Abstract
Highly efficient and cost-effective electrocatalysts are of critical significance in the domain of water electrolysis. In this study, a Ni3N-CeO2/NF heterostructure is synthesized through a facile hydrothermal technique followed by a subsequent nitridation process. This catalyst is endowed with an abundance of oxygen vacancies, thereby conferring a richer array of active sites. Therefore, the catalyst demonstrates a markedly low overpotential of 350 mV for the Oxygen Evolution Reaction (OER) at 50 mA cm−2 and a low overpotential of 42 mV for the Hydrogen Evolution Reaction (HER) at 10 mA cm−2. Serving as a dual-function electrode, this electrocatalyst is employed in overall water splitting in alkaline environments, demonstrating impressive efficiency at a cell voltage of 1.52 V of 10 mA cm−2. The in situ Raman spectroscopic analysis demonstrates that cerium dioxide (CeO2) facilitates the rapid reconfiguration of oxygen vacancy-enriched nickel oxyhydroxide (NiOOH), thereby enhancing the OER performance. This investigation elucidates the catalytic role of CeO2 in augmenting the OER efficiency of nickel nitride (Ni3N) for water electrolysis, offering valuable insights for the design of high-performance bifunctional catalysts tailored for water splitting applications.
1. Introduction
Over the past few decades, global energy consumption has increased at an alarming rate. The depletion of fossil fuels is driving our world toward a severe energy shortage. Consequently, the quest for novel energy sources holds immense importance for humanity’s sustainable growth. Hydrogen is the most ideal of all renewable energy sources because it has zero pollution and high energy density [1]. Among the hydrogen-forming methods, electrolytic water has attracted much attention from researchers as a result of its high utilization rate and lack of by-products. The electrochemical process of water splitting encompasses two distinct half reactions: HER at the cathode and OER at the anode. Precious metal catalysts (platinum, palladium, ruthenium, iridium) are active in hydrogen and oxygen evolution reactions but are currently expensive and difficult to apply in the industry [2,3]. Therefore, researchers must develop efficient and inexpensive electrocatalysts for electrochemical water separation [4].
When the element N is integrated into transition metal lattices, transition metal nitrides (TMNs), a type of mesenchymal substance, emerge, sharing characteristics with covalent compounds, ionic crystals, and transition metals [5]. Nanostructured TMNs, characterized by a large surface area, can be synthesized via temperature-regulated nitriding of oxide precursors by means of a “local structured reaction”, ensuring the oxide precursor’s crystal structure remains intact under stringent nitriding scenarios [6]. The introduction of N leads to an expansion of the metal lattice, a rise in metal spacing, and a reduction in the interaction force among metal atoms, culminating in the d-band shrinking and a rearrangement of state density near the Femi level [7,8]. Consequently, techniques like defect engineering, alloying, heteroatom doping, and the heterostructuring of TMNs represent sophisticated and beneficial approaches for nitride-based electrocatalysts [9]. As the count of valence electrons rises, the structure undergoes corresponding alterations [10]. TMNs are characterized by their distinct electronic configurations, featuring superior electrical conductivity, chemical robustness, electrocatalytic properties, and mechanical steadiness, making them versatile for various applications [11,12]. Typically, TMNs are characterized by their covalent, ionic, and metallic bonds, particularly those between transition metals and nitrogen atoms, which enlarge the parent metal lattice and reduce the size of the metallic d-band. The incorporation of nitrogen atoms in TMNs enhances their corrosion resistance, enabling them to function effectively in both acidic and alkaline environments. Compared to other compounds containing P, S, and Se, TMNs have advantages such as a large specific surface area, high chemical stability, and good electrical conductivity [13]. Ni3N, as a representative transition metal nitride (TMN), has been widely recognized as an electrocatalyst owing to its properties akin to those of noble metals, demonstrating respectable performance in the OER and HER [14,15,16]. Nevertheless, the intricate reaction mechanisms and high dissociation energy of water restrict its effectiveness. Presently, a range of augmentation techniques, including the formation of heterostructures, doping with heteroatoms, defect engineering, and alloying, have been employed to elevate the catalytic efficacy of Ni3N through the optimization of adsorption energies of intermediates. Despite these efforts, the catalytic performance of Ni3N remains significantly inferior to that of noble metal benchmarks in water splitting applications [17]. Concurrently, cerium dioxide (CeO2), recognized for its copious oxygen vacancies and the flexible valency shifts between Ce3+ and Ce4+, serves as an efficacious co-catalyst, augmenting both HER and OER efficiencies. Additionally, CeO2 is capable of activating water molecules in alkaline environments, boosting HER performance. Forming a composite structure is indeed helpful for electrocatalysis. Heterostructures have the following advantages in the field of electrocatalysis: enhancing catalytic activity, promoting charge separation, optimizing band structure, regulating interface properties, and improving stability [18]. Considering the unique benefits of Ni3N and CeO2, amalgamating them into a heterostructure might offer a viable strategy for the development of high-efficiency bifunctional catalysts [19,20].
