Sodium Alginate/UiO-66-NH2 Nanocomposite for Phosphate Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of UiO-66-NH2/SA@PEI
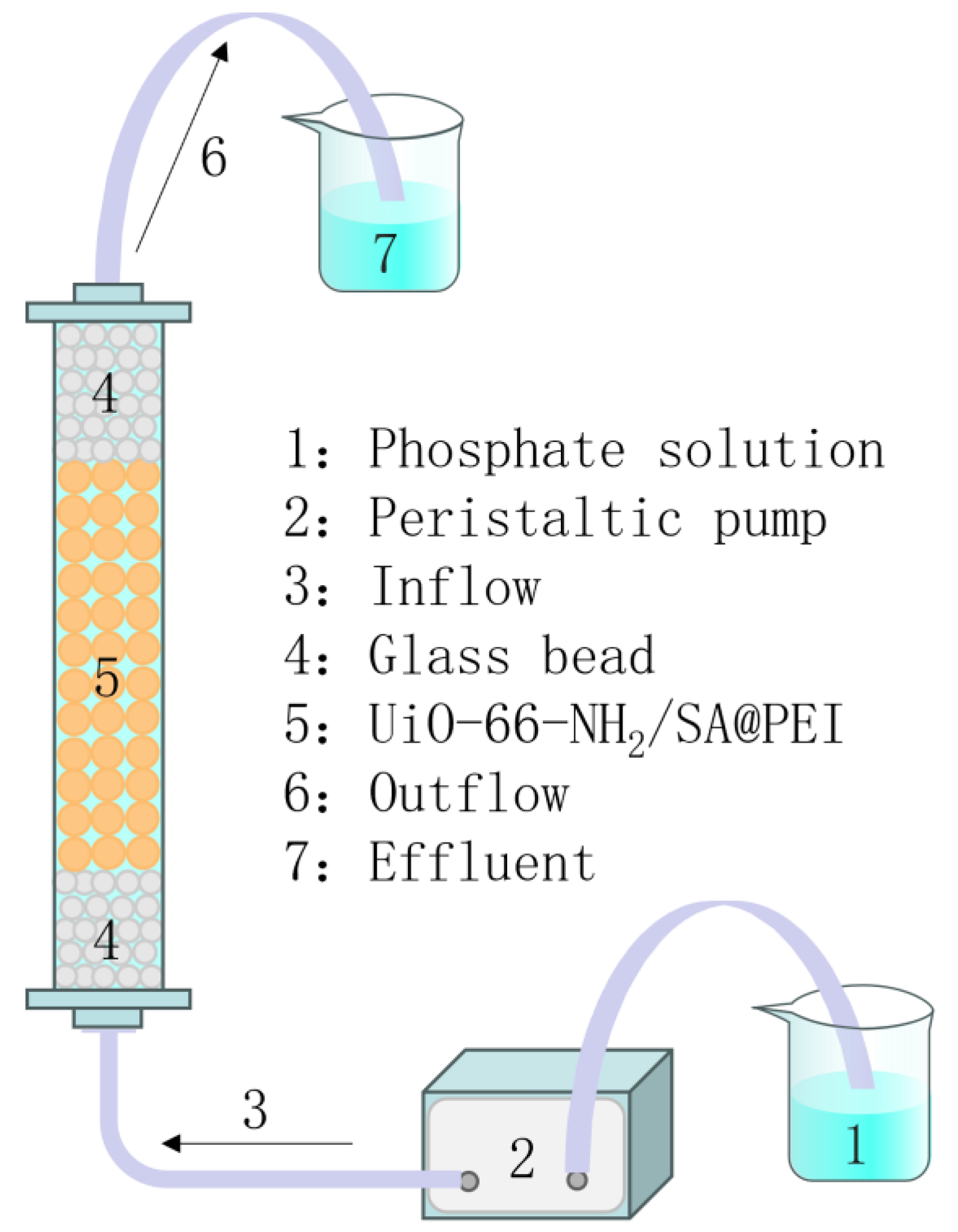
2.3. Adsorption of Phosphate by UiO-66-NH2/SA@PEI
2.4. Characterizations
3. Results
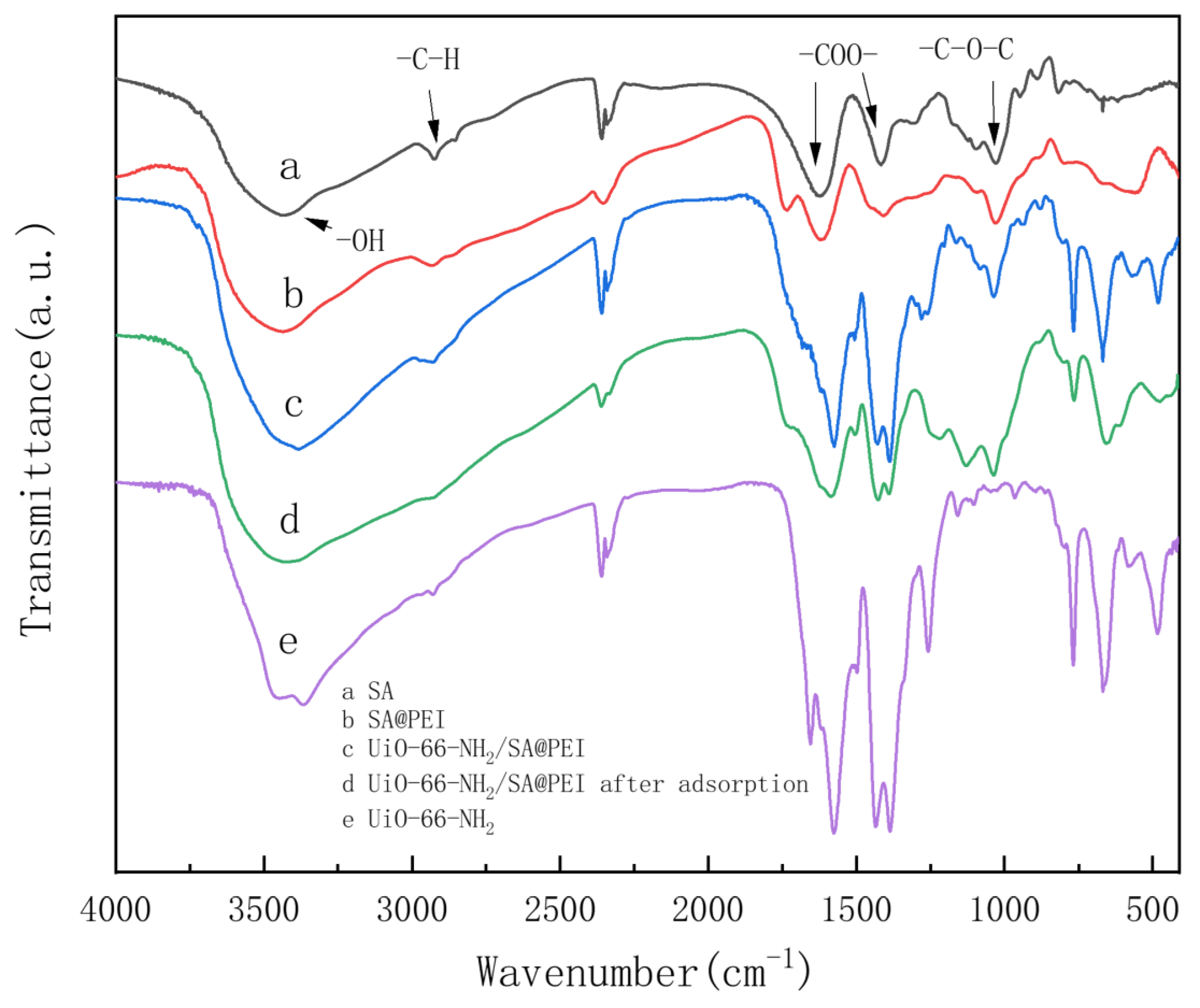
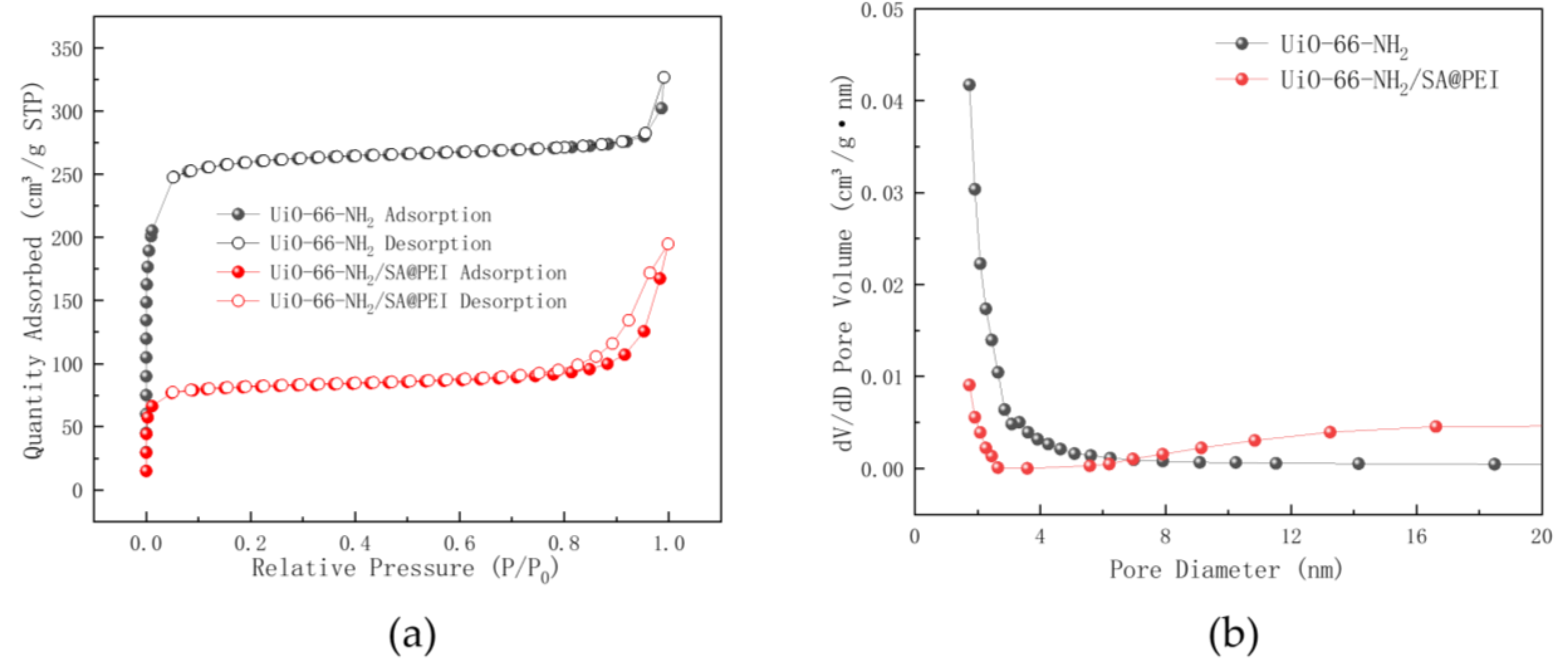
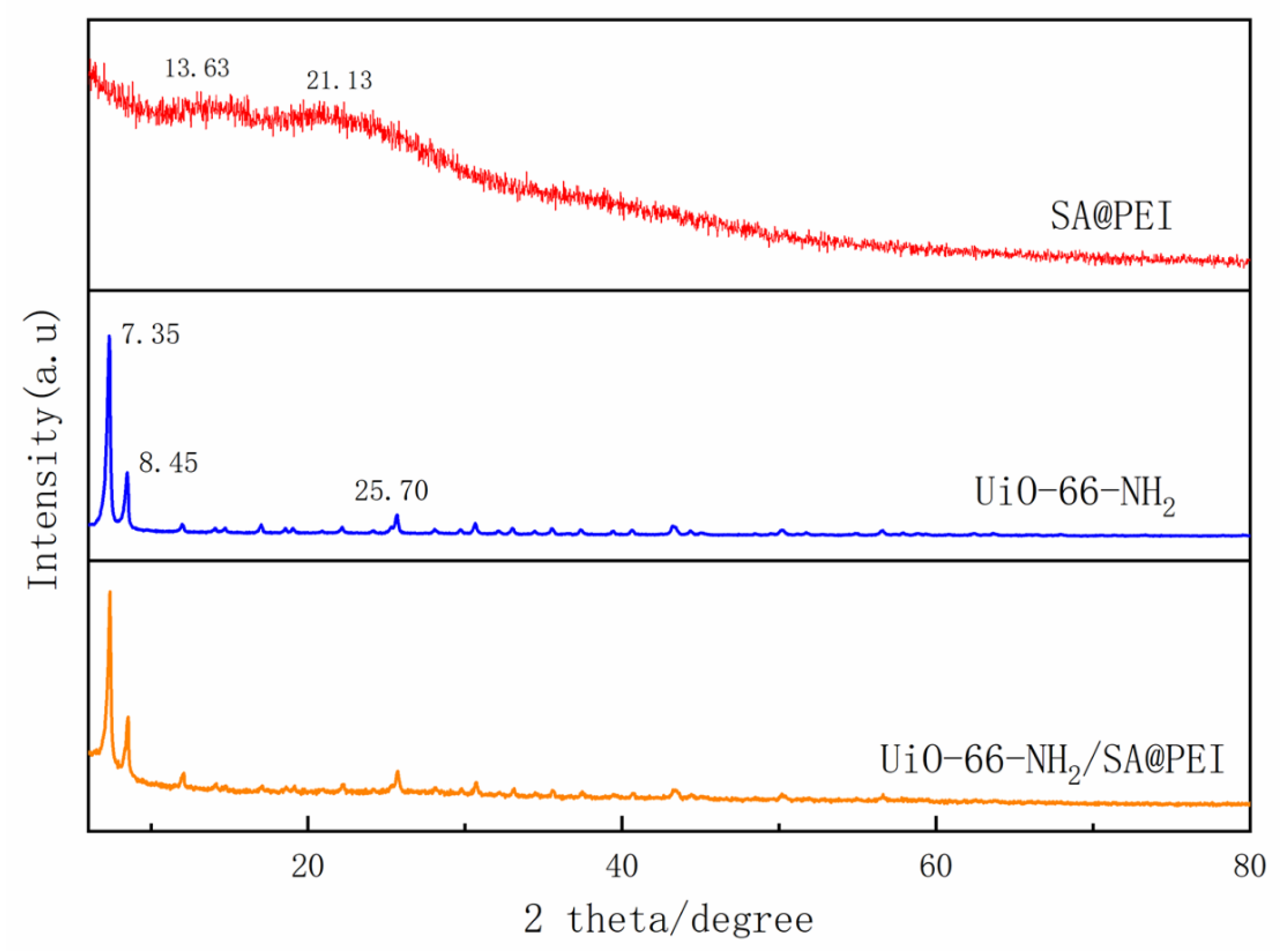
3.1. Characterization
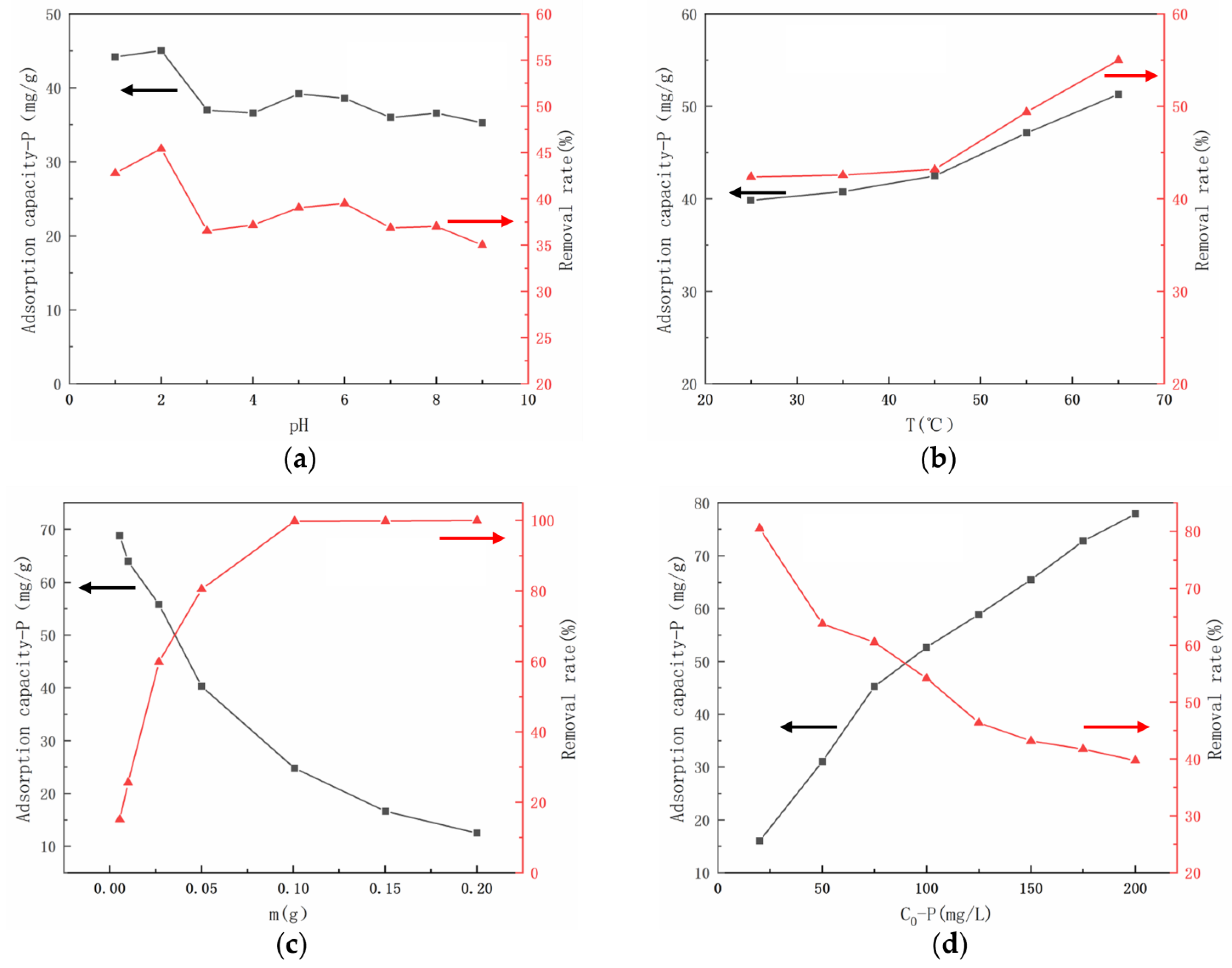
3.2. The Adsorption Capacity of UiO-66-NH2/SA@PEI under Different Conditions
3.3. Adsorption Kinetics of Phosphate by UiO-66-NH2/SA@PEI
3.4. The Adsorption Isotherm of Phosphate by UiO-66-NH2/SA@PEI
3.5. Cyclic Adsorption Performance of UiO-66-NH2/SA@PEI
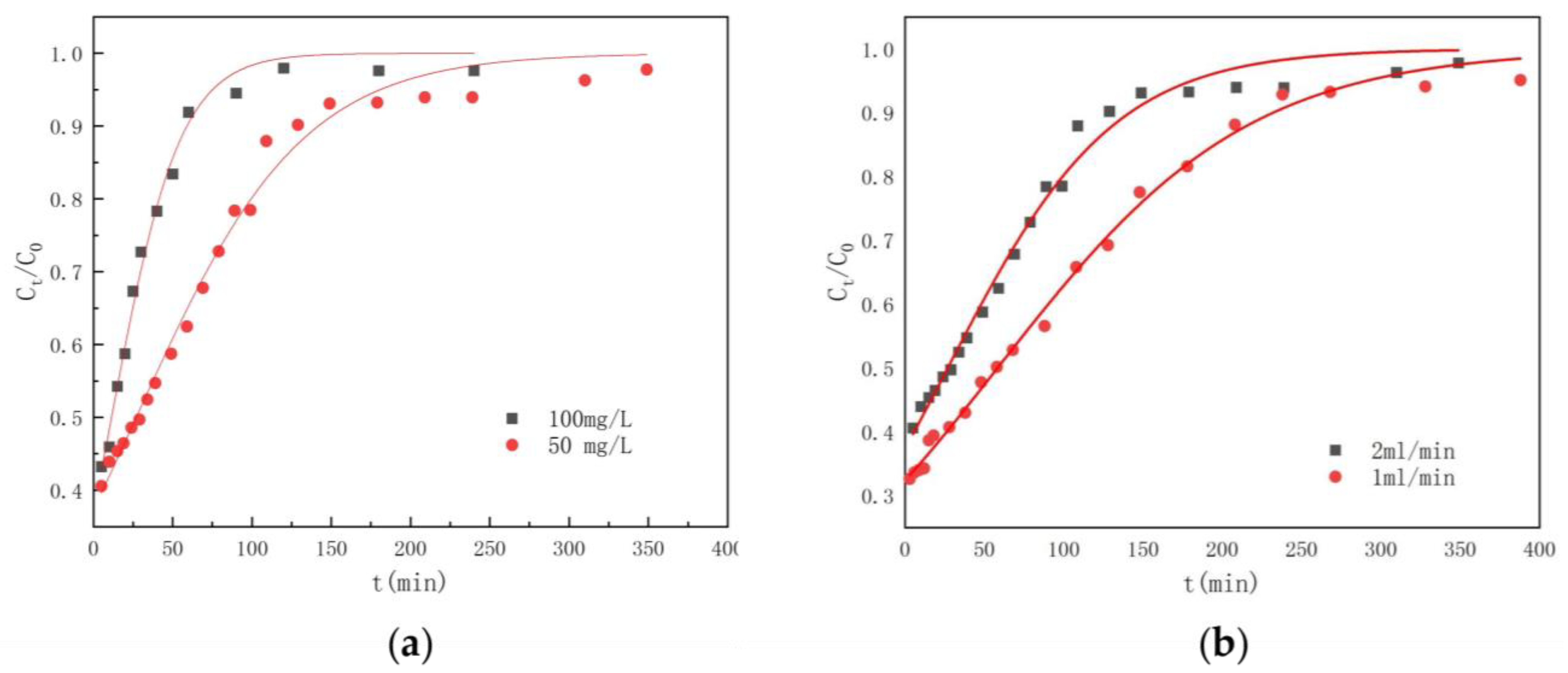
3.6. Dynamic Adsorption Capacity
3.7. Phosphate Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ignacimuthu, S. Management of phosphorus nutrient amid climate change for sustainable agriculture. J. Environ. Qual. 2021, 50, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xie, D.; Ma, Y.; Qin, W.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Size Modulation of Zirconium-Based Metal Organic Frameworks for Highly Efficient Phosphate Remediation. ACS Appl. Mater. Interfaces 2017, 9, 32151–32160. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.R.; Zhang, Y.M.; Yang, Q.; Deng, H.H.; Xu, J.; Hou, W.G. Ultrathin layered double hydroxide nanosheets prepared from a water-in-ionic liquid surfactant-free microemulsion for phosphate removal from aquatic systems. Chem. Eng. J. 2016, 302, 459–465. [Google Scholar] [CrossRef]
