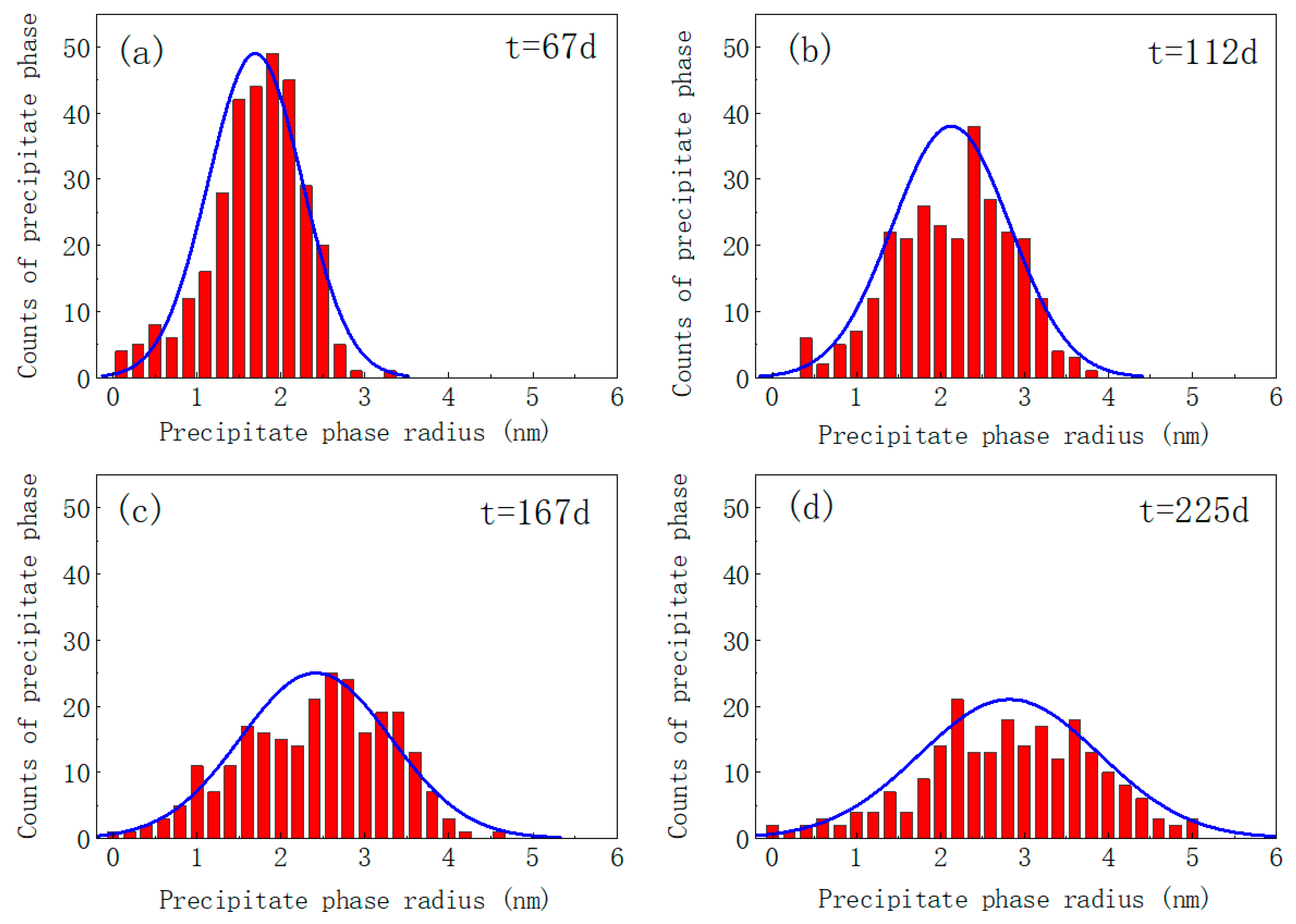
3.1. Irradiation-Accelerated Evolution of the α′ Phase
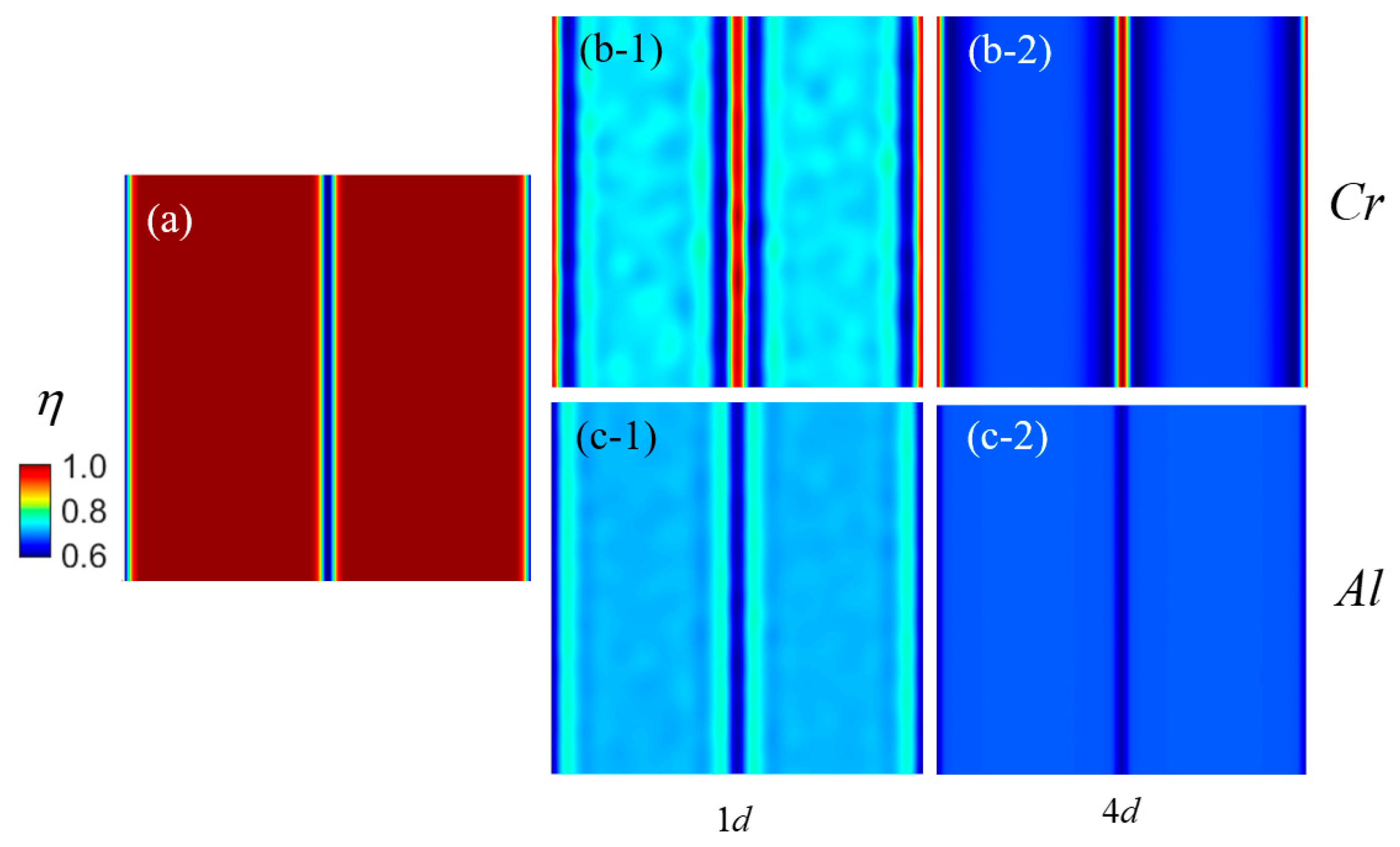
3.2. Effect of Grain Boundary on Segregation
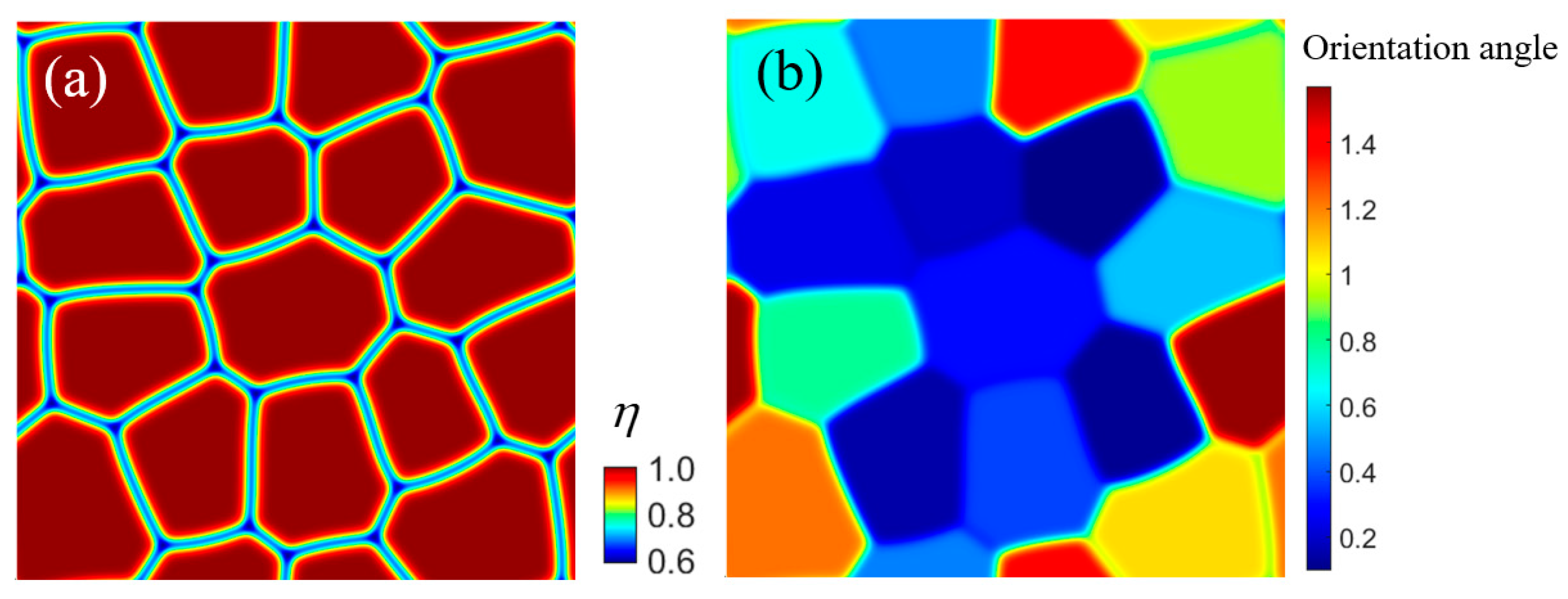
3.3. Element Segregation in Multigrain Fe-Cr-Al
4. Conclusions
- (1)
- The phase-field model enables the simulation of the acceleration effect of irradiation on precipitation and segregation, while elucidating the role of GBs in segregation. By introducing an irradiation vacancy source term related to irradiation intensity, the model enables the simulation of the irradiation-accelerated precipitation and segregation. Moreover, the model integrates polycrystalline grain boundaries to effectively depict the segregation of Fe-Cr-Al alloy along GBs under irradiation conditions.
- (2)
- The precipitation of the Cr-rich α’ second phase in Fe-Cr-Al alloy is simulated under varying irradiation intensities. The time and radius distributions of Cr-rich precipitates under different irradiation intensities were calculated. The results show that after 225 days at the irradiation intensity of 0.42 dpa/d, the average precipitation radius of the Fe-15Cr-6Al alloy is 2.802 nm and the number density is ~0.0147 nm−2. Long-time aging eventually leads to the dissolution of the precipitated phase within 2 nm.
