Bimodal MRI/Fluorescence Nanoparticle Imaging Contrast Agent Targeting Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
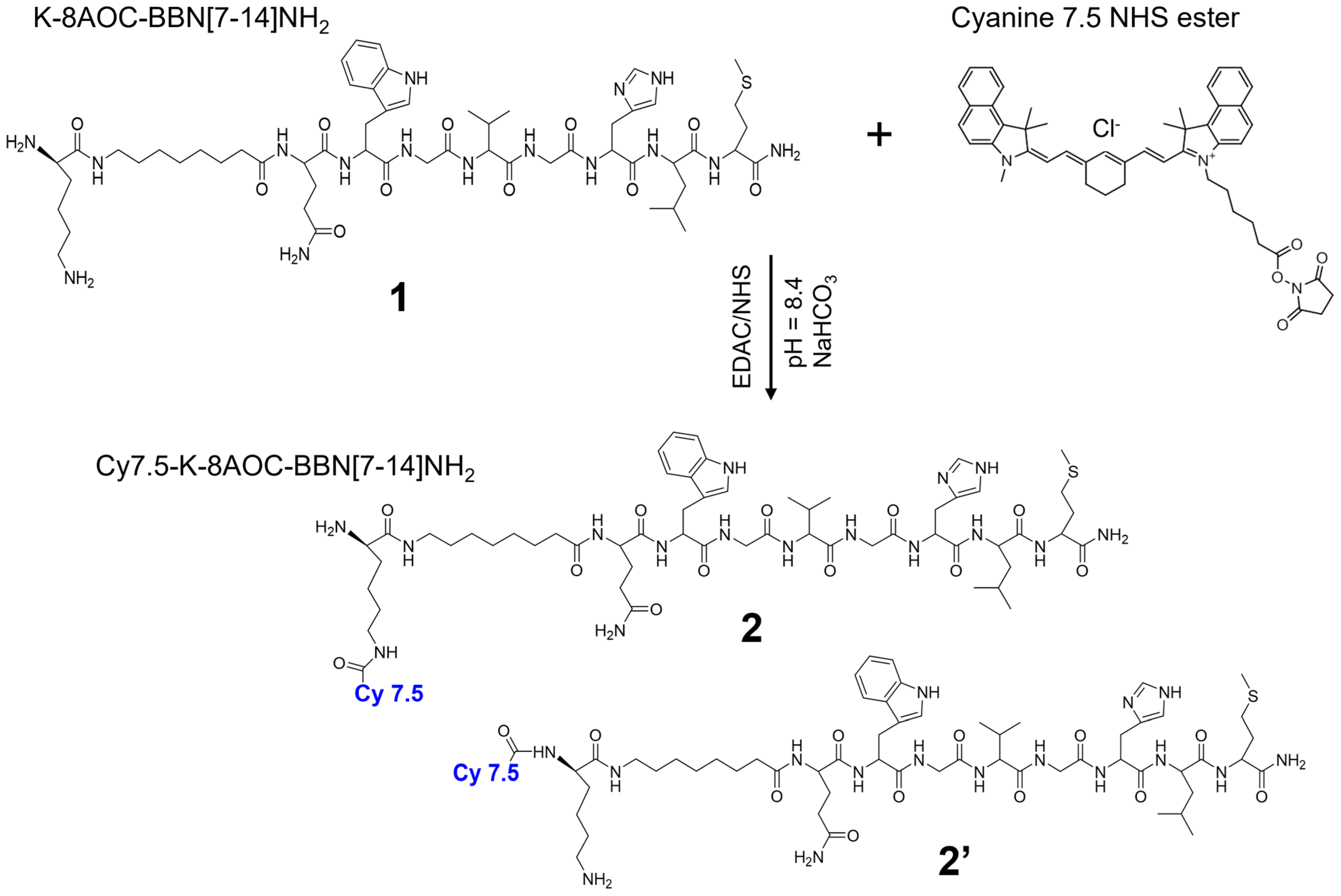
2.2. Synthesis and Purification of Cy7.5-K-8AOC-BBN [7-14]NH2
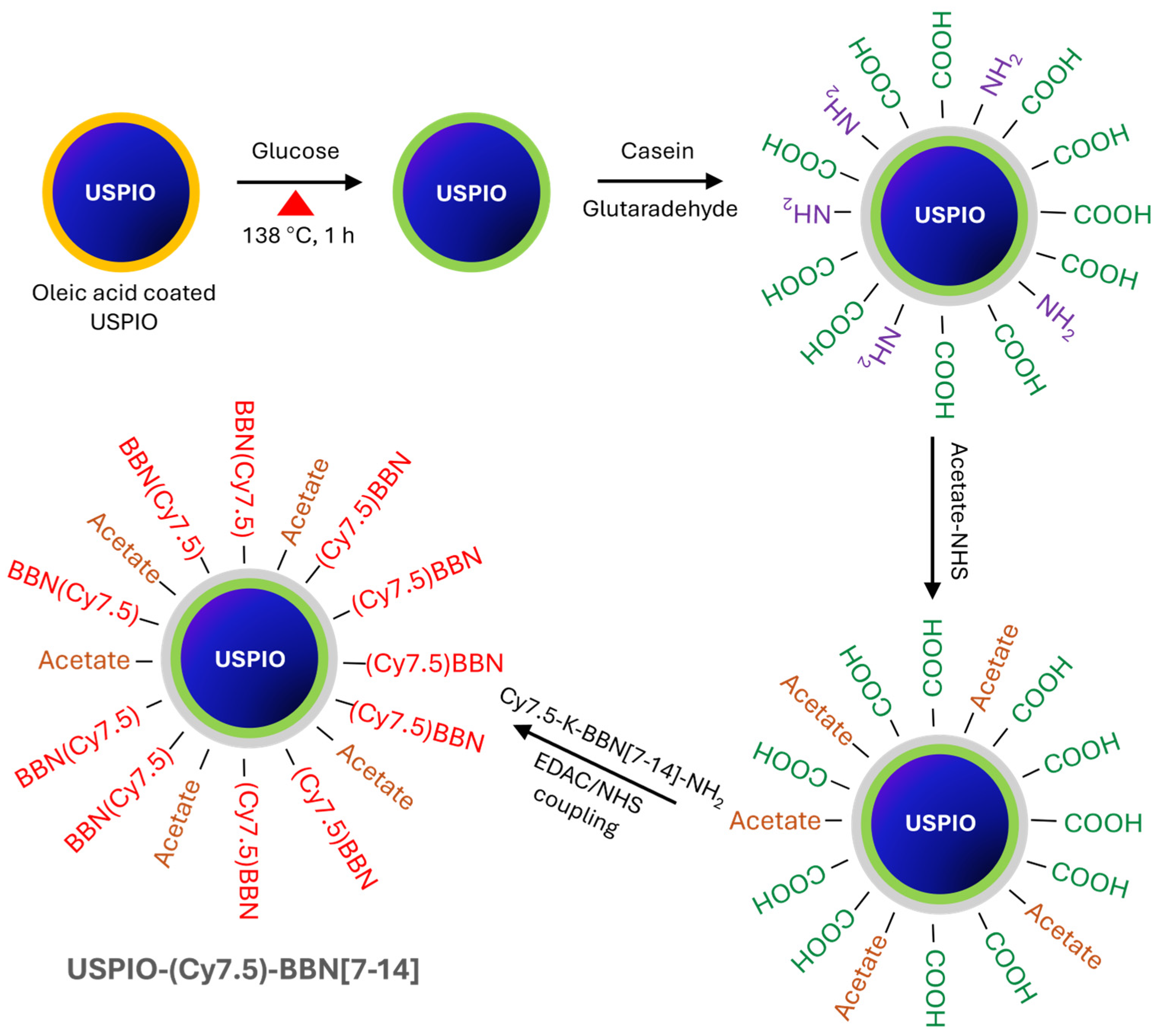
2.3. Synthesis and Purification of USPIO(Cy7.5)-BBN
2.4. Determination of Iron Content of Nanoparticles
2.5. Determination of Peptide to Nanoparticle Ratio
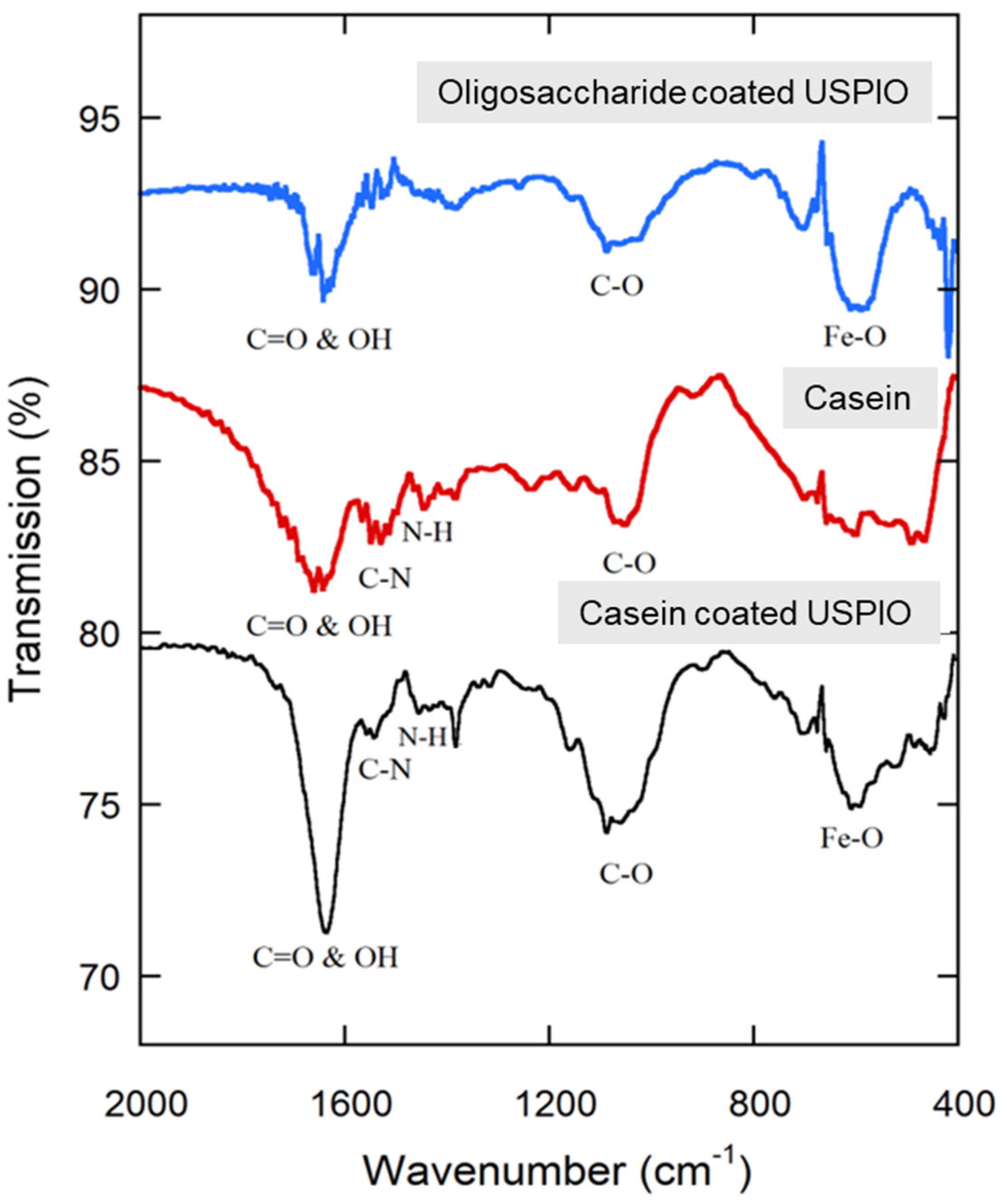
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
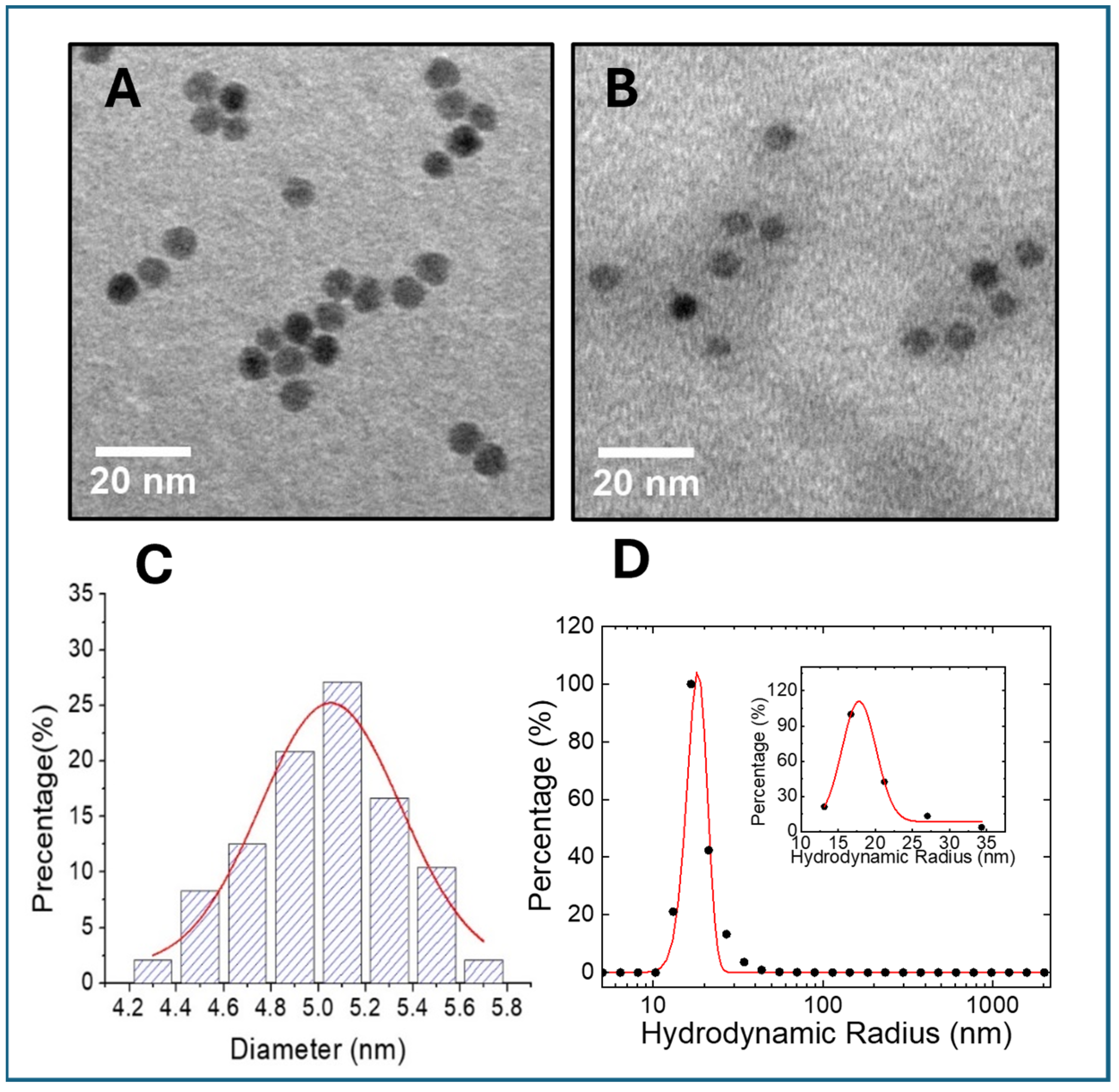
2.7. Transmission Electron Microscopy (TEM)
2.8. Dynamic Light Scattering (DLS)
2.9. MRI Relaxivity Determination
2.10. Cell Culture and In Vitro Binding Affinity Determination
2.11. In Vitro Cellular Microscopic Imaging: Uptake, Blocking, Internalization, and Prussian Blue Staining
2.12. Animal Model
2.13. In Vivo MRI
2.14. NIRF Molecular Imaging
2.15. Histopathology
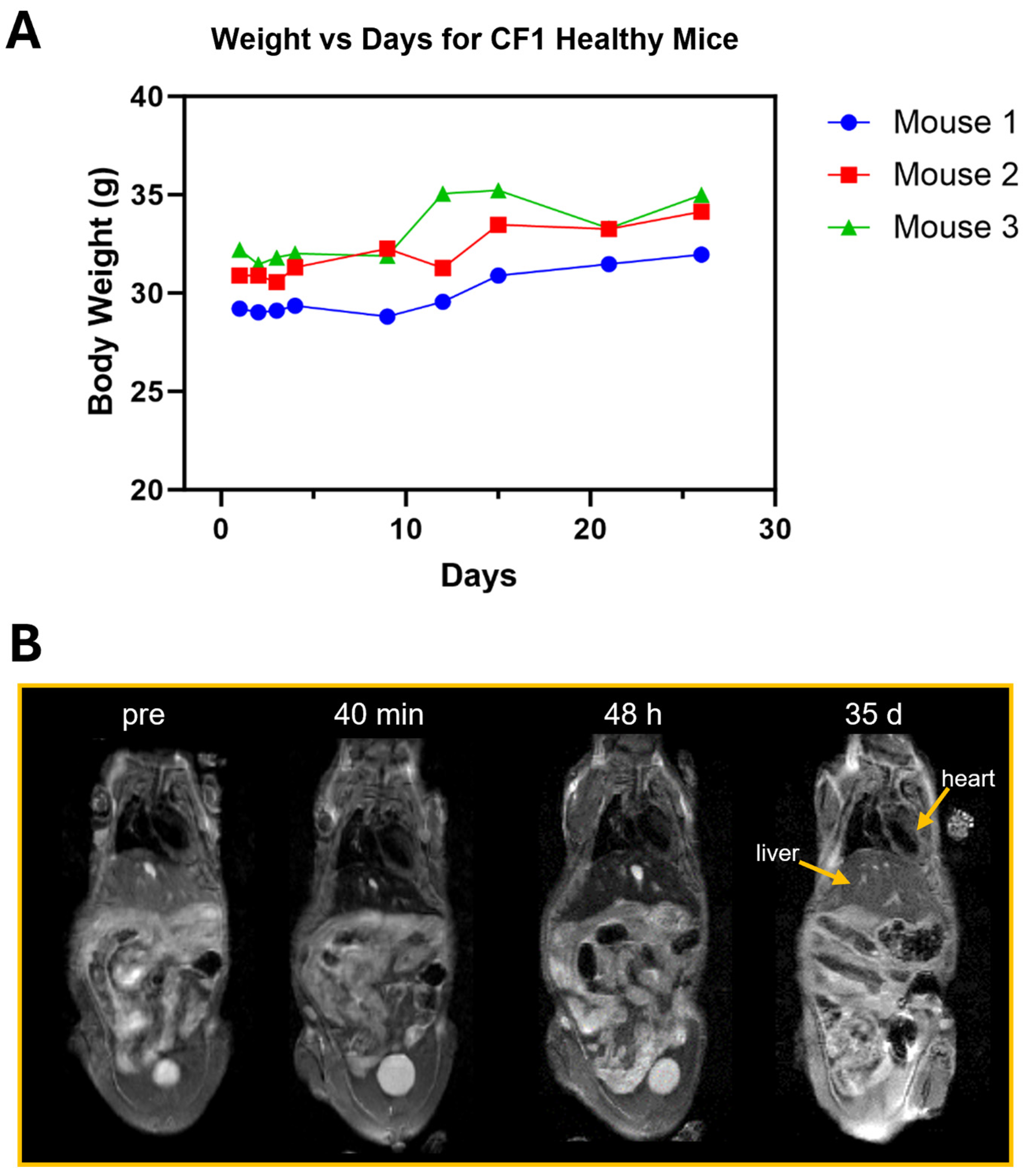
2.16. In Vivo Toxicity Assessment
2.17. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Purifications
3.2. Morphology, Core Size, Hydrodynamic Diameter, and Peptide-to-Nanoparticle Ratio Determination
3.3. MRI Relaxivity of USPIO(Cy7.5)-BBN
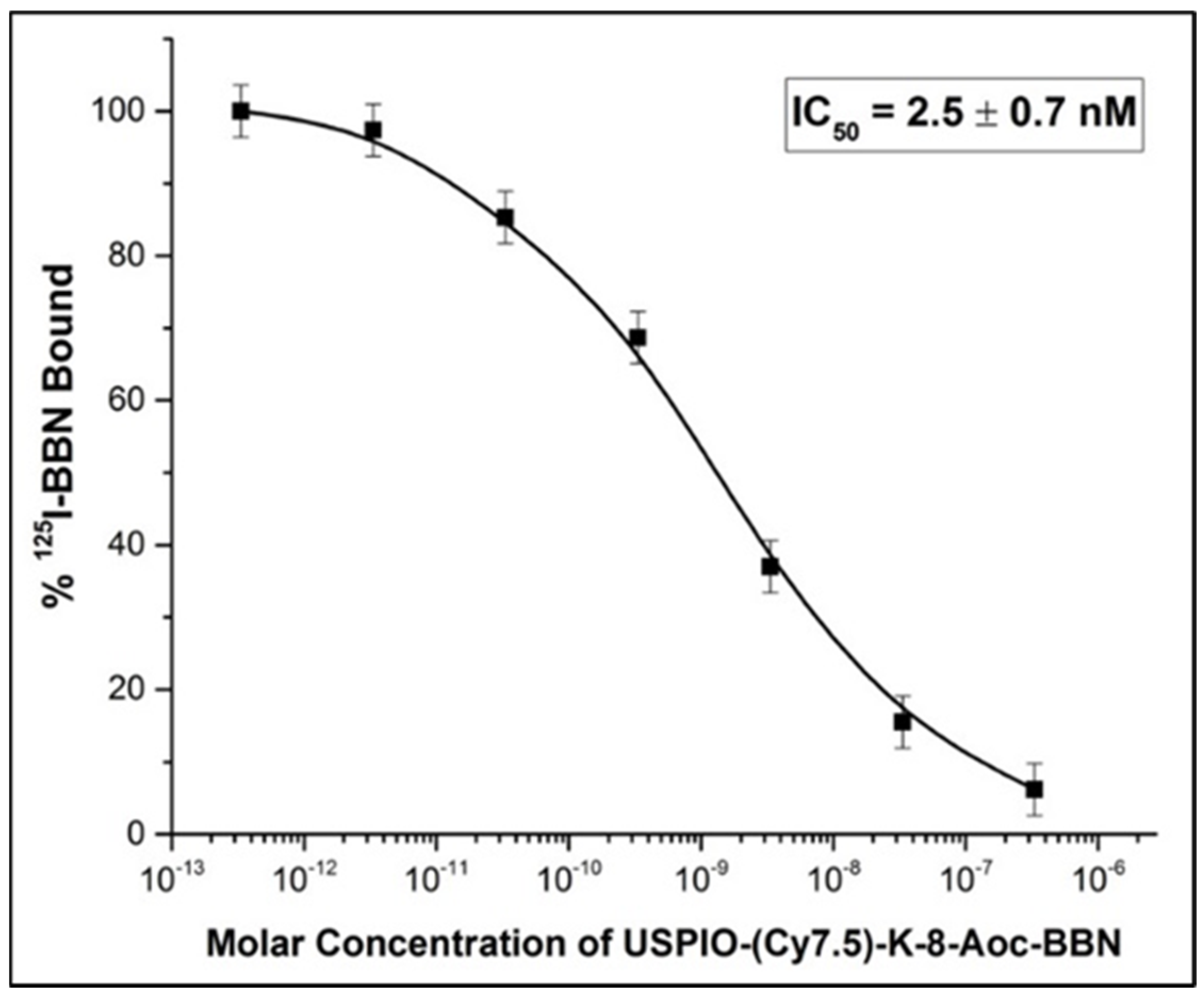
3.4. Binding Affinity of USPIO(Cy7.5)-BBN to Prostate Cancer Cells
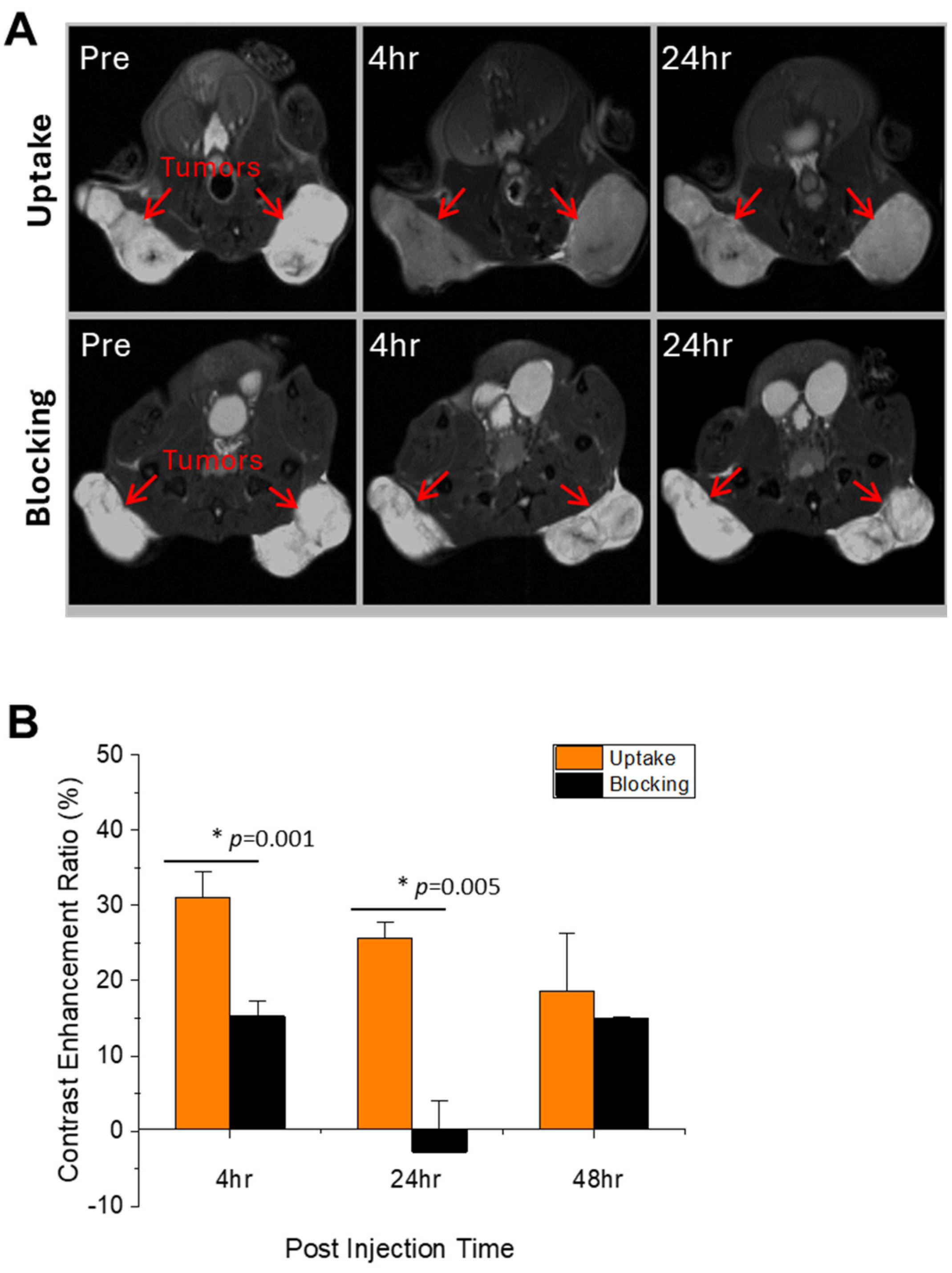
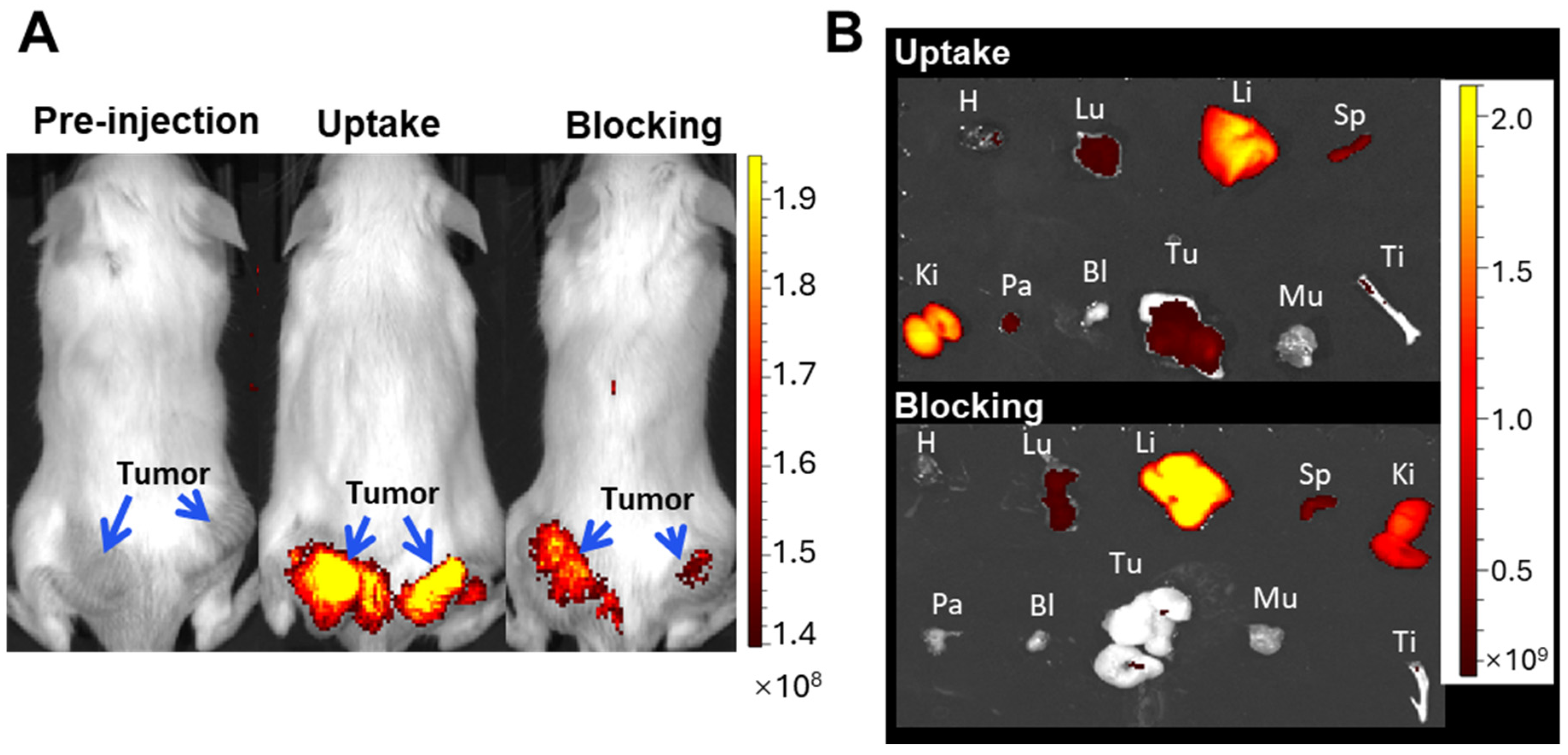
3.5. In Vivo NIRF & MRI Molecular Imaging
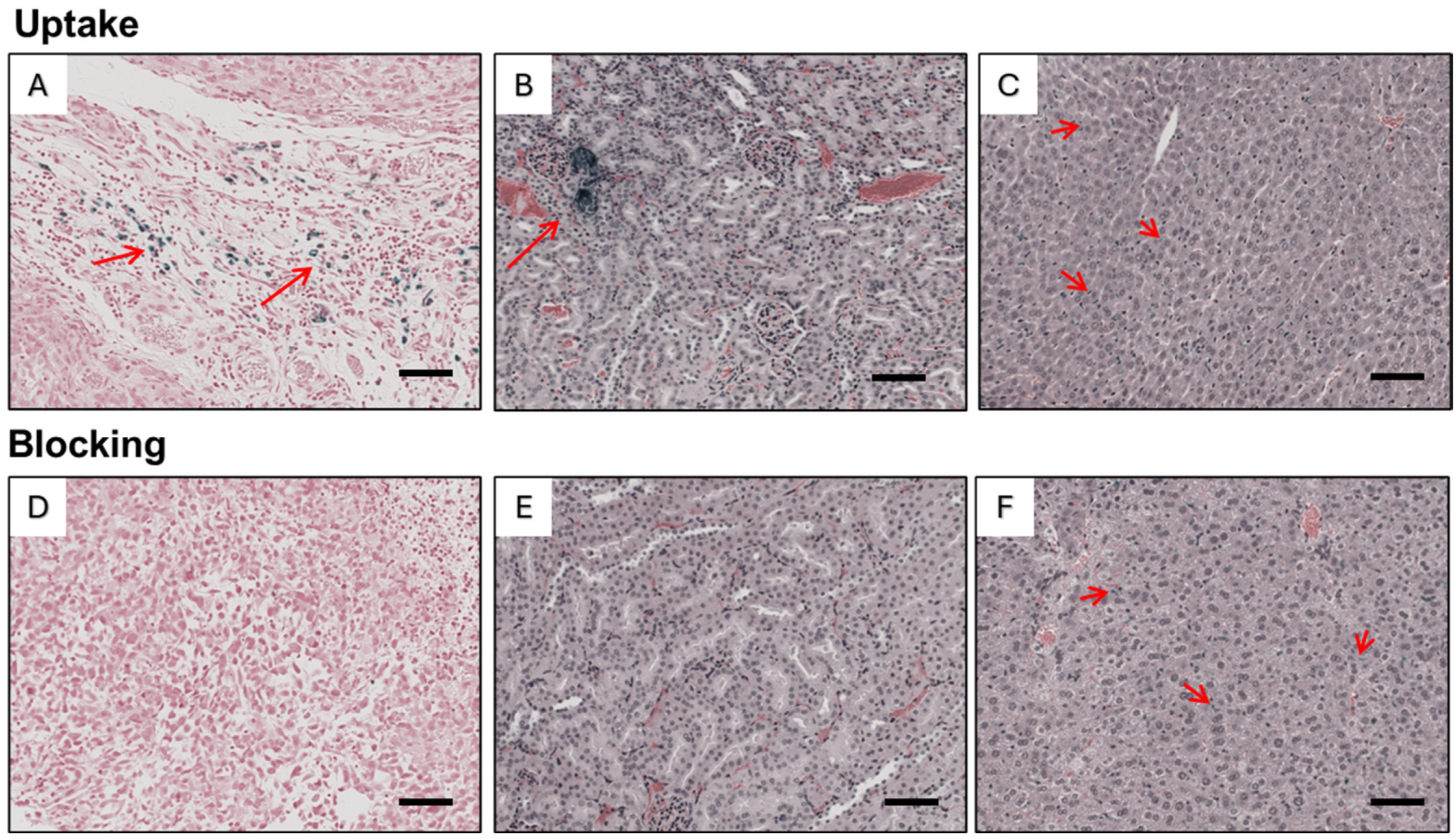
3.6. Histopathology