Herein, we produced Ni3N-CeO2/NF heterostructure catalysts. Due to the synergy between Ni3N and CeO2, Ni3N-CeO2/NF demonstrates superior catalytic proficiency in the context of water electrolysis, achieving remarkably low overpotentials of merely 42 mV at 10 mA cm−2 for the HER. Particularly, for the OER, the material exhibits an ultra-low overpotential (η50 = 350 mV). Through EPR testing, we can see that the catalyst also has abundant oxygen vacancies. The in situ Raman spectroscopy indicates that cerium dioxide (CeO2) significantly facilitates the formation of nickel oxyhydroxide (NiOOH) enriched with oxygen vacancies (OVs) during the OER. This comprehensive elucidation of the catalytic mechanism offers crucial insights for the subsequent development of heterostructured catalysts [21,22].
2. Material and Methods
2.1. Synthesis of Composites
Synthesis of Ni(OH)2-CeO2/NF Precursors on Ni foam. First, clean nickel foam was prepared, which was ultrasonically treated with 2 M HCl for 20 min to eliminate the oxides, and then ultrasonically treated with DI water and ethanol for 20 min. Then, we placed the clean nickel foam (2.0 cm × 4.0 cm) in a 50 mL Teflon autoclave and added 0.9 mmol Ni(NO3)2·6H2O, 1.8 mmol Ce(NO3)3·6H2O, 6 mmol CH4N2O, 4 mmol NH4F, and 30 mL DI water; after that, the mixture was stirred for 30 min to fully dissolve the medicine. Then, the Teflon autoclave was placed in an electric oven and maintained at 120 °C for 6 h. After dropping to room temperature, the precursor was rinsed with DI water several times and then placed in a vacuum drying oven at 70 °C to dry for 12 h.
Synthesis of Ni3N-CeO2/NF. A combustion boat containing Ni(OH)2-CeO2/NF precursors was calcined at 350 °C under ammonia atmosphere for 3 h in a tube furnace, and Ni3N-CeO2/NF was then obtained. Because the molar ratio of the synthesized Ce and Ni is different, we name it Ni3N-xCeO2/NF (Scheme 1).
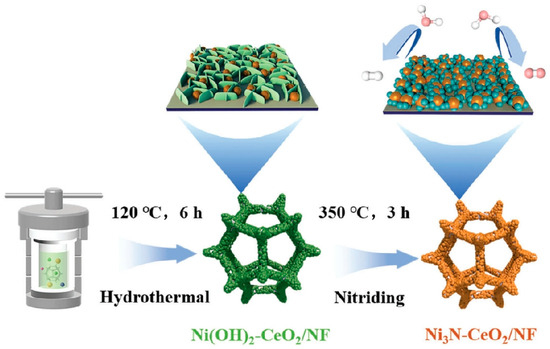
Scheme 1.
A schematic illustration of the synthesis method for Ni3N-CeO2/NF.
Synthesis of Ni3N/NF. First, we placed the clean nickel foam (2.0 cm × 4.0 cm) in a 50 mL Teflon autoclave and added 0.9 mmol Ni(NO3)2·6H2O, 6 mmol CH4N2O, 4 mmol NH4F, and 30 mL DI water; after that, the mixture was stirred for 20 min to fully dissolve the medicine. Then, the Teflon autoclave was placed in an electric oven and maintained at 120 °C for 6 h. After dropping to room temperature, the precursor was rinsed with DI water several times and then placed in a vacuum drying oven at 70 °C to dry for 12 h. Then, the precursor was heated to 350 °C for 3 h under ammonia atmosphere.
Synthesis of CeO2/NF. The synthesis process is the same as for Ni3N-CeO2/NF except that Ni(NO3)2·6H2O is not used.
2.2. Electrochemical Measurements
The electrocatalysts, synthesized and supported on nickel foam, were directly implemented as the working electrodes. The mercury/mercuric oxide electrode served as the reference electrode, while a polished graphite rod functioned as the counter electrode. The exposed area of the working electrode in contact with the electrolyte measured 1 cm2. Linear sweep voltammetry (LSV) measurements were conducted with a scan rate of 5 mV s−1. The obtained polarization curves were adjusted for iR compensation to account for the ohmic resistance presented by the electrolyte, with a compensation level set to 100%. Cyclic voltammetry (CV) profiles were acquired by executing CV assays across a spectrum of scan rates (20, 40, 60, 80, and 100 mV·s−1) within the potential windows from 0.1 to 0.2 V (vs. RHE) for the HER, and from 1.06 to 1.16 V (vs. RHE) for the OER. The stability performance test was conducted at 50 mA cm−2 for 40 h (not iR-corrected). Furthermore, the evaluation of overall water electrolysis was carried out utilizing a two-electrode configuration, employing two identical catalyst-coated electrodes serving concurrently as both the anode and cathode. The stability of the system was assessed at 10 mA cm−2 over a continuous duration of 34 h, with the measurements taken without applying iR compensation. Electrochemical impedance spectroscopy (EIS) measurements were conducted utilizing the aforementioned system across a frequency range from 10,000 Hz to 0.1 Hz. The Hg/HgO reference electrode was calibrated using a reversible hydrogen electrode (RHE). All test data associated with the Hg/HgO electrode were calibrated by using the Nernst equation: ERHE = EHg/HgO + 0.098 + 0.0591 × pH.