- (3)
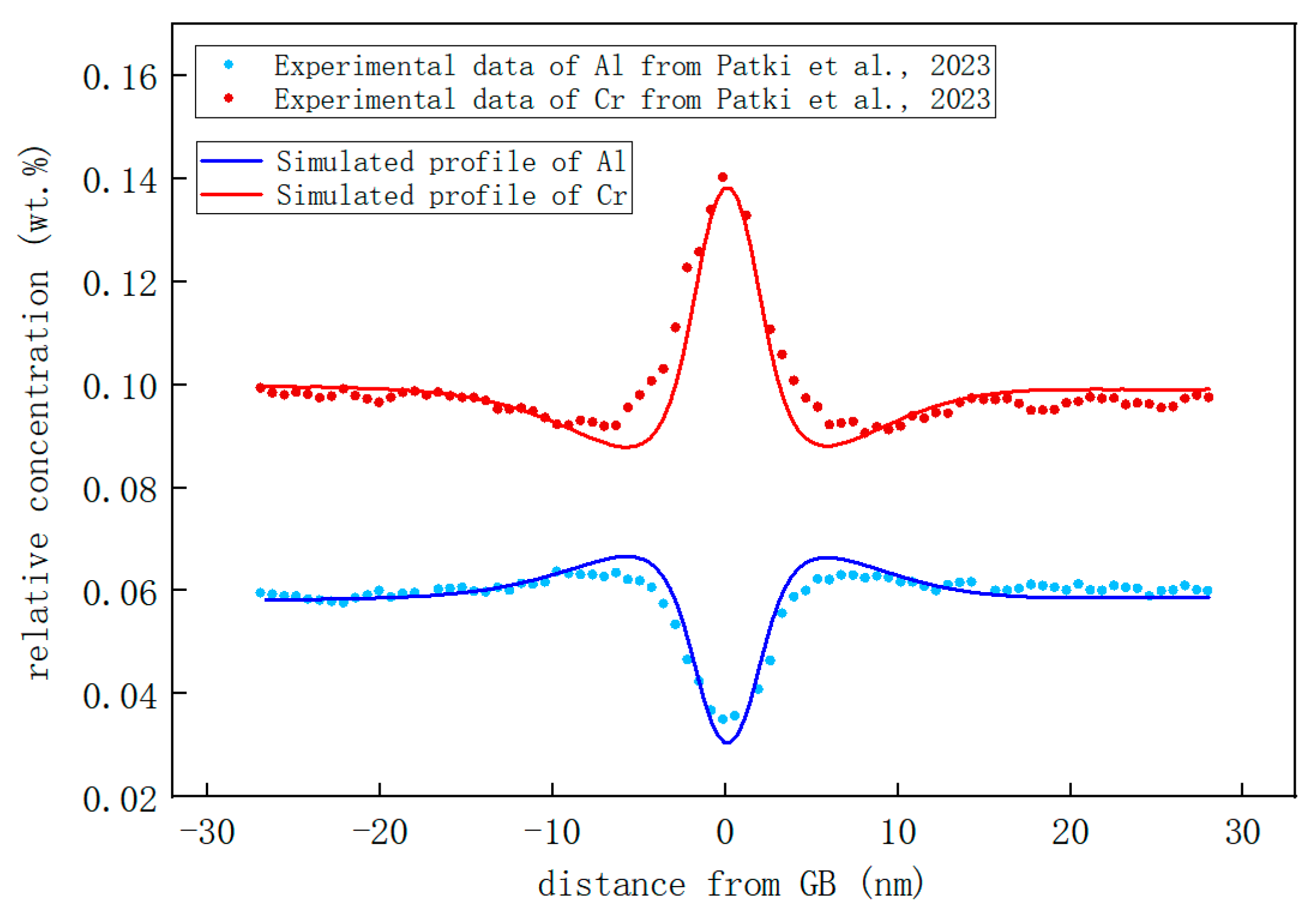
- The segregation of Cr and Al in Fe-Cr-Al is simulated in both twin and polycrystalline systems. Segregation at GBs leads to the formation of Cr-rich and Al-poor regions with a width of ~14 nm. Under the influence of an irradiation intensity of 0.12 dpa/d, the precipitation of Cr mainly happening in ~150 days is replaced with segregation mainly happening in ~20 days. The simulation results demonstrate excellent agreement with the corresponding experimental findings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Field, K.G.; Briggs, S.A.; Sridharan, K.; Yamamoto, Y.; Howard, R.H. Dislocation loop formation in model FeCrAl alloys after neutron irradiation below 1 dpa. J. Nucl. Mater. 2017, 495, 20–26. [Google Scholar] [CrossRef]
- Field, K.G.; Hu, X.X.; Littrell, K.C.; Yamamoto, Y.; Snead, L.L. Radiation tolerance of neutron-irradiated model Fe-Cr-Al alloys. J. Nucl. Mater. 2015, 465, 746–755. [Google Scholar] [CrossRef]
- Pint, B.A.; Terrani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High temperature oxidation of fuel cladding candidate materials in steam-hydrogen environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.G.; Yang, Y.; Snead, L.L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar] [CrossRef]
- Wolff, I.M.; Iorio, L.E.; Rumpf, T.; Scheers, P.V.T.; Potgieter, J.H. Oxidation and corrosion behaviour of Fe-Cr and Fe-Cr-Al alloys with minor alloying additions. Mat. Sci. Eng. A-Struct. 1998, 241, 264–276. [Google Scholar] [CrossRef]
- George, N.M.; Terrani, K.; Powers, J.; Worrall, A.; Maldonado, I. Neutronic analysis of candidate accident-tolerant cladding concepts in pressurized water reactors. Ann. Nucl. Energy 2015, 75, 703–712. [Google Scholar] [CrossRef]
- Edmondson, P.D.; Briggs, S.A.; Yamamoto, Y.; Howard, R.H.; Sridharan, K.; Terrani, K.A.; Field, K.G. Irradiation-enhanced α′ precipitation in model FeCrAl alloys. Scr. Mater. 2016, 116, 112–116. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takasugi, T. Mapping of 475 °C embrittlement in ferritic Fe–Cr–Al alloys. Scr. Mater. 2010, 63, 1104–1107. [Google Scholar] [CrossRef]
- Ejenstam, J.; Thuvander, M.; Olsson, P.; Rave, F.; Szakalos, P. Microstructural stability of Fe–Cr–Al alloys at 450–550 °C. J. Nucl. Mater. 2015, 457, 291–297. [Google Scholar] [CrossRef]
- Liao, X.; Chen, Y.; Gong, H.; Liu, T.; Deng, H.; Hu, W.; Gao, F. The stability and behavior of Cr-rich α’ precipitates under cascade damage in Fe-15Cr-8Al ternary alloys: An atomic-scale simulation study. J. Nucl. Mater. 2022, 570, 153955. [Google Scholar] [CrossRef]
- Briggs, S.A.; Edmondson, P.D.; Littrell, K.C.; Yamamoto, Y.; Howard, R.H.; Daily, C.R.; Terrani, K.A.; Sridharan, K.; Field, K.G. A combined APT and SANS investigation of α′ phase precipitation in neutron-irradiated model FeCrAl alloys. Acta Mater. 2017, 129, 217–228. [Google Scholar] [CrossRef]
