Hydroxyapatite-Coated Ti6Al4V ELI Alloy: In Vitro Cell Adhesion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Coating Morphology and Degradation
2.2.2. Protein Adhesion
2.2.3. Adhesion, Proliferation, and Differentiation of Human Osteoblasts and Adipose-Derived Stem Cells
Cell Proliferation and Differentiation
Cell Imaging
Osteogenic Characterization
3. Results and Discussion
3.1. SEM Analysis
3.2. Protein Adhesion
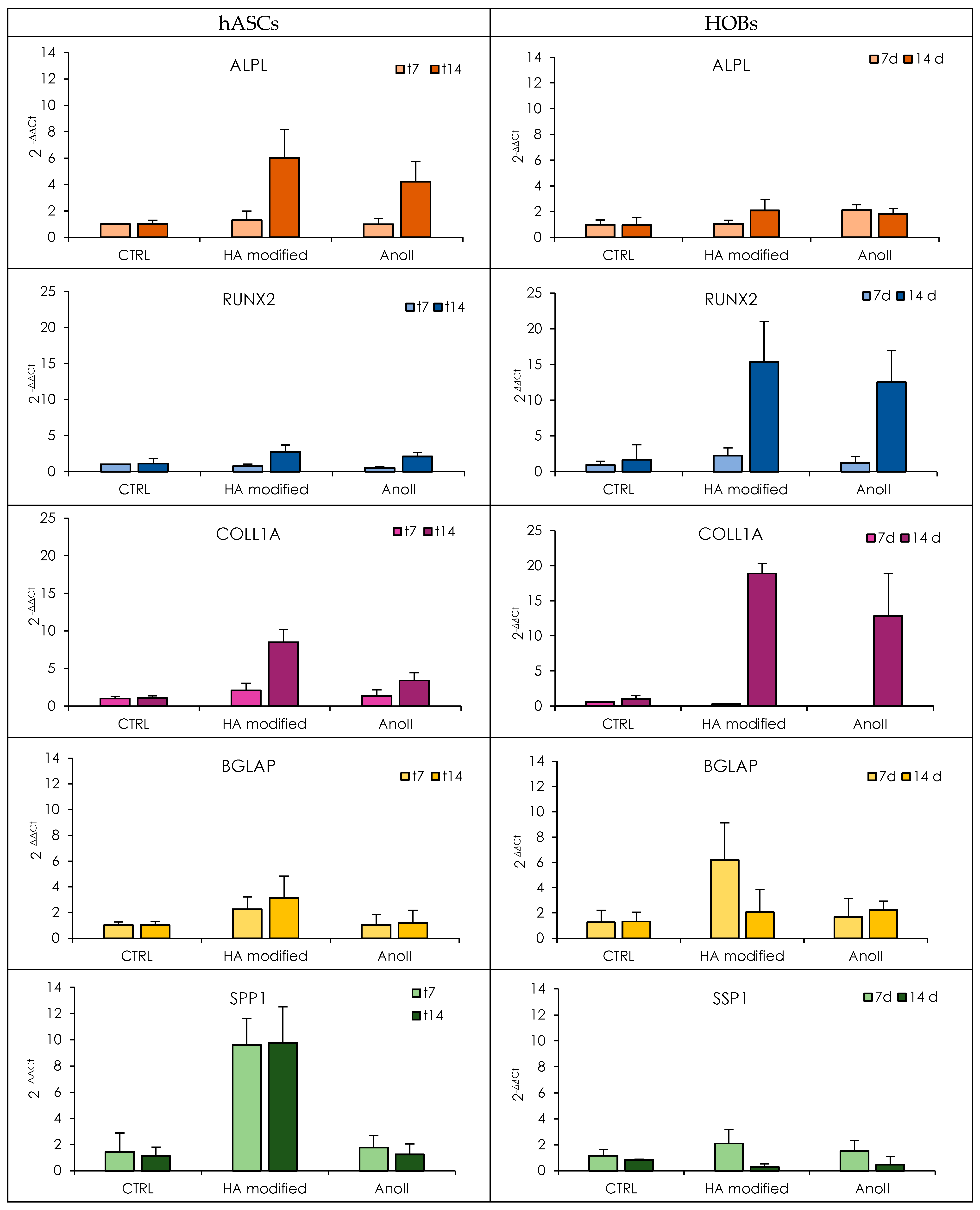
3.3. Proliferation, Adhesion, and Mineralization of Human Osteoblasts and Adipose-Derived Stem Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2020, 38, 1445–1459. [Google Scholar] [CrossRef]
- Raffa, M.L.; Nguyen, V.H.; Hernigou, P.; Flouzat-Lachaniette, C.H.; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef]
- Cruz, M.B.; Silva, N.; Marques, J.F.; Mata, A.; Silva, F.S.; Caramês, J. Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics 2022, 7, 74. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, e2000116. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C 2021, 118, 111505. [Google Scholar] [CrossRef]
- McCarthy, C.; Camci-Unal, G. Low Intensity Pulsed Ultrasound for Bone Tissue Engineering. Micromachines 2021, 12, 1488. [Google Scholar] [CrossRef]
- Xu, A.; Xie, Y.; Xu, J.; Li, J.; Wang, H.; He, F. Effects of strontium-incorporated micro/nano rough titanium surfaces on osseointegration via modulating polarization of macrophages. Colloids Surf. B 2021, 207, 111992. [Google Scholar] [CrossRef]
- Janßen, S.; Gach, S.; Kant, S.; Aveic, S.; Rütten, S.; Olschok, S.; Reisgen, U.; Fischer, H. Enhanced osteogenic differentiation of human mesenchymal stromal cells as response to periodical microstructured Ti6Al4V surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2218–2226. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol. Life Sci. 2017, 76, 2999–3009. [Google Scholar] [CrossRef]
- Mughal, M.P.; Farooq, M.U.; Mumtaz, J.; Mia, M.; Shareef, M.; Javed, M.; Jamil, M.; Pruncu, C.I. Surface modification for osseointegration of Ti6Al4V ELI using powder mixed sinking EDM. J. Mech. Behav. Biomed. Mater. 2021, 113, 104145. [Google Scholar] [CrossRef]
- Joy, N.; Prakash, S.; Krishnamoorthy, A.; Antony, A. Experimental investigation and analysis of drilling in Grade 5 Titanium alloy (Ti-6Al-4V). Mater. Today Proc. 2020, 21, 335–339. [Google Scholar] [CrossRef]