3.7. In Vivo Toxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Howrey, B.; Kuo, Y.-F.; Lin, Y.-L.; Goodwin, J.S. The impact of PSA screening on prostate cancer mortality and overdiagnosis of prostate cancer in the United States. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Prostate-cancer mortality at 11 years of follow-up. N. Engl. J. Med. 2012, 366, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Kwak, J.W.; Park, J.W. Nanotechnology for early cancer detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shiramoto, S.; Miyashita, M.; Fujishima, Y.; Kaneo, Y. Tumor targeting based on the effect of enhanced permeability and retention (EPR) and the mechanism of receptor-mediated endocytosis (RME). Int. J. Pharm. 2004, 277, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.G.; Moon, M.J.; Lee, H.; Sasikala, A.R.K.; Kim, C.S.; Park, I.-K.; Jeong, Y.Y. Hyaluronic acid conjugated superparamagnetic iron oxide nanoparticle for cancer diagnosis and hyperthermia therapy. Carbohydr. Polym. 2015, 131, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Elizondo, G.; Wittenberg, J.; Rabito, C.A.; Bengele, H.H.; Josephson, L. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for MR imaging. Radiology 1990, 175, 489–493. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.-L.; Xu, J.-H.; Cai, B.; Yu, G.-T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.-S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef] [PubMed]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Pouliquen, D.; Le Jeune, J.J.; Perdrisot, R.; Ermias, A.; Jallet, P. Iron oxide nanoparticles for use as an MRI contrast agent: Pharmacokinetics and metabolism. Magn. Reson. Imaging 1991, 9, 275–283. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Liang, X.J. Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc. Chem. Res. 2011, 44, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ito, N.; Kotake, F.; Mizokami, Y.; Matsuoka, T. Tumor-detecting capacity and clinical usefulness of SPIO-MRI in patients with hepatocellular carcinoma. J. Gastroenterol. 2000, 35, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Hahn, P.F.; Stark, D.D.; Elizondo, G.; Saini, S.; Todd, L.E.; Wittenberg, J.; Ferrucci, J.T. Superparamagnetic iron oxide: Enhanced detection of focal splenic tumors with MR imaging. Radiology 1988, 169, 399–403. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Lin, R.; Wang, A.Y.; Yang, L.; Kuang, M.; Qian, W.; Mao, H. Casein-coated iron oxide nanoparticles for high MRI contrast enhancement and efficient cell targeting. ACS Appl. Mater. Interfaces 2013, 5, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Biddlecombe, G.B.; Rogers, B.E.; De Visser, M.; Parry, J.J.; De Jong, M.; Erion, J.L.; Lewis, J.S. Molecular imaging of gastrin-releasing peptide receptor-positive tumors in mice using 64Cu- and 86Y-DOTA-(Pro1,Tyr 4)-bombesin(1-14). Bioconjugate Chem. 2007, 18, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.J.; Gali, H.; Smith, C.J.; Sieckman, G.L.; Hayes, D.L.; Owen, N.K.; Volkert, W.A. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J. Nucl. Med. 2003, 44, 823–831. [Google Scholar] [PubMed]