2.3. Characterization of Material
Characterizations: The morphology was detected by using a double-beam scanning electron microscope (SEM, JEOL JEM-7800F, Tokyo, Japan) and transmission electron microscopy (TEM, JEOL JEM-2100F, Tokyo, Japan). The lattice parameters originated from X-ray diffraction (XRD, Bruker D8 ADVANCE, Billerica, MA, USA) and high-resolution TEM (HR-TEM, JEOL JEM-2100F, Tokyo, Japan). The electronic structure as well as group structure were obtained from an X-ray photoelectron spectrometer (XPS, THERMO-Fisher ESCALAB250Xi, Waltham, MA, USA) and micro-Raman spectrometer (Renishaw in Via Reflex, Dundee, IL, USA) under an excitation of 532 nm laser light. We used a LabRam HR Evolution confocal Raman microscope to perform in situ Raman spectroscopy tests to determine the active phase and dynamic surface structure of Ni3FeN/NF. The oxygen vacancies were tested by using an electron paramagnetic resonance spectrometer (EPR, Bryker EMXplus-6/1, Billerica, MA, USA).
3. Results and Discussion
3.1. Characterization of Composites
The XRD patterns depicted in Figure 1a corroborate the successful synthesis of Ni3N–CeO2/NF, with the observed peaks aligning well with the standard diffraction patterns of Ni3N (#10-0280) and CeO2 (#34-0394) [23,24]. The XRD patterns (Figure S1) corroborate the successful production of Ni(OH)2–CeO2/NF, with the observed peaks aligning well with the standard diffraction patterns of Ni(OH)2 (#14-0117) and CeO2 (#34-0394). Furthermore, the SEM image (Figure S1) illustrates the nanosheet morphology of the compound. Additionally, the SEM and XRD analyses displayed in Figures S2 and S3 affirm that Ni3N/NF and CeO2/NF have been synthesized effectively. Figure 1b,c depict the morphology of the catalyst, whereas Figure 1d,e validate the homogeneous dispersion of the nanoparticle catalysts, each with a diameter less than 100 nm. The HRTEM images presented in Figure 1f illustrate numerous heterojunctions between Ni3N and CeO2. The lattice fringes exhibiting spacings of 0.312 nm and 0.214 nm are ascribed to the (111) plane of CeO2 and the (002) plane of Ni3N, respectively. It is observed that neighboring nanoparticles converged at the boundary seamlessly, indicating their tight interconnection, which guarantees effective electrical and mechanical interaction for stable and efficient catalysis [25,26]. The EDX spectroscopy elemental mapping of Ni3N-CeO2/NF demonstrates a uniform dispersion of nickel (Ni), cerium (Ce), oxygen (O), and nitrogen (N) throughout the analyzed area, as depicted in Figure 1g. The EDX mapping presented in Figure 1g illustrates that the Ni3N nanoparticles are in complete contact with CeO2, evidenced by the extensive overlap in the mapping. This configuration facilitates the formation of numerous heterogeneous interfaces and increases the availability of active sites, thereby enhancing catalytic activity. The EDX spectroscopy micrographs presented in Supplementary Figure S4 substantiate the presence of nickel (Ni), cerium (Ce), oxygen (O), and nitrogen (N) elements within the analyzed specimen.
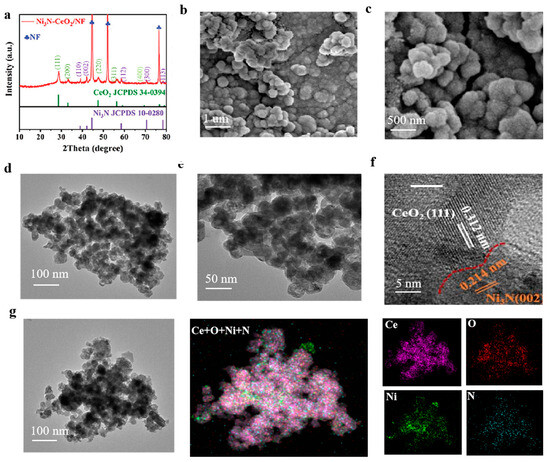
Figure 1.
Structural characterization of Ni3N-CeO2/NF. (a) XRD, (b,c) SEM, and (d,e) TEM images of Ni3N-CeO2/NF. (f) HRTEM of Ni3N-CeO2/NF. (g) Corresponding EDS mapping images of Ni, Ce, O, and N in Ni3N-CeO2/NF at 100 nm.
