Enhancing the Stability and Initial Coulombic Efficiency of Silicon Anodes through Coating with Glassy ZIF-4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Material Characterization
2.3. Electrochemical Characterization
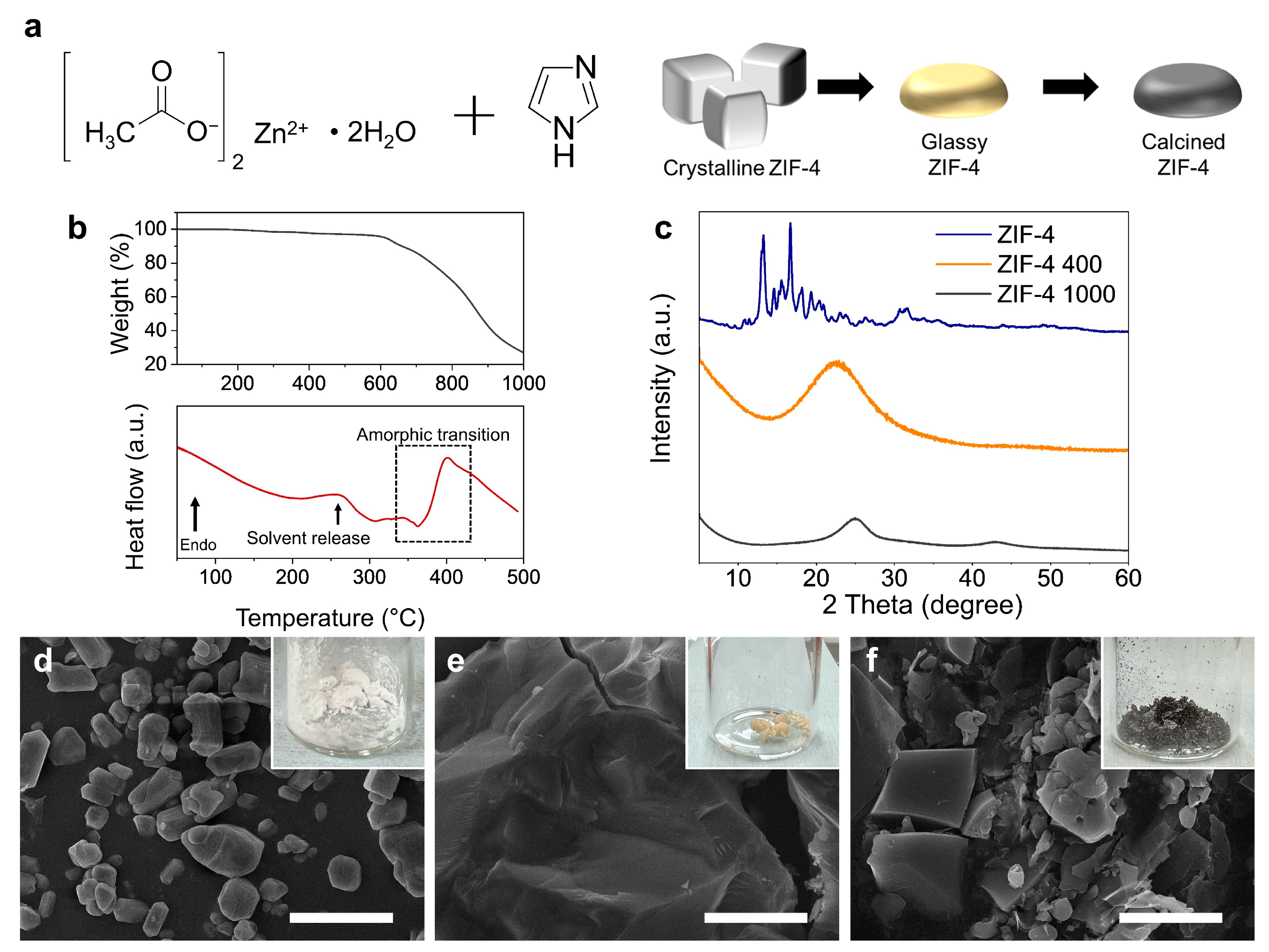
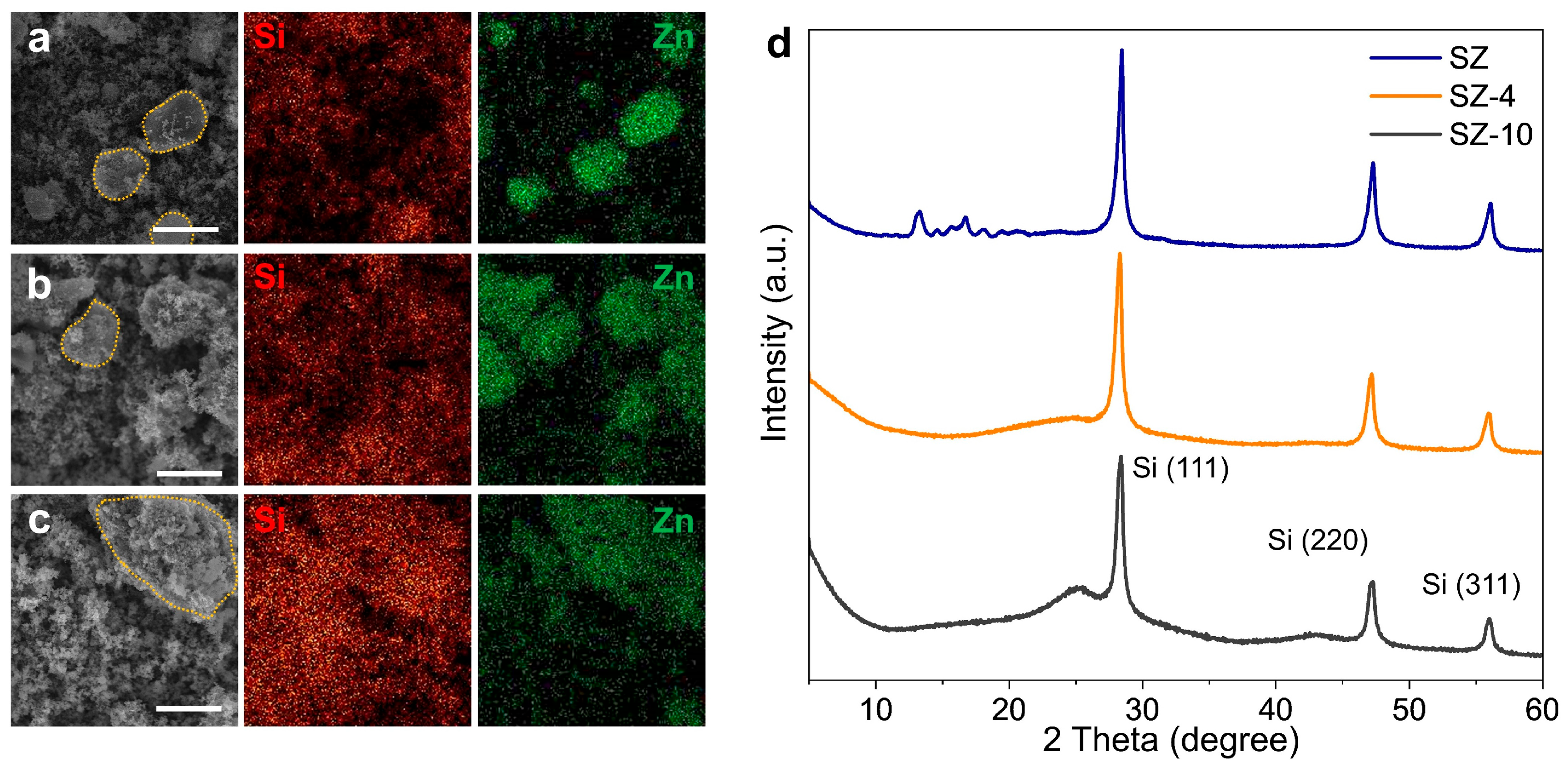
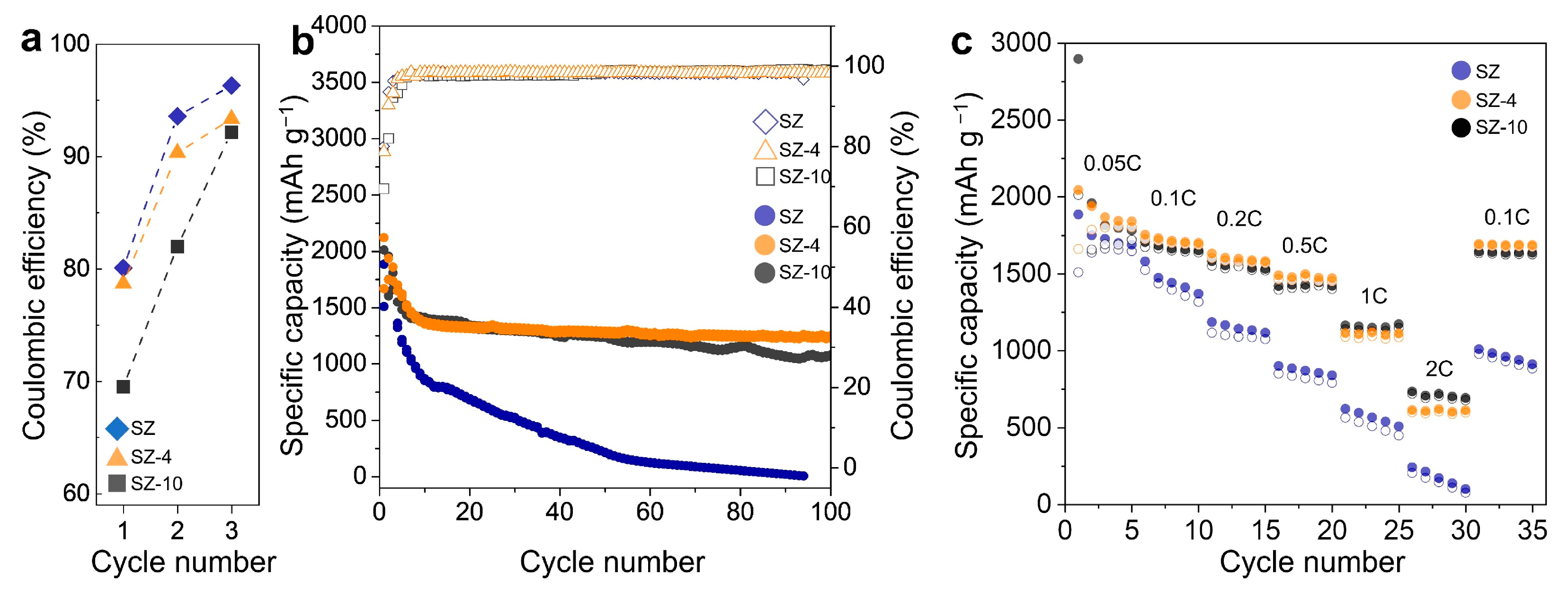
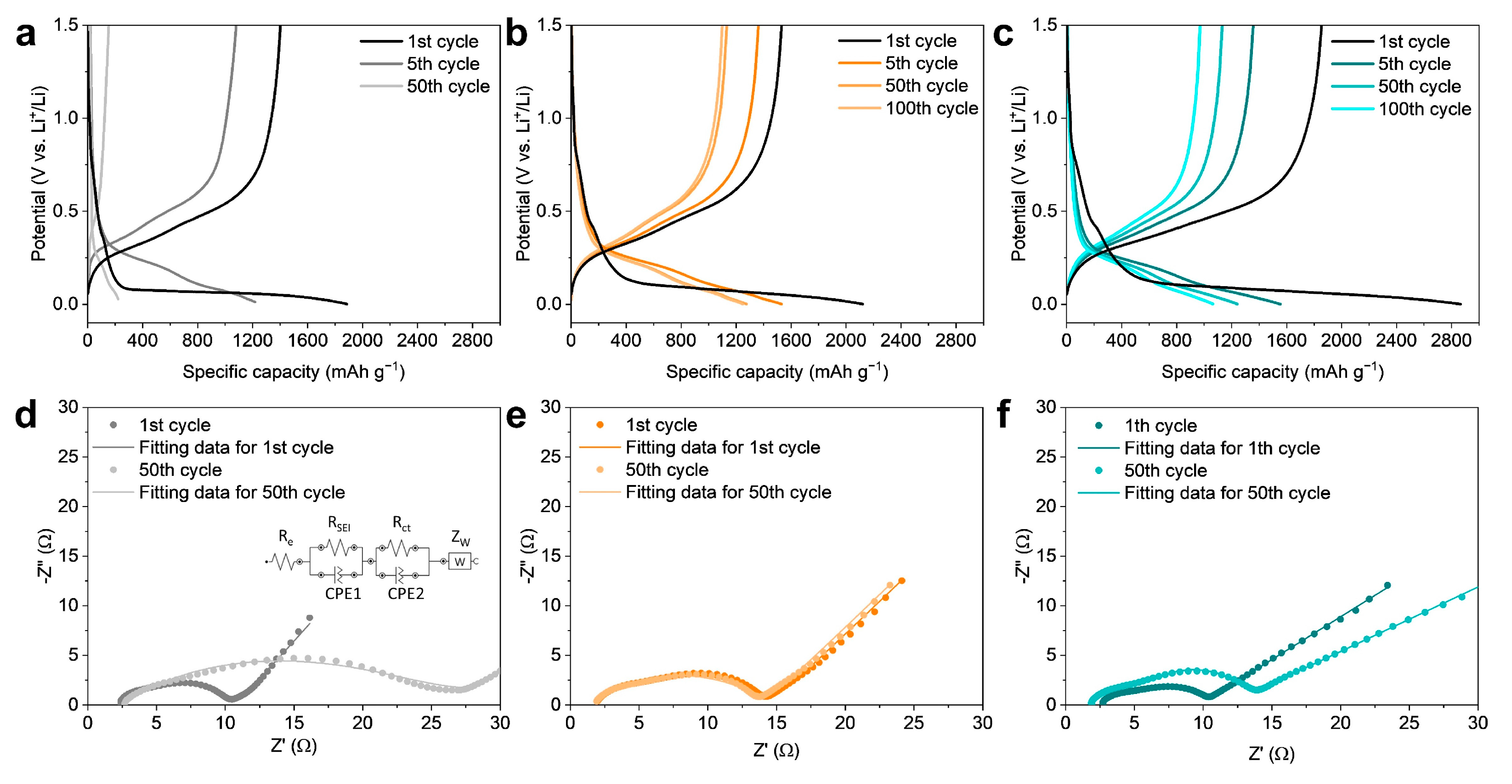
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103. [Google Scholar] [CrossRef]
- Ozanam, F.; Rosso, M. Silicon as anode material for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 2–11. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.T.; Lee, S.W.; Harris, J.T.; Korgel, B.A.; Wang, C.; Nix, W.D.; Cui, Y. In Situ TEM of Two-Phase Lithiation of Amorphous Silicon Nanospheres. Nano Lett. 2013, 13, 758–764. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; Vullum, P.E.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon-Carbon composite anodes from industrial battery grade silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles during Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Lu, Z.; Wong, T.; Ng, T.-W.; Wang, C. Facile synthesis of carbon decorated silicon nanotube arrays as anode material for high-performance lithium-ion batteries. RSC Adv. 2014, 4, 2440–2446. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, X.; Chen, L.; Yue, J.; Xu, H.; Yang, J.; Qian, Y. Novel mesoporous silicon nanorod as an anode material for lithium ion batteries. Electrochim. Acta 2014, 127, 252–258. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, Y.; Zheng, X.; Or, T.; Ma, Q.; Qian, L.; Deng, Y.; Yu, A.; Li, J.; Chen, Z. Design Criteria for Silicon-Based Anode Binders in Half and Full Cells. Adv. Energy Mater. 2022, 12, 2200850. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.-K. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Figgemeier, E. Confronting the Challenges of Next-Generation Silicon Anode-Based Lithium-Ion Batteries: Role of Designer Electrolyte Additives and Polymeric Binders. ChemSusChem 2019, 12, 2515–2539. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zheng, X.; Qu, Q.; Huang, Y.; Zheng, H. Electrolyte Design Enabling a High-Safety and High-Performance Si Anode with a Tailored Electrode–Electrolyte Interphase. Adv. Mater. 2021, 33, 2103178. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhai, W.; Sun, Q.; Ai, Q.; Li, J.; Cheng, J.; Dai, L.; Ci, L. Facilely tunable core-shell Si@SiOx nanostructures prepared in aqueous solution for lithium ion battery anode. Electrochim. Acta 2020, 342, 136068. [Google Scholar] [CrossRef]
- Murugesan, S.; Harris, J.T.; Korgel, B.A.; Stevenson, K.J. Copper-Coated Amorphous Silicon Particles as an Anode Material for Lithium-Ion Batteries. Chem. Mater. 2012, 24, 1306–1315. [Google Scholar] [CrossRef]
- Mei, S.; Guo, S.; Xiang, B.; Deng, J.; Fu, J.; Zhang, X.; Zheng, Y.; Gao, B.; Chu, P.K.; Huo, K. Enhanced ion conductivity and electrode–electrolyte interphase stability of porous Si anodes enabled by silicon nitride nanocoating for high-performance Li-ion batteries. J. Energy Chem. 2022, 69, 616–625. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Li, C.-H.; Wang, D.-Y.; Chen, C.-C. Chemical doping of a core–shell silicon nanoparticles@polyaniline nanocomposite for the performance enhancement of a lithium ion battery anode. Nanoscale 2016, 8, 1280–1287. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, S.; Wang, H.; Qian, J.; Yang, H.; Ai, X.; Liu, J. Surface-Bound Silicon Nanoparticles with a Planar-Oriented N-Type Polymer for Cycle-Stable Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2019, 11, 13251–13256. [Google Scholar] [CrossRef] [PubMed]