- Liu, Z.; Li, Z.B.; Cao, Q.; Liu, S.; Wang, F.; Chen, X. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J. Nucl. Med. 2009, 50, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, Y.; Chin, F.T.; Wang, F.; Chen, X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-Labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J. Med. Chem. 2009, 52, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Wang, X.; Forrer, F.; Waser, B.; Cescato, R.; Graham, K.; Borkowski, S.; Reubi, J.C.; Maecke, H.R. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Pinski, J.; Halmos, G.; Yano, T.; Szepeshazi, K.; Qin, Y.; Ertl, T.; Schally, A.V. Inhibition of growth of MKN45 human gastric-carcinoma xenografts in nude mice by treatment with bombesin/gastrin-releasing-peptide antagonist (RC-3095) and somatostatin analogue RC-160. Int. J. Cancer 1994, 57, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.E.; Bigott, H.M.; McCarthy, D.W.; Della Manna, D.; Kim, J.; Sharp, T.L.; Welch, M.J. MicroPET imaging of a gastrin-releasing peptide receptor-positive tumor in a mouse model of human prostate cancer using a 64Cu-labeled bombesin analogue. Bioconjugate Chem. 2003, 14, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.E.; Manna, D.D.; Safavy, A. In Vitro and In Vivo Evaluation of a 64Cu-Labeled Polyethylene Glycol-Bombesin Conjugate. Cancer Biother. Radio. 2004, 19, 25–34. [Google Scholar] [CrossRef]
- Stott Reynolds, T.J.; Schehr, R.; Liu, D.; Xu, J.; Miao, Y.; Hoffman, T.J.; Rold, T.L.; Lewis, M.R.; Smith, C.J. Characterization and evaluation of DOTA-conjugated Bombesin/RGD-antagonists for prostate cancer tumor imaging and therapy. Nucl. Med. Biol. 2015, 42, 99–108. [Google Scholar] [CrossRef]