The XPS was utilized to elucidate the surface chemical compositions of Ni3N-CeO2/NF, Ni3N/NF, and CeO2/NF. The comprehensive XPS spectra shown in Figure 2a reveal the presence of Ni, Ce, N, O, and C elements in Ni3N-CeO2/NF, whereas Ni3N/NF contains only Ni, O, N, and C, and CeO2/NF comprises Ce, O, and C. In Figure 2b, the Ce 3d XPS spectra of Ni3N-CeO2/NF reveal peak distributions spanning 883–894 eV and 896–924 eV, respectively, associated with the Ce 3d5/2 and Ce 3d3/2 orbital states [27,28]. These peaks suggest the coexistence of Ce3+ and Ce4+ states, along with satellite features. The Ce 3d5/2 peak in Ni3N-CeO2/NF exhibits a downward shift of approximately 0.88 eV when compared to CeO2/NF. The high-resolution Ni 2p spectrum of Ni3N-CeO2/NF, illustrated in Figure 2c, delineates peaks at 871.0 and 853.3 eV, attributed to Ni 2p1/2 and Ni 2p3/2, respectively, associated with Ni-N bonding [29,30]. Compared to Ni3N/NF, the Ni 2p spectrum of Ni3N-CeO2/NF shows an upward shift by about 0.6 eV, suggesting electron donation from Ni2+ to Ce4+ across the heterojunction. As depicted in Figure 2d, the O1s X-ray photoelectron spectroscopy (XPS) spectra decompose into three distinct peaks: O1, O2, and O3, which correspond to metal–oxygen bonds, oxygen vacancies, and adsorbed surface oxygen, respectively [31,32]. Particularly, Ni3N-CeO2/NF exhibits a higher proportion of the O2 peak compared to CeO2/NF, indicating an enhancement in oxygen vacancy formation due to the presence of Ni3N. Based on this, we conducted EPR tests on both Ni3N-CeO2/NF and CeO2. The comparative results indicate that the oxygen vacancy content in Ni3N-CeO2/NF is greater than that in CeO2, further confirming that the presence of Ni3N enhances the formation of oxygen vacancies and verifying the substantial oxygen vacancies in Ni3N-CeO2/NF (Figure 2e). Furthermore, the XPS N 1s spectra of Ni3N-CeO2/NF, presented in Figure 2f, exhibit two distinct peaks at 397.7 and 399.5 eV, which correspond, respectively, to N–Ni and N–H bonding [33,34].
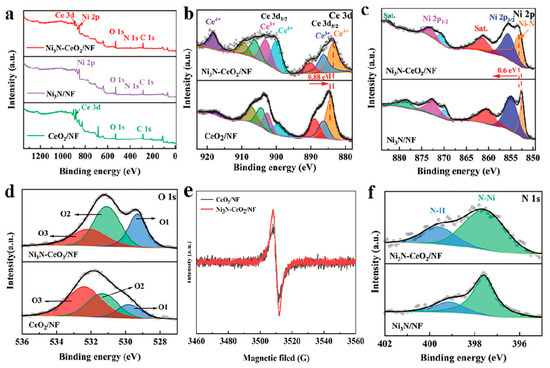
Figure 2.
XPS spectra of Ni3N-CeO2/NF. (a) Full spectrum. (b) Ce 3d, (c) Ni 2p, and (d) O 1s. (e) Room temperature EPR of Ni3N-CeO2 and CeO2. (f) N 1s.
3.2. Electrocatalytic Performance for HER
To investigate the influence of the Ce/Ni molar ratio on the HER activity, a variety of samples with varying ratios (Ni3N-xCeO2/NF) were synthesized. The sample Ni3N-2CeO2/NF, where Ce/Ni = 2, demonstrates optimal HER performance, as depicted in Figure 3f. Consequently, Ni3N-2CeO2/NF was selected for subsequent experiments and denoted as Ni3N–CeO2/NF.
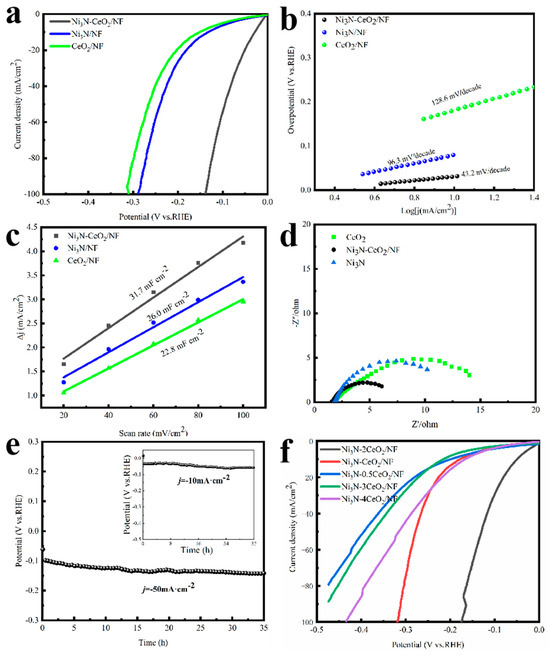
Figure 3.
HER performance in 1 M KOH. (a) LSV, (b) Tafel plots, (c) Cdl, (d) EIS, and (e) stability tests. (f) Molar ratio on HER activity.