- Nzabahimana, J.; Liu, Z.; Guo, S.; Wang, L.; Hu, X. Top-Down Synthesis of Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries: Mechanical Milling and Etching. ChemSusChem 2020, 13, 1923–1946. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tian, Z.; Fan, R.; Shao, L.; Zhang, D.; Cao, G.; Kou, L.; Bai, Y. Research progress on silicon/carbon composite anode materials for lithium-ion battery. J. Energy Chem. 2018, 27, 1067–1090. [Google Scholar] [CrossRef]
- Chae, S.; Xu, Y.; Yi, R.; Lim, H.-S.; Velickovic, D.; Li, X.; Li, Q.; Wang, C.; Zhang, J.-G. A Micrometer-Sized Silicon/Carbon Composite Anode Synthesized by Impregnation of Petroleum Pitch in Nanoporous Silicon. Adv. Mater. 2021, 33, 2103095. [Google Scholar] [CrossRef] [PubMed]
- Rage, B.; Delbegue, D.; Louvain, N.; Lippens, P.-E. Engineering of Silicon Core-Shell Structures for Li-ion Anodes. Chem. Eur. J. 2021, 27, 16275–16290. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Miao, C.; He, Y.-B.; Liang, G.; Zhou, D.; Liu, M.; Han, C.; Li, B.; Kang, F. A honeycomb-cobweb inspired hierarchical core–shell structure design for electrospun silicon/carbon fibers as lithium-ion battery anodes. Carbon 2016, 98, 582–591. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Y.; He, L.; Zhu, Y.; Tian, Q.; Guo, X.; Chen, J.; Li, L.; Wang, Q.; Song, G.; et al. Scalable synthesis of nano-Si embedded in porous C and its enhanced performance as anode of Li-ion batteries. Electrochim. Acta 2017, 249, 166–172. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial Coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Cho, K.Y.; An, H.; Do, X.H.; Choi, K.; Yoon, H.G.; Jeong, H.-K.; Lee, J.S.; Baek, K.-Y. Synthesis of amine-functionalized ZIF-8 with 3-amino-1,2,4-triazole by postsynthetic modification for efficient CO2-selective adsorbents and beyond. J. Mater. Chem. A 2018, 6, 18912–18919. [Google Scholar] [CrossRef]