- Sun, B.; Schally, A.V.; Halmos, G. The presence of receptors for bombesin/GRP and mRNA for three receptor subtypes in human ovarian epithelial cancers. Regul. Pept. 2000, 90, 77–84. [Google Scholar] [CrossRef]
- Xu, H.; Bandari, R.P.; Lee, L.; Li, R.; Yu, P.; Smith, C.J.; Ma, L. Design, Synthesis, and in Vitro and in Vivo Evaluation of High Affinity and Specificity Near-Infrared Fluorescent Bombesin Antagonists for Tumor Imaging. J. Med. Chem. 2018, 61, 7657–7670. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Yu, P.; Besch-Williford, C.; Smith, C.J.; Sieckman, G.L.; Hoffman, T.J.; Ma, L. Near-infrared fluorescence imaging of gastrin releasing peptide receptor targeting in prostate cancer lymph node metastases. Prostate 2013, 73, 842–854. [Google Scholar] [CrossRef]
- Li, R.; Gao, R.; Wang, Y.; Liu, Z.; Xu, H.; Duan, A.; Zhang, F.; Ma, L. Gastrin releasing peptide receptor targeted nano-graphene oxide for near-infrared fluorescence imaging of oral squamous cell carcinoma. Sci. Rep. 2020, 10, 11434. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gao, R.; Zhao, Y.; Zhang, F.; Wang, X.; Li, B.; Wang, L.; Ma, L.; Du, J. pH-responsive graphene oxide loaded with targeted peptide and anticancer drug for OSCC therapy. Front. Oncol. 2022, 12, 930920. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yu, P.; Veerendra, B.; Rold, T.L.; Retzloff, L.; Prasanphanich, A.; Sieckman, G.; Hoffman, T.J.; Volkert, W.A.; Smith, C.J. In vitro and in vivo evaluation of Alexa Fluor 680-bombesin [7-14]NH2 peptide conjugate, a high-affinity fluorescent probe with high selectivity for the gastrin-releasing peptide receptor. Mol. Imaging 2007, 6, 171–180. [Google Scholar] [CrossRef]