- Zahed, M.A.; Salehi, S.; Tabari, Y.; Farraji, H.; Ataei-Kachooei, S.; Zinatizadeh, A.A.; Kamali, N.; Mahjouri, M. Phosphorus removal and recovery: State of the science and challenges. Environ. Sci. Pollut. Res. 2022, 29, 58561–58589. [Google Scholar] [CrossRef] [PubMed]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Wang, X.Q.; Doug, L.; Li, Z.L.; Yang, L.; Yu, J.Y.; Ding, B. Flexible Hierarchical ZrO2 Nanoparticle-Embedded SiO2 Nanofibrous Membrane as a Versatile Tool for Efficient Removal of Phosphate. ACS Appl. Mater. Interfaces 2016, 8, 34668–34676. [Google Scholar] [CrossRef]
- Qiu, H.; Liang, C.; Yu, J.H.; Zhang, Q.R.; Song, M.X.; Chen, F.H. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem. Eng. J. 2017, 315, 345–354. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Smith, R.L., Jr.; Qi, X. Black liquor-derived calcium-activated biochar for recovery of phosphate from aqueous solutions. Bioresour. Technol. 2019, 294, 122198. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2020, 413, 127530. [Google Scholar] [CrossRef]
- Alrebdi, T.A.; Ahmed, H.A.; Alrefaee, S.H.; Pashameah, R.A.; Toghan, A.; Mostafa, A.M.; Alkallas, F.H.; Rezk, R.A. Enhanced adsorption removal of phosphate from water by Ag-doped PVA-NiO nanocomposite prepared by pulsed laser ablation method. J. Mater. Res. Technol. JmrT 2022, 20, 4356–4364. [Google Scholar] [CrossRef]