In contrast to others, the Ni3N-CeO2/NF composite exhibits a markedly reduced overpotential of just 42 mV at 10 mA cm−2 and 115 mV at 50 mA cm−2. This is substantially lower compared to the overpotentials recorded for the pristine Ni3N (156 mV and 230 mV), and CeO2 (186 mV and 260 mV), as depicted in Figure 3a. To assess the kinetics of the HER, the Tafel slope was calculated [35]. Figure 3b illustrates through Tafel plots that Ni3N-CeO2/NF shows the smallest Tafel gradient at 43.2 mV decade−1, which implies rapid mass transfer kinetics. The Cdl, ascertained in the non-Faradaic region through cyclic voltammetry (Figure S5), was employed to assess the ECSA. The activated Ni3N-CeO2/NF exhibits the peak Cdl measurement of 31.7 mF cm−2, surpassing the levels of Ni3N (26.0 mF cm−2), and CeO2 (22.8 mF cm−2) (Figure 3c). Moreover, EIS was employed as an investigative tool to probe the reaction kinetics of the Ni3N-CeO2/NF composite, utilizing a three-electrode cell configuration in conjunction with a 1 M KOH electrolyte solution. When compared to others, Ni3N-CeO2/N exhibits a significant decrease in its charge-transfer resistance at an overpotential of 100 mV (Figure 3d). The findings from EIS indicate that Ni3N-CeO2/N demonstrates the quickest charge-transfer mechanism among the four catalysts, aligning well with its superior HER efficiency and minimal Tafel slope. The Ni3N-CeO2/NF composite exhibits robust stability, maintaining performance for 34 h at 50 mA cm−2 (Figure 3e). Post-stability testing, both SEM and XRD analyses (Figures S6 and S7), further confirmed its structural integrity [36].
3.3. Electrocatalytic Performance for OER
The sample Ni3N-2CeO2/NF, where Ce/Ni = 2, demonstrates optimal OER performance, as depicted in Figure 4f. Consequently, Ni3N-2CeO2/NF was selected for subsequent experiments and denoted as Ni3N–CeO2/NF. In contrast to others, the Ni3N-CeO2/NF composite exhibits a markedly reduced overpotential of just 350 mV at 50 mA cm−2. This is substantially lower compared to the overpotentials recorded for the pristine Ni3N (430 mV), and CeO2 (450 mV), as depicted in Figure 4a. The reverse scanned LSV curve is used to avoid oxidation peak effects during the OER and determine the overpotential of the electrocatalyst at a low-current density (Figure S8). To assess the kinetics of the OER, the Tafel slope was calculated [37]. Figure 4b illustrates through Tafel plots that Ni3N-CeO2/NF shows the smallest Tafel gradient at 65.2 mV decade−1. The electrochemical double-layer capacitances (Cdl), ascertained in the non-Faradaic region through cyclic voltammetry (Figure S9), were employed to assess the ECSA. The activated Ni3N-CeO2/NF exhibits the peak Cdl measurement of 32.3 mF cm−2, surpassing the levels of Ni3N (26.3 mF cm−2), and CeO2 (21.9 mF cm−2) (Figure 4c). Moreover, EIS was employed as an investigative tool to probe the reaction kinetics of the Ni3N-CeO2/NF composite, utilizing a three-electrode cell configuration in conjunction with a 1 M KOH electrolyte solution. When compared to others, Ni3N-CeO2/N exhibits a significant decrease in its charge-transfer resistance at an overpotential of 300 mV (Figure 4d). The findings from EIS indicate that Ni3N-CeO2/N demonstrates the quickest charge-transfer mechanism among the four catalysts, aligning well with its superior OER efficiency and minimal Tafel slope. The Ni3N-CeO2/NF composite demonstrates substantial stability, sustaining performance for 40 h at 50 mA cm−2, as illustrated in Figure 4e. Subsequent stability assessments using SEM and XRD (Figures S10 and S11) further verified its enduring stability, with no detectable structural alterations [38].
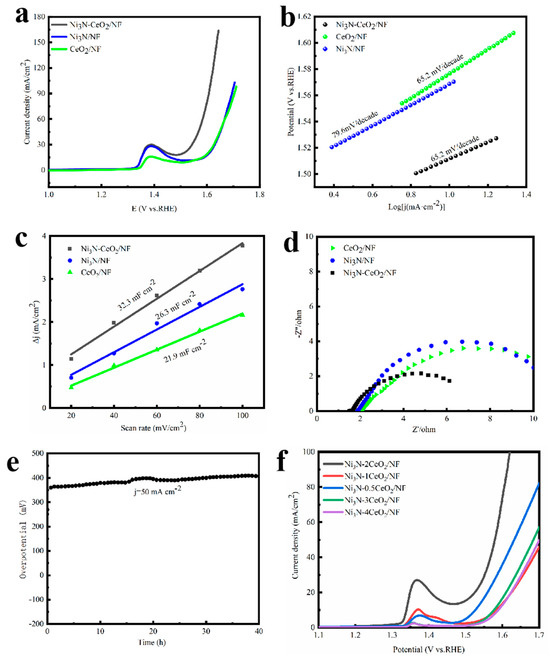
Figure 4.
OER performance in 1 M KOH. (a) LSV, (b) Tafel plots, (c) Cdl, (d) EIS, and (e) stability tests. (f) Molar ratio on OER activity.