- Field, K.G.; Littrell, K.C.; Briggs, S.A. Precipitation of α′ in neutron irradiated commercial FeCrAl alloys. Scr. Mater. 2018, 142, 41–45. [Google Scholar] [CrossRef]
- Reese, E.R.; Bachhav, M.; Wells, P.; Yamamoto, T.; Odette, G.R.; Marquis, E.A. On α′ precipitate composition in thermally annealed and neutron-irradiated Fe-9-18Cr alloys. J. Nucl. Mater. 2018, 500, 192–198. [Google Scholar] [CrossRef]
- Capdevila, C.; Miller, M.K.; Chao, J. Phase separation kinetics in a Fe-Cr-Al alloy. Acta Mater. 2012, 60, 4673–4684. [Google Scholar] [CrossRef]
- Capdevila, C.; Miller, M.K.; Pimentel, G.; Chao, J. Influence of recrystallization on phase separation kinetics of oxide dispersion strengthened Fe-Cr-Al alloy. Scr. Mater. 2012, 66, 254–257. [Google Scholar] [CrossRef]
- Patki, P.V.; Pownell, T.J.; Bazarbayev, Y.; Zhang, D.L.; Field, K.G.; Wharry, J.P. Systematic study of radiation-induced segregation in neutron-irradiated FeCrAl alloys. J. Nucl. Mater. 2023, 574, 154205. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Wang, F.; Setyawan, W.; Long, X.; Liu, Y.; Dong, L.; Gao, N.; Gao, F.; Wang, X. Coupled effect of Cr and Al on interactions between a prismatic interstitial dislocation loop and an edge dislocation line in Fe-Cr-Al alloy. Acta Mater. 2023, 245, 118651. [Google Scholar] [CrossRef]
- Liu, X.X.; Liu, W.B.; Li, B.Y.; He, X.F.; Yang, Z.X.; Yun, D. Calculation of Critical Nucleus Size and Minimum Energy Path of Cu-Riched Precipitates During Radiation in Fe-Cu Alloy Using String Method. Acta. Metall. Sin. 2022, 58, 943–955. [Google Scholar]
- Li, Y.S.; Li, S.X.; Zhang, T.Y. Effect of dislocations on spinodal decomposition in Fe-Cr alloys. J. Nucl. Mater. 2009, 395, 120–130. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhang, L.; Li, C.L.; Li, Q.L.; Chen, J.; Shu, G.G.; Weng, Y.Q.; Xu, B.; Hu, S.Y.; Liu, W. The effect of Mn/Ni on thermodynamic properties of critical nucleus in Fe-Cu-Mn (Ni) ternary alloys. J. Nucl. Mater. 2018, 507, 59–67. [Google Scholar] [CrossRef]
- Yang, Z.X.; Liu, W.B.; Zhang, C.Y.; He, X.F.; Sun, Z.Y.; Jia, L.X.; Shi, T.T.; Yun, D. Phase field simulation of grain boundary segregation and radiation-enhanced segregation in Fe-Cr alloys. Acta Phys. Sin. 2021, 70, 116101. [Google Scholar] [CrossRef]
- Yang, Z.X.; Liu, W.B.; Liu, X.X.; Jiang, Y.B.; Yun, D. Effects of grain boundaries and temperature on spinodal decomposition in a binary Fe-Cr alloy: A phase-field simulation. Ann. Nucl. Energy 2023, 193, 110030. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.S.; Shi, S.J.; Jin, S.S. Quantitative Phase-Field Simulation of Composition Partition and Separation Kinetics of Nanoscale Phase in Fe-Cr-Al Alloy. J. Nanomater. 2019, 2019, 6862390. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.; Chang, K.O. Effect of Al Concentration on the Microstructural Evolution of Fe-Cr-Al Systems: A Phase-Field Approach. Metals 2021, 11, 4. [Google Scholar] [CrossRef]