- He, Q.; Zhao, H.; Teng, Z.; Wang, Y.; Li, M.; Hoffmann, M.R. Phosphate removal and recovery by lanthanum-based adsorbents: A review for current advances. Chemosphere 2022, 303, 134987. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.N.; Adil, S.; Byambaa, B.; Sohail, M.; Wang, J.; Li, C. Progress, challenges, and prospects of MOF-based adsorbents for phosphate recovery from wastewater. J. Water Process Eng. 2024, 63, 105530. [Google Scholar] [CrossRef]
- Gutov, O.V.; Molina, S.; Escudero-Adan, E.C.; Shafir, A. Modulation by Amino Acids: Toward Superior Control in the Synthesis of Zirconium Metal-Organic Frameworks. Chem. A Eur. J. 2016, 22, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chi, L.; Wang, X.; Wang, Y.; Sui, Y.; Xie, T.; Arandiyan, H. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019, 357, 159–168. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, J.; Zhang, S.; Alsaedi, A.; Hayat, T.; Li, J.; Song, Y. Efficient removal of metal contaminants by EDTA modified MOF from aqueous solutions. J. Colloid Interface Sci. 2019, 555, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sang, Z.; Li, Q.; Gong, J.; Peng, X.; Li, L.; Zhang, Z.; Zhang, B.; Li, S.; Yang, X. Facile fabrication of La/Ca bimetal-organic frameworks for economical and efficient remove phosphorus from water. J. Mol. Liq. 2022, 356, 119024. [Google Scholar] [CrossRef]
- Cox, C.S.; Galicia, V.C.; Lessio, M. Computational Insights into As(V) Removal from Water by the UiO-66 Metal-Organic Framework. J. Phys. Chem. C 2021, 125, 3157–3168. [Google Scholar] [CrossRef]