- Bratanovic, I.J.; Zhang, C.; Zhang, Z.; Kuo, H.T.; Colpo, N.; Zeisler, J.; Merkens, H.; Uribe, C.; Lin, K.S.; Bénard, F. A Radiotracer for Molecular Imaging and Therapy of Gastrin-Releasing Peptide Receptor-Positive Prostate Cancer. J. Nucl. Med. 2022, 63, 424–430. [Google Scholar] [CrossRef]
- Mansi, R.; Minamimoto, R.; Mäcke, H.; Iagaru, A.H. Bombesin-Targeted PET of Prostate Cancer. J. Nucl. Med. 2016, 57 (Suppl. S3), 67s–72s. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, G.; Ananias, H.J.; Yu, Z.; Hoving, H.D.; Helfrich, W.; Dierckx, R.A.; Liu, S.; de Jong, I.J.; Elsinga, P.H. Preclinical evaluation of a novel ¹¹¹In-labeled bombesin homodimer for improved imaging of GRPR-positive prostate cancer. Mol. Pharm. 2013, 10, 1716–1724. [Google Scholar] [CrossRef]
- Van de Wiele, C.; Phonteyne, P.; Pauwels, P.; Goethals, I.; Van den Broecke, R.; Cocquyt, V.; Dierckx, R.A. Gastrin-releasing peptide receptor imaging in human breast carcinoma versus immunohistochemistry. J. Nucl. Med. 2008, 49, 260–264. [Google Scholar] [CrossRef]
- Kähkönen, E.; Jambor, I.; Kemppainen, J.; Lehtiö, K.; Grönroos, T.J.; Kuisma, A.; Luoto, P.; Sipilä, H.J.; Tolvanen, T.; Alanen, K.; et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou-Strauss, A.; Hohenberger, P.; Pan, L.; Kasper, B.; Roumia, S.; Strauss, L.G. Dynamic PET with FDG in patients with unresectable aggressive fibromatosis: Regression-based parametric images and correlation to the FDG kinetics based on a 2-tissue compartment model. Clin. Nucl. Med. 2012, 37, 943–948. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A.; Hohenberger, P.; Haberkorn, U.; Mäcke, H.R.; Eisenhut, M.; Strauss, L.G. 68Ga-Labeled Bombesin Studies in Patients with Gastrointestinal Stromal Tumors: Comparison with 18F-FDG. J. Nucl. Med. 2007, 48, 1245. [Google Scholar] [CrossRef]
- Duan, H.; Davidzon, G.A.; Moradi, F.; Liang, T.; Song, H.; Iagaru, A. Modified PROMISE criteria for standardized interpretation of gastrin-releasing peptide receptor (GRPR)-targeted PET. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 4087–4095. [Google Scholar] [CrossRef]
- Koller, L.; Joksch, M.; Schwarzenböck, S.; Kurth, J.; Heuschkel, M.; Holzleitner, N.; Beck, R.; von Amsberg, G.; Wester, H.J.; Krause, B.J.; et al. Preclinical Comparison of the 64Cu- and 68Ga-Labeled GRPR-Targeted Compounds RM2 and AMTG, as Well as First-in-Humans [68Ga]Ga-AMTG PET/CT. J. Nucl. Med. 2023, 64, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Montaño, A.R.; Masillati, A.M.; Jones, J.A.; Barth, C.W.; Combs, J.R.; Kumarapeli, S.U.; Shams, N.A.; van den Berg, N.S.; Antaris, A.L.; et al. Nerve Visualization using Phenoxazine-Based Near-Infrared Fluorophores to Guide Prostatectomy. Adv. Mater. 2024, 36, e2304724. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Niu, G.; Wang, F.; Liu, S.; Chen, X. Optical imaging of integrin alphavbeta3 expression with near-infrared fluorescent RGD dimer with tetra(ethylene glycol) linkers. Mol. Imaging 2010, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V.; Melchiorri, P.; Sopranzi, N. The action of bombesin on the kidney of the anaesthetized dog. Br. J. Pharmacol. 1973, 48, 438–455. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Yang, C.Y.; Hsiao, J.K.; Tai, M.F.; Chen, S.T.; Cheng, H.Y.; Wang, J.L.; Liu, H.M. Direct labeling of hMSC with SPIO: The long-term influence on toxicity, chondrogenic differentiation capacity, and intracellular distribution. Mol. Imaging Biol. 2011, 13, 443–451. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yang, M.; Ma, L.; Sauer, M.; Avella, D.; Kaifi, J.T.; Bryan, J.; Cheng, K.; Staveley-O’Carroll, K.F.; Kimchi, E.T.; et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J. Immunother. Cancer 2020, 8, e001038. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Yu, P.; Bandari, R.P.; Smith, C.J.; Aro, M.R.; Singh, A.; Ma, L. Bimodal MRI/Fluorescence Nanoparticle Imaging Contrast Agent Targeting Prostate Cancer. Nanomaterials 2024, 14, 1177. https://doi.org/10.3390/nano14141177
Xu H, Yu P, Bandari RP, Smith CJ, Aro MR, Singh A, Ma L. Bimodal MRI/Fluorescence Nanoparticle Imaging Contrast Agent Targeting Prostate Cancer. Nanomaterials. 2024; 14(14):1177. https://doi.org/10.3390/nano14141177
Chicago/Turabian StyleXu, Hang, Ping Yu, Rajendra P. Bandari, Charles J. Smith, Michael R. Aro, Amolak Singh, and Lixin Ma. 2024. "Bimodal MRI/Fluorescence Nanoparticle Imaging Contrast Agent Targeting Prostate Cancer" Nanomaterials 14, no. 14: 1177. https://doi.org/10.3390/nano14141177
APA StyleXu, H., Yu, P., Bandari, R. P., Smith, C. J., Aro, M. R., Singh, A., & Ma, L. (2024). Bimodal MRI/Fluorescence Nanoparticle Imaging Contrast Agent Targeting Prostate Cancer. Nanomaterials, 14(14), 1177. https://doi.org/10.3390/nano14141177