- Mathai, S.; Shaji, P.S. Bioactive conductive polymeric nanocomposite coating for titanium implants. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, J.; Huang, X.; Weir, M.D.; Masri, R.; Oates, T.W.; Xu, H.H.K.; Cheng, L. Novel antibacterial titanium implant healing abutment with dimethylaminohexadecyl methacrylate to combat implant-related infections. Dental. Mater. 2024, 40, 244–253. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2021, 7, e10239. [Google Scholar] [CrossRef]
- Kaewmanee, R.; Wang, F.; Pan, Y.; Mei, S.; Meesane, J.; Li, F.; Wu, Z.; Wei, J. Microporous surface containing flower-like molybdenum disulfide submicro-spheres of sulfonated polyimide with antibacterial effect and promoting bone regeneration and osteointegration. Biomater. Sci. 2022, 10, 4243–4256. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Kavasi, R.-M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef]
- Schönweger, F.; Sprecher, C.M.; Milz, S.; Dommann-Scherrer, C.; Meier, C.; Dommann, A.; Neels, A.; Wahl, P. New Insights into Osteointegration and Delamination from a Multidisciplinary Investigation of a Failed Hydroxyapatite-Coated Hip Joint Replacement. Materials 2020, 13, 4713. [Google Scholar] [CrossRef]
- Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials 2015, 8, 3815–3830. [Google Scholar] [CrossRef]
- Ruggeri, M.; Vigani, B.; Boselli, C.; Icaro Cornaglia, A.; Colombo, D.; Sànchez-Espejo, R.; Del Favero, E.; Mandras, N.; Roana, J.; Cavallo, L.; et al. Smart nano-in-microparticles to tackle bacterial infections in skin tissue engineering. Mater. Today Bio 2022, 16, 100418. [Google Scholar] [CrossRef]
- Ruggeri, M.; Lenzuni, M.; Suarato, G.; Vigani, B.; Boselli, C.; Icaro Cornaglia, A.; Colombo, D.; Grisoli, P.; Ricci, C.; Del Favero, E.; et al. Polysaccharide-protein microparticles based-scaffolds to recover soft tissue loss in mild periodontitis. Int. J. Pharm. 2023, 640, 123015. [Google Scholar] [CrossRef]
- Faccendini, A.; Bianchi, E.; Ruggeri, M.; Vigani, B.; Perotti, C.; Pavesi, F.C.; Caliogna, L.; Natali, F.; Del Favero, E.; Cantu’, L.; et al. Smart Device for Biologically Enhanced Functional Regeneration of Osteo–Tendon Interface. Pharmaceutics 2021, 13, 1996. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.J.; Aparicio, C. Adsorption of fibronectin, fibrinogen, and albumin on TiO2: Time-resolved kinetics, structural changes, and competition study. Biointerphases 2012, 7, 48. [Google Scholar] [CrossRef]