- Shams, M.; Dehghani, M.H.; Nabizadeh, R.; Mesdaghinia, A.; Alimohammadi, M.; Najafpoor, A.A. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: Modeling, mechanical agitation versus sonication. J. Mol. Liq. 2016, 224, 151–157. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Zhou, Z.; Li, D.; Wang, T.; Su, P.; Yang, Y. Highly Effective Removal of Pharmaceutical Compounds from Aqueous Solution by Magnetic Zr-Based MOFs Composites. Ind. Eng. Chem. Res. 2019, 58, 3876–3884. [Google Scholar] [CrossRef]
- Assaad, N.; Sabeh, G.; Hmadeh, M. Defect Control in Zr-Based Metal-Organic Framework Nanoparticles for Arsenic Removal from Water. ACS Appl. Nano Mater. 2020, 3, 8997–9008. [Google Scholar] [CrossRef]
- Sonal, S.; Mishra, B.K. A comprehensive review on the synthesis and performance of different zirconium-based adsorbents for the removal of various water contaminants. Chem. Eng. J. 2021, 424, 130509. [Google Scholar] [CrossRef]
- Tao, Y.; Fang, F.; Lv, Q.; Qin, W.; He, X.; Zhang, Y.; Zhou, Y.; Li, X.; Li, J. Highly efficient removal of glyphosate from water by hierarchical-pore UiO-66: Selectivity and effects of natural water particles. J. Environ. Manag. 2022, 316, 115301. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Gil-San-Millan, R.; Navarro, J.A.R.; Maldonado, C.R.; Barea, E.; Carmona, F.J. Green synthesis of zirconium MOF-808 for simultaneous phosphate recovery and organophosphorus pesticide detoxification in wastewater. J. Mater. Chem. A 2022, 10, 19606–19611. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Pan, L.; Zhang, X.; Zou, J.-J. Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: A review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Payno, D.; Lezama, L.; Laza, J.M.; Wuttke, S.; de Luis, R.F. Multivariate Functionalization of UiO-66 for Photocatalytic Water Remediation. Adv. Sustain. Syst. 2022, 6, 2200024. [Google Scholar] [CrossRef]