- Li, H.-H.; Saini, A.; Xu, R.-Y.; Wang, N.; Lv, X.-X.; Wang, Y.-P.; Yang, T.; Chen, L.; Jiang, H.-B. Hierarchical Fe3O4@C nanofoams derived from metal–organic frameworks for high-performance lithium storage. Rare Met. 2020, 39, 1072–1081. [Google Scholar] [CrossRef]
- Jia, H.; Yao, Y.; Zhao, J.; Gao, Y.; Luo, Z.; Du, P. A novel two-dimensional nickel phthalocyanine-based metal–organic framework for highly efficient water oxidation catalysis. J. Mater. Chem. A 2018, 6, 1188–1195. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Yu, X.; Tang, S.; Ozawa, K.; Sasaki, T.; Qin, L.-C. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy 2020, 70, 104444. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Dai, K.; Feng, Y. Silicon particles coated with a metal–organic framework as anodes for enhanced lithium-ion batteries. New J. Chem. 2022, 46, 21756–21761. [Google Scholar] [CrossRef]
- Shi, Z.; Arramel, A.; Bennett, T.D.; Yue, Y.; Li, N. The deformation of short-range order leading to rearrangement of topological network structure in zeolitic imidazolate framework glasses. iScience 2022, 25, 104351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Qu, C.; Xu, F.; Zhang, E.; Lu, Q.; Cai, X.; Hausdorf, S.; Wang, H.; Kaskel, S. Glassy Metal–Organic-Framework-Based Quasi-Solid-State Electrolyte for High-Performance Lithium-Metal Batteries. Adv. Funct. Mater. 2021, 31, 2104300. [Google Scholar] [CrossRef]