3.4. In Situ Raman Spectroscopy
Prior studies have demonstrated that metal nitrides typically undergo a phase transformation during oxygen evolution reaction (OER) testing, thereby generating the genuine active sites of metal oxyhydroxides. In this context, we conducted in situ Raman spectroscopic analyses to investigate the structural dynamics of Ni3N-CeO2/NF post-OER testing. The in situ Raman spectra depicted in Figure 5a reveal that upon elevating the voltage to 1.34 V relative to the reversible hydrogen electrode, the peak initially located at 544 cm−1 progressively shifts to 553 cm−1, signaling the onset of oxidation in Ni3N, corroborating the peak observed (Figure 5a) in the linear sweep voltammetry. Upon increasing the voltage to 1.39 V, two distinct peaks are observed at 476 and 558 cm−1, which are attributed to the Eg and A1g vibrational bands of NiOOH, respectively. A comparative analysis of the NiOOH vibrational peak at 558 cm−1 in Figure 5a,b reveals that when CeO2 is present, the half-peak width of NiOOH increases by approximately 20%. This suggests that CeO2 promotes the development of NiOOH that is enriched with a higher density of oxygen vacancies.
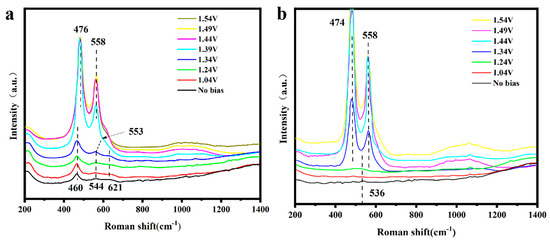
Figure 5.
(a,b) In situ Raman spectroscopy of Ni3N–CeO2/NF and Ni3N/NF at various potentials.
3.5. Overall Water Splitting in an Alkaline Electrolyte
Given the superior electrochemical properties of the HER and OER, Ni3N-CeO2/NF, once activated, acts as dual-function catalysts, facilitating the formation of a two-electrode system for comprehensive water division (Figure 6a) [39,40]. At 10 mA cm−2, Ni3N-CeO2/NF||Ni3N-CeO2/NF demonstrates a low cell voltage of 1.52 V in 1 M KOH at 25 °C (Figure 6a). It is observed that Ni3N-CeO2/NF’s efficiency surpasses that of Pt/C/NF||IrO2/NF (1.63 V) and other previously documented electrocatalysts, underscoring the feasibility of effective overall water splitting (Figure 6c) [41,42,43]. Furthermore, the battery has demonstrated remarkable steadiness. At 10 mA cm−2, the battery can sustain its current density for over 34 h without experiencing voltage loss, and it retains 93.52% of it (Figure 6b).
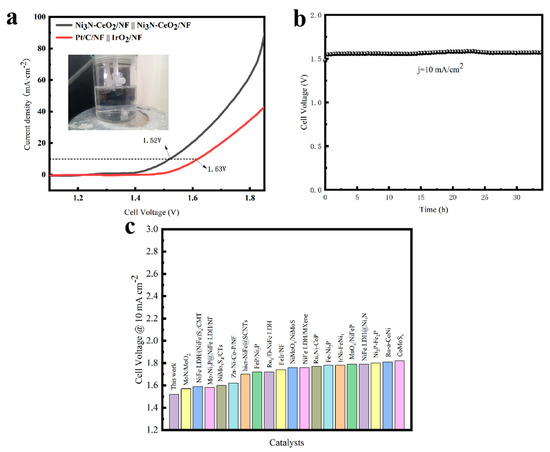
Figure 6.
Overall water-splitting performance. (a) Polarization curves. (b) Stability tests. (c) Comparison of potential with other electrocatalysts at 10 mA cm−2.
4. Conclusions
In conclusion, we have developed bifunctional heterojunction catalysts containing abundant oxygen vacancies. The material demonstrates superior catalytic proficiency in the context of water electrolysis, achieving remarkably low overpotentials of merely 42 mV at 10 mA cm−2 for the HER. Particularly, the Ni3N-CeO2/NF exhibits an ultra-low overpotential for the OER (η50 = 350 mV). Through EPR testing, we can see that the catalyst also has abundant oxygen vacancies. The in situ Raman analysis distinctly demonstrated that cerium dioxide (CeO2) significantly enhanced the formation of nickel oxyhydroxide (NiOOH) with a high concentration of oxygen vacancies (Ov). This study furnishes innovative perspectives that may inform the future conceptualization of electrocatalysts characterized by enhanced activity, stability, and bifunctionality for the application of water electrolysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano14110935/s1, Figure S1. XRD of Ni(OH)2-CeO2/NF and SEM image of Ni(OH)2-CeO2/NF. Figure S2. XRD of Ni3N/NF and SEM image of Ni3N/NF. Figure S3. XRD of CeO2 and SEM image of CeO2. Figure S4. The corresponding energy dispersive Xtray (EDX) images of the Ni3N-CeO2/NF. Figure S5. CV curves of at 0.125-0.225 V vs RHE with the non-Faradaic potential regions from 20-100 mV/s for (a) Ni3N-CeO2/NF, (b) Ni3N/NF, and (c) CeO2/NF. Figure S6. SEM images of Ni3N-CeO2/NF after HER stability test. Figure S7. XRD images of Ni3N-CeO2/NF after HER stability test. Figure S8. Backscatter polarization curves of OER for Ni3N-CeO2/NF, Ni3N/NF and CeO2/NF. Figure S9. CV curves of at 0.125-0.225 V vs RHE with the non-Faradaic potential regions from 20-100 mV/s for (a) Ni3N-CeO2/NF, (b) Ni3N/NF, and (c) CeO2/NF. Figure S10. SEM images of Ni3N-CeO2/NF after HER stability test. Figure S11. XRD images of Ni3N-CeO2/NF after HER stability test.
