Mechanism Exploration of the Effect of Polyamines on the Polishing Rate of Silicon Chemical Mechanical Polishing: A Study Combining Simulations and Experiments
Abstract
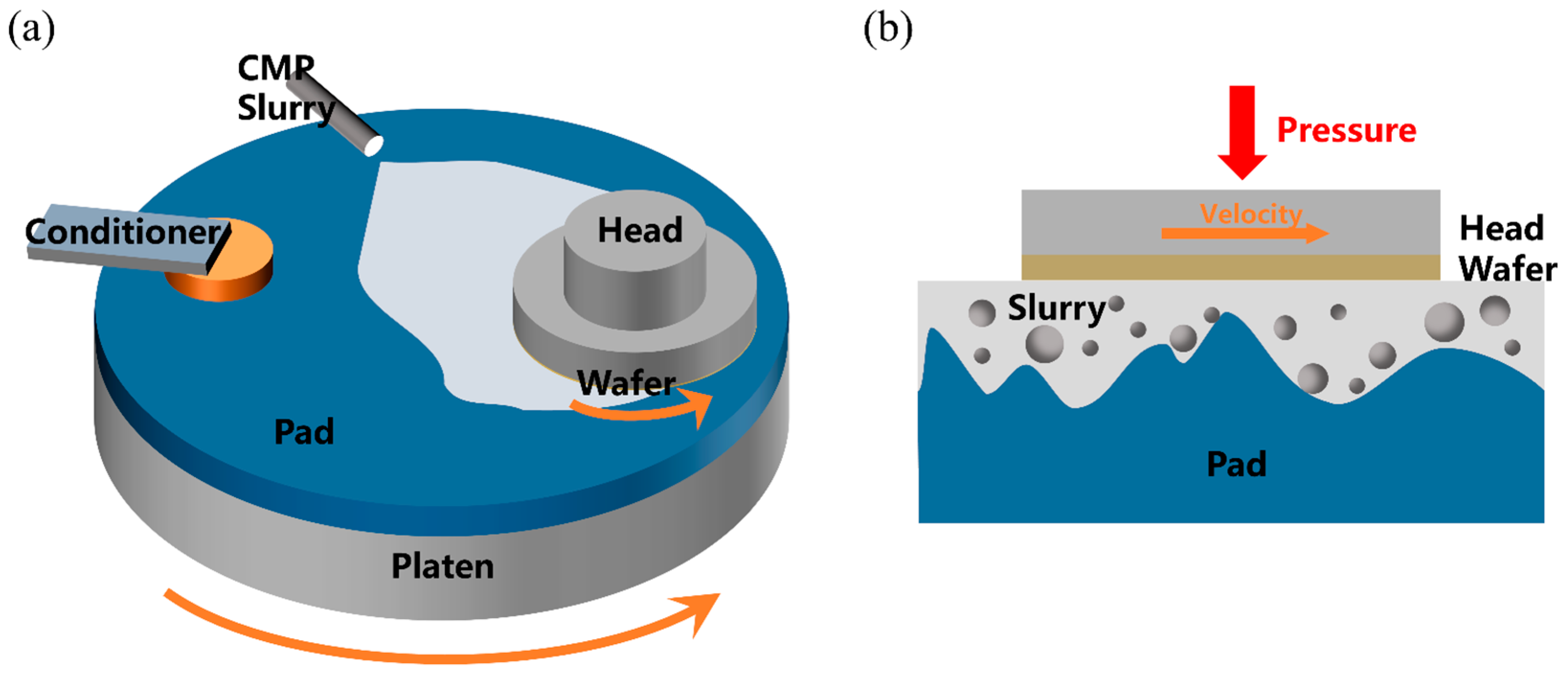
1. Introduction
2. Materials and Methods
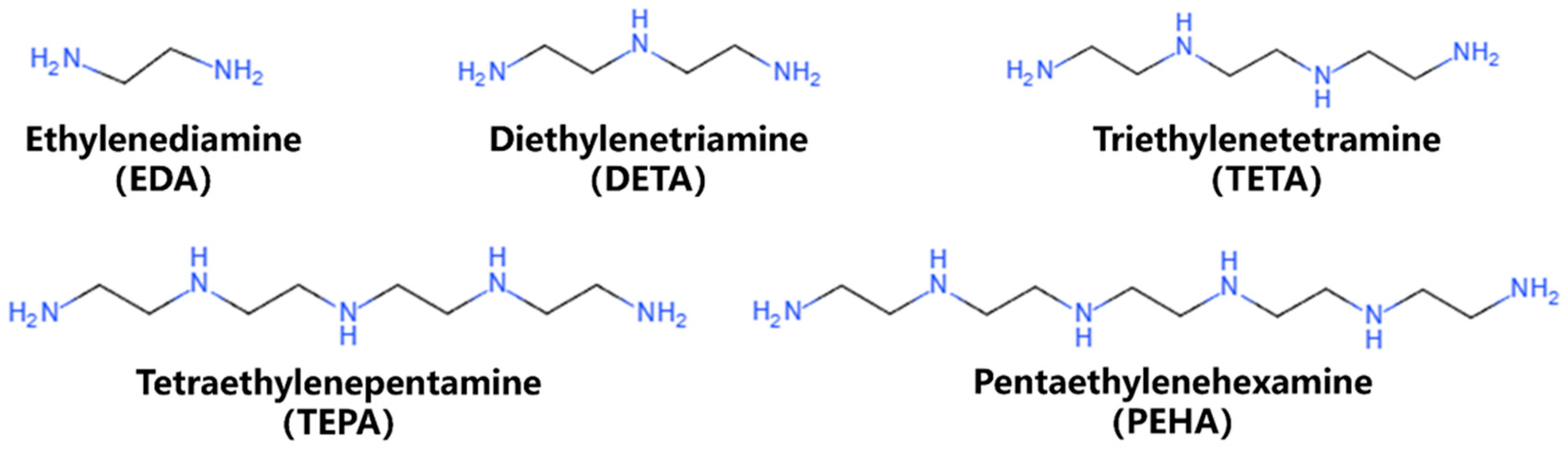
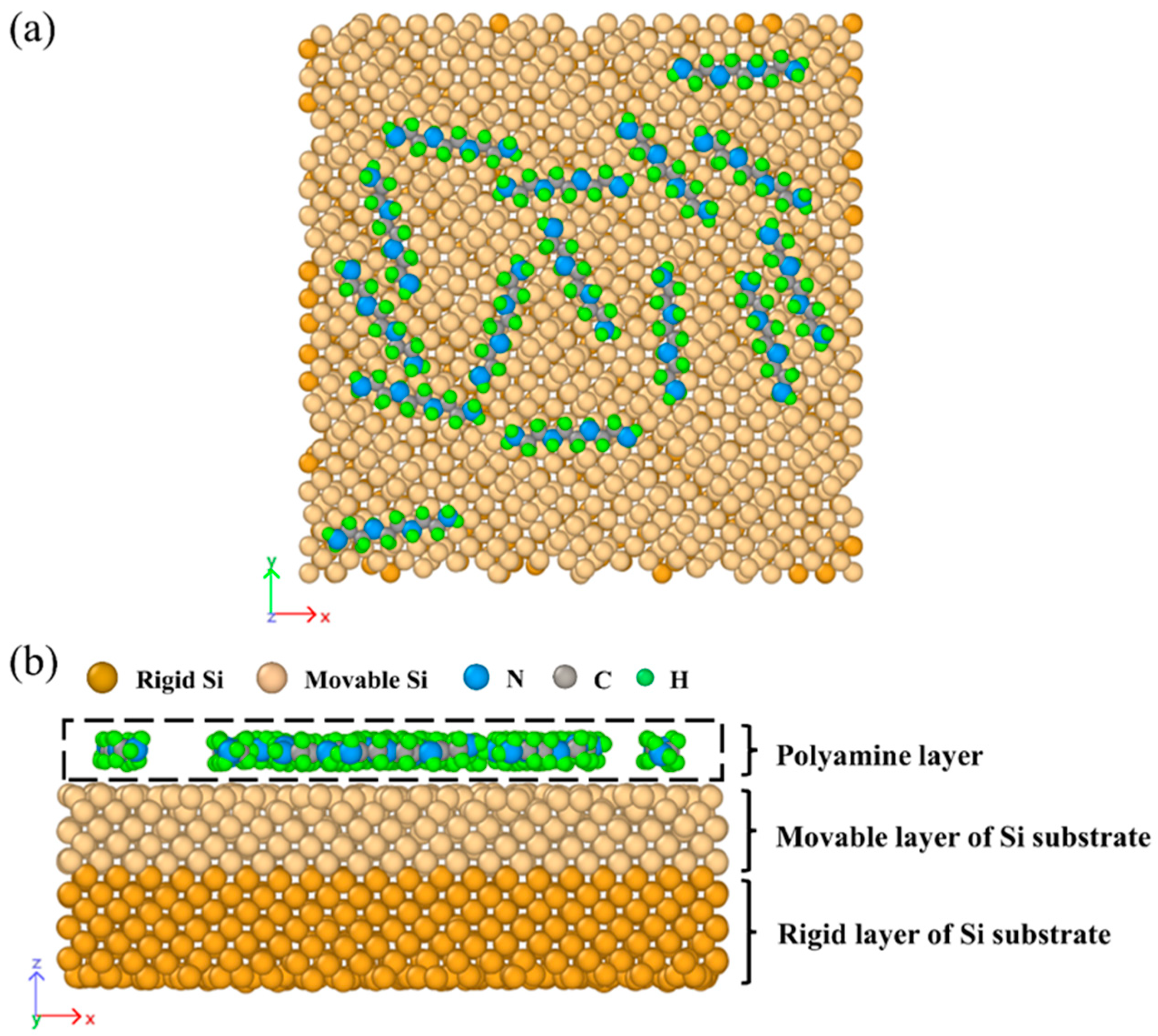