- Swain, S.K.; Sarkar, K.D. Study of BSA protein adsorption/release on hydroxyapatite nanoparticles. Appl. Surf. Sci. 2013, 286, 99–103. [Google Scholar] [CrossRef]
- Shotorbani, B.B.; Alizadeh, E.; Salehi, R.; Barzegar, A. Adhesion of mesenchymal stem cells to biomimetic polymers: A review. Mat. Sci. Eng. C 2017, 71, 1192–1200. [Google Scholar] [CrossRef]
- Uggeri, J.; Guizzardi, S.; Scandroglio, R.; Gatti, R. Adhesion of human osteoblasts to titanium: A morpho-functional analysis with confocal microscopy. Micron 2010, 41, 210–219. [Google Scholar] [CrossRef]
- Iline-Vul, T.; Nanda, R.; Mateos, B.; Hazan, S.; Matlahov, I.; Perelshtein, I.; Keinan-Adamsky, K.; Althoff-Ospelt, G.; Konrat, R.; Goobes, G. Osteopontin regulates biomimetic calcium phosphate crystallization from disordered mineral layers covering apatite crystallites. Sci. Rep. 2020, 10, 15722. [Google Scholar] [CrossRef]
- Knabe, C.; Howlett, C.R.; Klar, F.; Zreiqat, H. The effect of different titanium and hydroxyapatite-coated dental implant surfaces on phenotypic expression of human bone-derived cells. J. Biomed. Mater. Res. A 2004, 71, 98–107. [Google Scholar] [CrossRef]
- Lopez, H.B.; Souza, A.T.P.; Freitas, G.P.; Elias, C.N.; Rosa, A.L.; Beloti, M.M. Effect of focal adhesion kinase inhibition on osteoblastic cells grown on titanium with different topographies. J. Appl. Oral Sci. 2020, 28, e20190156. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggeri, M.; Miele, D.; Caliogna, L.; Bianchi, E.; Jepsen, J.M.; Vigani, B.; Rossi, S.; Sandri, G. Hydroxyapatite-Coated Ti6Al4V ELI Alloy: In Vitro Cell Adhesion. Nanomaterials 2024, 14, 1181. https://doi.org/10.3390/nano14141181
Ruggeri M, Miele D, Caliogna L, Bianchi E, Jepsen JM, Vigani B, Rossi S, Sandri G. Hydroxyapatite-Coated Ti6Al4V ELI Alloy: In Vitro Cell Adhesion. Nanomaterials. 2024; 14(14):1181. https://doi.org/10.3390/nano14141181
Chicago/Turabian StyleRuggeri, Marco, Dalila Miele, Laura Caliogna, Eleonora Bianchi, Johannes Maui Jepsen, Barbara Vigani, Silvia Rossi, and Giuseppina Sandri. 2024. "Hydroxyapatite-Coated Ti6Al4V ELI Alloy: In Vitro Cell Adhesion" Nanomaterials 14, no. 14: 1181. https://doi.org/10.3390/nano14141181
APA StyleRuggeri, M., Miele, D., Caliogna, L., Bianchi, E., Jepsen, J. M., Vigani, B., Rossi, S., & Sandri, G. (2024). Hydroxyapatite-Coated Ti6Al4V ELI Alloy: In Vitro Cell Adhesion. Nanomaterials, 14(14), 1181. https://doi.org/10.3390/nano14141181