- Hovestadt, M.; Vargas Schmitz, J.; Weissenberger, T.; Reif, F.; Kaspereit, M.; Schwieger, W.; Hartmann, M. Scale-up of the Synthesis of Zeolitic Imidazolate Framework ZIF-4. Chem. Ing. Tech. 2017, 89, 1374–1378. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Li, J.; Fan, S.; Xiu, H.; Wu, H.; Huang, S.; Wang, S.; Yin, D.; Deng, Z.; Xiong, C. TiO2-Coated Silicon Nanoparticle Core-Shell Structure for High-Capacity Lithium-Ion Battery Anode Materials. Nanomaterials 2023, 13, 1144. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Qiao, Y.; Peng, G.; Ren, Y.; Xie, Z.; Qu, M. Pomegranate-Like Structured Si@SiOx Composites With High-Capacity for Lithium-Ion Batteries. Front. Chem. 2020, 8, 666. [Google Scholar] [CrossRef]
- Hovestadt, M.; Schwegler, J.; Schulz, P.S.; Hartmann, M. Synthesis of the zeolitic imidazolate framework ZIF-4 from the ionic liquid 1-butyl-3-methylimidazolium imidazolate. J. Chem. Phys. 2018, 148, 193837. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, M.; Sheikhshoaie, I.; Beitollahi, H. Electrochemical sensor based on Fe3O4/ZIF-4 nanoparticles for determination of bisphenol A. J. Food Meas. Charact. 2023, 17, 1109–1118. [Google Scholar] [CrossRef]
- Rajan, A.S.; Sampath, S.; Shukla, A.K. An in situ carbon-grafted alkaline iron electrode for iron-based accumulators. Energy Environ. Sci. 2014, 7, 1110–1116. [Google Scholar] [CrossRef]
- Sivakumar, A.; Jude Dhas, S.S.; Pazhanivel, T.; Almansour, A.I.; Kumar, R.S.; Arumugam, N.; Raj, C.J.; Dhas, S.A.M.B. Phase Transformation of Amorphous to Crystalline of Multiwall Carbon Nanotubes by Shock Waves. Cryst. Growth Des. 2021, 21, 1617–1624. [Google Scholar] [CrossRef]
- Eom, G.H.; Han, S.A.; Suh, J.H.; Kim, J.H.; Park, M.-S. Enriched Cavities to ZIF-8-Derived Porous Carbon for Reversible Metallic Lithium Storage. ACS Appl. Energy Mater. 2021, 4, 14520–14525. [Google Scholar] [CrossRef]