- Moumen, E.; Bazzi, L.; El Hankari, S. Metal-organic frameworks and their composites for the adsorption and sensing of phosphate. Coord. Chem. Rev. 2022, 454, 214376. [Google Scholar] [CrossRef]
- Hosoya, K.; Ohtsuki, C.; Kawai, T.; Kamitakahara, M.; Ogata, S.-I.; Miyazaki, T.; Tanihara, M. A novel covalently crosslinked gel of alginate and silane with the ability to form bone-like apatite. J. Biomed. Mater. Res. Part A 2004, 71, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Li, Q.; Yang, Y.; Zhang, J.; Guo, F.; Wang, X.; Xu, S.; Ruan, S. Highly effective removal of phosphate from complex water environment with porous Zr-bentonite alginate hydrogel beads: Facile synthesis and adsorption behavior study. Appl. Clay Sci. 2021, 201, 105919. [Google Scholar] [CrossRef]
- Shan, S.; Tang, H.; Zhao, Y.; Wang, W.; Cui, F. Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal. Chem. Eng. J. 2019, 359, 779–789. [Google Scholar] [CrossRef]
- Zhao, Y.; Gai, L.; Liu, H.; An, Q.; Xiao, Z.; Zhai, S. Network interior and surface engineering of alginate-based beads using sorption affinity component for enhanced phosphate capture. Int. J. Biol. Macromol. 2020, 162, 301–309. [Google Scholar] [CrossRef]
- Zeng, Z.; Sorescu, D.C.; White, D.L.; Hwang, S.I.; Shao, W.; He, X.; Schulte, Z.M.; Rosi, N.L.; Star, A. Heterogeneous Growth of UiO-66-NH2 on Oxidized Single-Walled Carbon Nanotubes to form “Beads-on-a-String” Composites. ACS Appl. Mater. Interfaces 2021, 13, 15482–15489. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Alvarez-Ramirez, J.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Hernández-Marín, N.Y. Characterization of corn starch-calcium alginate xerogels by microscopy, thermal, XRD, and FTIR analyses. Starch-Stärke 2021, 73, 2000282. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Crupi, V.; Majolino, D.; Fiorati, A.; Punta, C. FTIR-ATR analysis of the H-bond network of water in branched polyethyleneimine/TEMPO-oxidized cellulose nano-fiber xerogels. Cellulose 2020, 27, 8605–8618. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Zhu, X.; Wang, Z.; Li, Y.; Zhuang, Q.; Shi, J.; Gu, J. Metal-organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Strauss, I.; Chakarova, K.; Mundstock, A.; Mihaylov, M.; Hadjiivanov, K.; Guschanski, N.; Caro, J. UiO-66 and UiO-66-NH2 based sensors: Dielectric and FTIR investigations on the effect of CO2 adsorption. Microporous Mesoporous Mater. 2020, 302, 110227. [Google Scholar] [CrossRef]
- Wang, L.; Wen, X.; Li, J.; Zeng, P.; Song, Y.; Yu, H. Roles of defects and linker exchange in phosphate adsorption on UiO-66 type metal organic frameworks: Influence of phosphate concentration. Chem. Eng. J. 2021, 405, 126681. [Google Scholar] [CrossRef]
- Karim, K.H. Copper adsorption behavior in some calcareous soils using Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich models. J. Soil Sci. Agric. Eng. 2020, 11, 27–34. [Google Scholar] [CrossRef]