- Cahn, J.W.; Hilliard, J.E. Free Energy of a Nonuniform System. I. Interfacial Free Energy. J. Chem. Phys. 1958, 28, 258–267. [Google Scholar] [CrossRef]
- Chen, L.Q. Phase-field models for microstructure evolution. Annu. Rev. Mater. Res. 2002, 32, 113–140. [Google Scholar] [CrossRef]
- Lass, E.A.; Johnson, W.C.; Shiflet, G.J. Correlation between CALPHAD data and the Cahn-Hilliard gradient energy coefficient κ and exploration into its composition dependence. Calphad 2006, 30, 42–52. [Google Scholar] [CrossRef]
- Yan, Z.W.; Shi, S.J.; Li, Y.S.; Chen, J.; Maqbool, S. Vacancy and interstitial atom evolution with the separation of the nanoscale phase in Fe-Cr alloys: Phase-field simulations. Phys. Chem. Chem. Phys. 2020, 22, 3611–3619. [Google Scholar] [CrossRef]
- Ramanarayan, H.; Abinandanan, T.A. Phase field study of grain boundary effects on spinodal decomposition. Acta Mater. 2003, 51, 4761–4772. [Google Scholar] [CrossRef]
- Ramanarayan, H.; Abinandanan, T.A. Grain boundary effects on spinodal decomposition II. Discontinuous microstructures. Acta Mater. 2004, 52, 921–930. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Jacobs, M.H.G.; Schmid-Fetzer, R.; Markus, T.; Motalov, V.; Borchardt, G.; Spitzer, K.H. Thermodynamics and diffusion in ternary Fe-Al-Cr alloys, Part I: Thermodynamic modeling. Intermetallics 2008, 16, 995–1005. [Google Scholar] [CrossRef]
- Martínez, E.; Senninger, O.; Fu, C.C.; Soisson, F. Decomposition kinetics of Fe-Cr solid solutions during thermal aging. Phys. Rev. B 2012, 86, 224109. [Google Scholar] [CrossRef]
- Wu, K.; Morral, J.E.; Wang, Y. A phase field study of microstructural changes due to the Kirkendall effect in two-phase diffusion couples. Acta Mater. 2001, 49, 3401–3408. [Google Scholar] [CrossRef]
- Cahn, J.W. On spinodal decomposition. Acta Metall. Sin.-Eng. 1961, 9, 795–801. [Google Scholar] [CrossRef]
- Huang, C.; Delacruz, M.O.; Swift, B.W. Phase-separation of ternary mixtures—symmetrical polymer blends. Macromolecules 1995, 28, 7996–8005. [Google Scholar] [CrossRef]
- Koyama, T.; Onodera, H. Modeling of microstructure changes in Fe-Cr-Co magnetic alloy using the phase-field method. J. Phase Equilibria Diffu. 2006, 27, 22–29. [Google Scholar] [CrossRef]
- Hu, S.Y.; Henager, C.H. Phase-field modeling of void lattice formation under irradiation. J. Nucl. Mater. 2009, 394, 155–159. [Google Scholar] [CrossRef]