Author Contributions
Conceptualization, X.M.; Methodology, X.M.; Formal analysis, X.Z., Y.M. and Q.X.; Investigation, X.M.; Resources, Q.L.; Writing—original draft, X.M.; Writing—review & editing, Q.L.; Supervision, Q.L.; Project administration, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge funding from the National Science Foundation of China (grant numbers 22172098 and 91745112) and the Science and Technology Commission of Shanghai Municipality (23ZR1424900, 22010501200, 21ZR1425000, and 19DZ2271100).
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, Q.; Zhou, Y.; Shou, H.; Wang, X.; Zhang, P.; Xu, W.; Qiao, S.; Wu, C.; Liu, H.; Liu, D.; et al. Synergic Reaction Kinetics over Adjacent Ruthenium Sites for Superb Hydrogen Generation in Alkaline Media. Adv. Mater. 2022, 34, e2110604. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Qu, J.; Xiao, Y.; Zhao, S.; Chen, H.; Dai, L. Carbon Nanomaterials for Energy and Biorelated Catalysis: Recent Advances and Looking Forward. ACS Cent. Sci. 2019, 5, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, T.J.; Fjällström, V.; Sahlberg, M.; Edoff, M.; Edvinsson, T. A monolithic device for solar water splitting based on series interconnected thin film absorbers reaching over 10% solar-to-hydrogen efficiency. Energy Environ. Sci. 2013, 6, 3676–3683. [Google Scholar] [CrossRef]
- Ji, Q.; Kong, Y.; Tan, H.; Duan, H.; Li, N.; Tang, B.; Wang, Y.; Feng, S.; Lv, L.; Wang, C.; et al. Operando Identification of Active Species and Intermediates on Sulfide Interfaced by Fe3O4 for Ultrastable Alkaline Oxygen Evolution at Large Current Density. ACS Catal. 2022, 12, 4318–4326. [Google Scholar] [CrossRef]
- Jiang, M.; Zhai, H.; Chen, L.; Mei, L.; Tan, P.; Yang, K.; Pan, J. Unraveling the Synergistic Mechanism of Bi-Functional Nickel–Iron Phosphides Catalysts for Overall Water Splitting. Adv. Funct. Mater. 2023, 33, 2302621. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Wu, Y.; Zhang, G.; Wu, H.; Lyu, F.; He, J.; Fan, J.; Lu, J.; Li, Y.Y. Identifying Fe as OER Active Sites and Ultralow-Cost Bifunctional Electrocatalysts for Overall Water Splitting. Small 2023, 19, e2301715. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of Heterostructure Materials in Energy Storage: A Review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Q.; Xie, L.; Zeng, H.; Zhou, S.; Huang, Y.; Yan, M.; Zhang, X.; Liu, T.; Zeng, J.; et al. Superassembly of Surface-Enriched Ru Nanoclusters from Trapping–Bonding Strategy for Efficient Hydrogen Evolution. ACS Nano 2022, 16, 7993–8004. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, T.; Zhang, J.; Zheng, X.; Mao, J.; Liu, H.; Li, Y.; Hao, Q. Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution. Appl. Catal. B Environ. 2020, 262, 118245. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Liu, X.; Lu, H.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Xu, W.; Liang, Y.; Long, G.; Jiang, H. Alloying-Triggered Phase Engineering of NiFe System via Laser-Assisted Al Incorporation for Full Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202300800. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yu, Y.; Wu, Y.; Ma, Z.; Ma, X.; Jiang, Y.; Shen, W.; He, R.; Su, W.; Li, M. Realizing efficient oxygen evolution at low overpotential via dopant-induced interfacial coupling enhancement effect. Appl. Catal. B Environ. 2023, 336, 122928. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Xu, X.M.; Pan, Y.L.; Ge, L.; Chen, Y.B.; Mao, X.; Guan, D.Q.; Li, M.R.; Zhong, Y.J.; Hu, Z.W.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.-J.; Park, N.; Jeong, H.Y.; Baek, J.-B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Mir, Z.M.; Bastos, A.; Höche, D.; Zheludkevich, M.L. Recent Advances on the Application of Layered Double Hydroxides in Concrete—A Review. Materials 2020, 13, 1426. [Google Scholar] [CrossRef]
- Ojha, K.; Saha, S.; Kumar, B.; Hazra, K.S.; Ganguli, A.K. Controlling the Morphology and Efficiency of Nanostructured Molybdenum Nitride Electrocatalysts for the Hydrogen Evolution Reaction. ChemCatChem 2016, 8, 1218–1225. [Google Scholar] [CrossRef]
- Ortel, E.; Reier, T.; Strasser, P.; Kraehnert, R. Mesoporous IrO2 Films Templated by PEO-PB-PEO Block-Copolymers: Self-Assembly, Crystallization Behavior, and Electrocatalytic Performance. Chem. Mater. 2011, 23, 3201–3209. [Google Scholar] [CrossRef]