- Sivonxay, E.; Aykol, M.; Persson, K.A. The lithiation process and Li diffusion in amorphous SiO2 and Si from first-principles. Electrochim. Acta 2020, 331, 135344. [Google Scholar] [CrossRef]
- Tritsaris, G.A.; Zhao, K.; Okeke, O.U.; Kaxiras, E. Diffusion of Lithium in Bulk Amorphous Silicon: A Theoretical Study. J. Phys. Chem. C 2012, 116, 22212–22216. [Google Scholar] [CrossRef]
- Moon, J.; Lee, B.; Cho, M.; Cho, K. Ab initio and kinetic Monte Carlo simulation study of lithiation in crystalline and amorphous silicon. J. Power Sources 2014, 272, 1010–1017. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.-H. Systematic electrochemical characterizations of Si and SiO anodes for high-capacity Li-Ion batteries. J. Power Sources 2019, 413, 20–28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-H.; Jin, Y.; Jang, H.-S.; Whang, D. Enhancing the Stability and Initial Coulombic Efficiency of Silicon Anodes through Coating with Glassy ZIF-4. Nanomaterials 2024, 14, 18. https://doi.org/10.3390/nano14010018
Lee I-H, Jin Y, Jang H-S, Whang D. Enhancing the Stability and Initial Coulombic Efficiency of Silicon Anodes through Coating with Glassy ZIF-4. Nanomaterials. 2024; 14(1):18. https://doi.org/10.3390/nano14010018
Chicago/Turabian StyleLee, In-Hwan, Yongsheng Jin, Hyeon-Sik Jang, and Dongmok Whang. 2024. "Enhancing the Stability and Initial Coulombic Efficiency of Silicon Anodes through Coating with Glassy ZIF-4" Nanomaterials 14, no. 1: 18. https://doi.org/10.3390/nano14010018
APA StyleLee, I.-H., Jin, Y., Jang, H.-S., & Whang, D. (2024). Enhancing the Stability and Initial Coulombic Efficiency of Silicon Anodes through Coating with Glassy ZIF-4. Nanomaterials, 14(1), 18. https://doi.org/10.3390/nano14010018