- Guan, T.; Li, X.; Fang, W.; Wu, D. Efficient removal of phosphate from acidified urine using UiO-66 metal-organic frameworks with varying functional groups. Appl. Surf. Sci. 2020, 501, 144074. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, S.-Y.; Jochems, A.P. Zirconium-based metal organic frameworks: Highly selective adsorbents for removal of phosphate from water and urine. Mater. Chem. Phys. 2015, 160, 168–176. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, S.; Yang, L. Magnetic zirconium-based metal-organic frameworks for selective phosphate adsorption from water. J. Colloid Interface Sci. 2019, 552, 134–141. [Google Scholar] [CrossRef]
- Afridi, M.N.; Kim, J.-O. Statistical optimization of Mg-doped UiO-66-NH2 synthesis for resource recovery from wastewater using response surface methodology. Appl. Surf. Sci. 2022, 606, 154973. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Tang, N.; Wang, Y.; Yang, X.; Wang, S. Highly efficient capture of phosphate from water via cerium-doped metal-organic frameworks. J. Clean. Prod. 2020, 265, 121782. [Google Scholar] [CrossRef]




| Kinetic Model | Initial Concentration of Phosphate Solution (mg/L) | |||
|---|---|---|---|---|
| 20 | 50 | 100 | ||
| Pseudo-first-order model | k1 | 0.03877 | 0.05502 | 0.04844 |
| Qe | 19.7897 | 33.7230 | 49.9256 | |
| R2 | 0.9970 | 0.9959 | 0.9971 | |
| Quasi-secondary model | k2 | 0.00117 | 0.0012 | 0.00066 |
| Qe | 27.4322 | 43.6677 | 66.1599 | |
| R2 | 0.9978 | 0.9973 | 0.9993 | |
| Elovich model | α | 2.6000 | 6.2009 | 8.1156 |
| β | 0.2031 | 0.1160 | 0.0787 | |
| R2 | 0.9472 | 0.9701 | 0.9654 | |
| Langmuir model | Qm | KL | R2 |
| 96.7882 | 0.0275 | 0.9680 | |