- Xia, B.; Wang, T.; Chi, X.; Yu, X.; Liu, P.; Zhang, J.; Xi, S.; Du, Y.; Gao, D. Anion vacancy-mediated ferromagnetism in atomic-thick Ni3N nanosheets. Appl. Phys. Lett. 2017, 111, 232402. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Zhao, H.; Zhang, Y.; Huang, Y.; Yin, Z.; Du, Y. Regulating the active species of Ni(OH)2 using CeO2: 3D CeO2/Ni(OH)2/carbon foam as an efficient electrode for the oxygen evolution reaction. Chem. Sci. 2017, 8, 3211–3217. [Google Scholar] [CrossRef]
- Park, S.; Shviro, M.; Hartmann, H.; Besmehn, A.; Mayer, J.; Stolten, D.; Carmo, M. Nickel Structures as a Template Strategy to Create Shaped Iridium Electrocatalysts for Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 13576–13585. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Chen, J.; Yu, T.; Liu, J.; Luo, L.; Yin, S. Three-Phase Heterojunction NiMo-Based Nano-Needle for Water Splitting at Industrial Alkaline Condition. Nano-Micro Lett. 2021, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Dai, R.; Mateen, M.; Hassan, Z.; Zhuang, Z.; Liu, C.; Israr, M.; Cheong, W.C.; Hu, B.; Tu, R.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. Int. Ed. 2021, 61, e202114951. [Google Scholar] [CrossRef]
- Shen, S.; Wang, Z.; Lin, Z.; Song, K.; Zhang, Q.; Meng, F.; Gu, L.; Zhong, W. Crystalline-Amorphous Interfaces Coupling of CoSe2/CoP with Optimized d-Band Center and Boosted Electrocatalytic Hydrogen Evolution. Adv. Mater. 2022, 34, 2110631. [Google Scholar] [CrossRef]
- Singh, B.; Gawande, M.B.; Kute, A.D.; Varma, R.S.; Fornasiero, P.; McNeice, P.; Jagadeesh, R.V.; Beller, M.; Zbořil, R. Single-Atom (Iron-Based) Catalysts: Synthesis and Applications. Chem. Rev. 2021, 121, 13620–13697. [Google Scholar] [CrossRef]
- Su, P.; Pei, W.; Wang, X.; Ma, Y.; Jiang, Q.; Liang, J.; Zhou, S.; Zhao, J.; Liu, J.; Lu, G.Q. Exceptional Electrochemical HER Performance with Enhanced Electron Transfer between Ru Nanoparticles and Single Atoms Dispersed on a Carbon Substrate. Angew. Chem. Int. Ed. 2021, 60, 16044–16050. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3 nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, X.; Zhang, W.; Hu, J.; Gao, Q. Ceria-Promoted Reconstruction of Ni-Based Electrocatalysts toward Efficient Oxygen Evolution. ACS Catal. 2022, 12, 13951–13960. [Google Scholar] [CrossRef]
- Tan, X.; Geng, S.; Ji, Y.; Shao, Q.; Zhu, T.; Wang, P.; Li, Y.; Huang, X. Closest Packing Polymorphism Interfaced Metastable Transition Metal for Efficient Hydrogen Evolution. Adv. Mater. 2020, 32, e2002857. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.D.; Chuong, N.D.; Hien, H.V.; Kshetri, T.; Tuan, L.H.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85. [Google Scholar] [CrossRef]
- Tyndall, D.; Craig, M.J.; Gannon, L.; McGuinness, C.; McEvoy, N.; Roy, A.; García-Melchor, M.; Browne, M.P.; Nicolosi, V. Demonstrating the source of inherent instability in NiFe LDH-based OER electrocatalysts. J. Mater. Chem. A 2023, 11, 4067–4077. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B.; et al. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, M.; Zhang, J.; Chen, Z.; Zheng, X.; Tian, Z.; Zhao, N.; Han, X.; Zaghib, K.; et al. Highly Active and Durable Single-Atom Tungsten-Doped NiS0.5Se0.5 Nanosheet NiS0.5Se0.5 Nanorod Heterostructures for Water Splitting. Adv. Mater. 2022, 34, 2107053. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Gong, Y.; Liu, D.; Liu, S.; Xiao, W.; Xiao, Z.; Li, Z.; Wu, Z.; Wang, L. Amorphous high-valence Mo-doped NiFeP nanospheres as efficient electrocatalysts for overall water-splitting under large-current density. Chem. Eng. J. 2023, 468, 143833. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, B.; Lin, Z.; Xu, Y.; Lin, Y.; Meng, F.; Zhang, Q.; Gu, L.; Fang, B.; Guo, S.; et al. PtSe2/Pt Heterointerface with Reduced Coordination for Boosted Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 23388–23393. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zou, W.; Tang, H. Nb-Doped nickel nitride-derived catalysts for electrochemical water splitting. Catal. Sci. Technol. 2021, 11, 6455–6461. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).