- Allen, S.M.; Cahn, J.W. A microscopic theory for antiphase boundary motion and its application to antiphase domain coarsening. Acta Metall. Sin.-Eng. 1979, 27, 1085–1095. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.-Q.; Shen, J.; Tikare, V. Coarsening kinetics from a variable-mobility Cahn-Hilliard equation: Application of a semi-implicit Fourier spectral method. Phys. Rev. E 1999, 60, 3564–3572. [Google Scholar] [CrossRef]
- Li, Y.P.; Ran, G.; Pei, K.F.; Huang, X.Y.; Zhang, R.Q.; Wang, Q.; Niu, B. In-situ TEM study on composition change and amorphous transformation of Laves phase precipitates in FeCrAl alloy during Fe+ irradiation. J. Nucl. Mater. 2022, 563, 153620. [Google Scholar] [CrossRef]
- Lescoat, M.L.; Ribis, J.; Chen, Y.; Marquis, E.A.; Bordas, E.; Trocellier, P.; Serruys, Y.; Gentils, A.; Kaïtasov, O.; de Carlan, Y.; et al. Radiation-induced Ostwald ripening in oxide dispersion strengthened ferritic steels irradiated at high ion dose. Acta Mater. 2014, 78, 328–340. [Google Scholar] [CrossRef]
- Massey, C.P.; Edmondson, P.D.; Field, K.G.; Hoelzer, D.T.; Dryepondt, S.N.; Terrani, K.A.; Zinkle, S.J. Post irradiation examination of nanoprecipitate stability and α′ precipitation in an oxide dispersion strengthened Fe-12Cr-5Al alloy. Scr. Mater. 2019, 162, 94–98. [Google Scholar] [CrossRef]
- Nychka, J.A.; Clarke, D.R. Quantification of aluminum outward diffusion during oxidation of FeCrAl alloys. Oxid. Met. 2005, 63, 325–352. [Google Scholar] [CrossRef]


| Parameter | Value | Reference |
|---|---|---|
| GFe0 | 1225.71 + 124.09T − 23.49TInT − 4.40e−3T2 − 5.88e−8T3 + 77,358T−1 J/m3 | [31] |
| GCr0 | −8856.90 + 157.50T − 26.90TInT + 1.89e−3T2 − 1.48e−6T3 + 139,250T−1 J/m3 | [31] |
| GAl0 | −1193.24 + 218.24T − 38.58TInT + 1.85e−2T2 − 5.64e−6T3 + 741T−1 J/m3 | [31] |
| LFeCr | 20,500 − 9.68T | [32] |
| LFeAl | −54,900 + 10.00T | [32] |
| LCrAl | −122,452.90 + 31.65T | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shen, W.; Liu, W. Phase-Field Simulation of Precipitation and Grain Boundary Segregation in Fe-Cr-Al Alloys under Irradiation. Nanomaterials 2024, 14, 1198. https://doi.org/10.3390/nano14141198
Liu X, Shen W, Liu W. Phase-Field Simulation of Precipitation and Grain Boundary Segregation in Fe-Cr-Al Alloys under Irradiation. Nanomaterials. 2024; 14(14):1198. https://doi.org/10.3390/nano14141198
Chicago/Turabian StyleLiu, Xuxi, Wenlong Shen, and Wenbo Liu. 2024. "Phase-Field Simulation of Precipitation and Grain Boundary Segregation in Fe-Cr-Al Alloys under Irradiation" Nanomaterials 14, no. 14: 1198. https://doi.org/10.3390/nano14141198
APA StyleLiu, X., Shen, W., & Liu, W. (2024). Phase-Field Simulation of Precipitation and Grain Boundary Segregation in Fe-Cr-Al Alloys under Irradiation. Nanomaterials, 14(14), 1198. https://doi.org/10.3390/nano14141198