| Freundlich model | KF | 1/nF | R2 |
| 9.3226 | 0.4431 | 0.9901 | |
| Temkin model | KT | B | R2 |
| 0.4552 | 18.0698 | 0.9470 | |
| D–R model | Qm | KD | R2 |
| 70.3320 | 2.3737×10−5 | 0.8157 |
| C0 | v | m | te | R | Thomas Model | ||
|---|---|---|---|---|---|---|---|
| KT | Q | R2 | |||||
| 50 | 1 | 2 | 291 | 29.19 | 0.2531 | 1.4594 | 0.9951 |
| 50 | 2 | 2 | 182 | 26.54 | 0.3830 | 1.3494 | 0.9873 |
| 100 | 2 | 2 | 89 | 23.79 | 0.4622 | 1.1422 | 0.9896 |
| Materials | Specific Surface Area (m2/g) | Pore Size (nm) | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|
| UiO-66 | 990 | —— | 27.70 | [39] |
| UiO-66-NH2 | 815 | —— | 30.00 | |
| MFC@UiO-66 | —— | —— | 7.83 | [40] |
| Mg-doped UiO-66-NH2 | 397 | 1.96 | 68.00 | [41] |
| Ce-doping UiO-66-NH2 | 557.7 | 1.96 | 69.10 | [42] |
| UiO-66-NH2/SA@PEI | 325.1 | 3.7 | 68.75 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Xiong, Y.; Dong, F. Sodium Alginate/UiO-66-NH2 Nanocomposite for Phosphate Removal. Nanomaterials 2024, 14, 1176. https://doi.org/10.3390/nano14141176
Lin X, Xiong Y, Dong F. Sodium Alginate/UiO-66-NH2 Nanocomposite for Phosphate Removal. Nanomaterials. 2024; 14(14):1176. https://doi.org/10.3390/nano14141176
Chicago/Turabian StyleLin, Xiaohang, Yuzhu Xiong, and Fuping Dong. 2024. "Sodium Alginate/UiO-66-NH2 Nanocomposite for Phosphate Removal" Nanomaterials 14, no. 14: 1176. https://doi.org/10.3390/nano14141176
APA StyleLin, X., Xiong, Y., & Dong, F. (2024). Sodium Alginate/UiO-66-NH2 Nanocomposite for Phosphate Removal. Nanomaterials, 14(14), 1176. https://doi.org/10.3390/nano14141176