2.1. Model Construction
2.2. Simulation Details
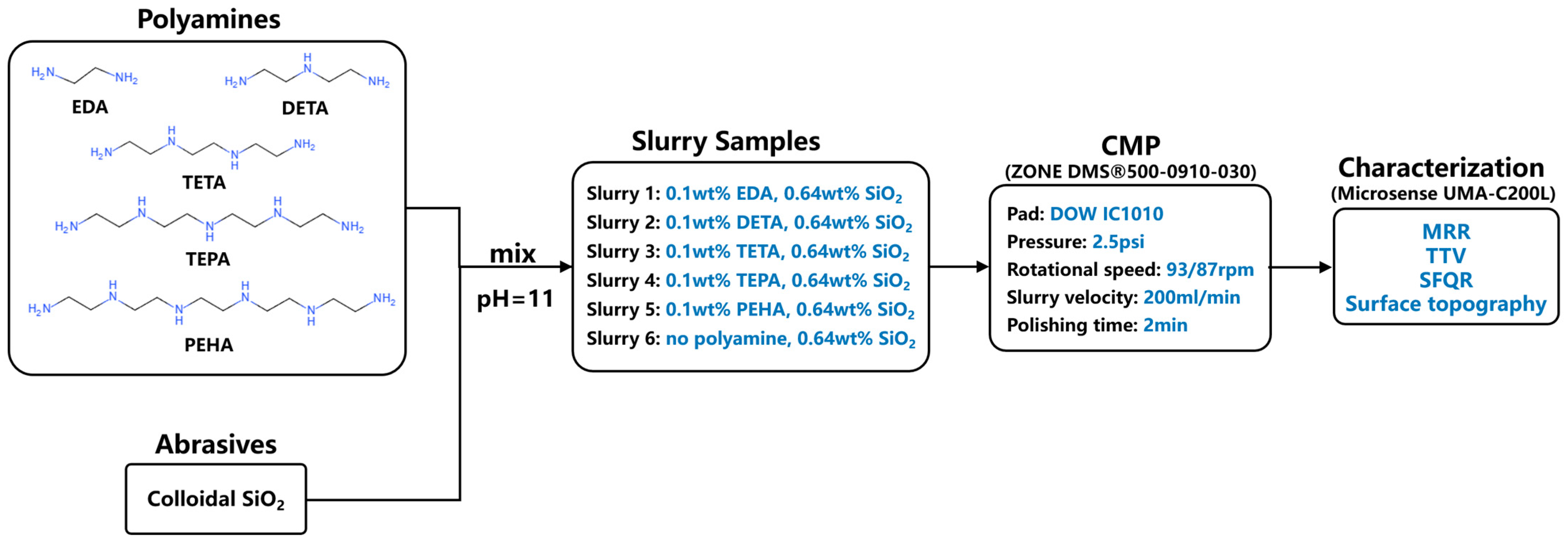
2.3. Experimental Details
3. Results and Discussion
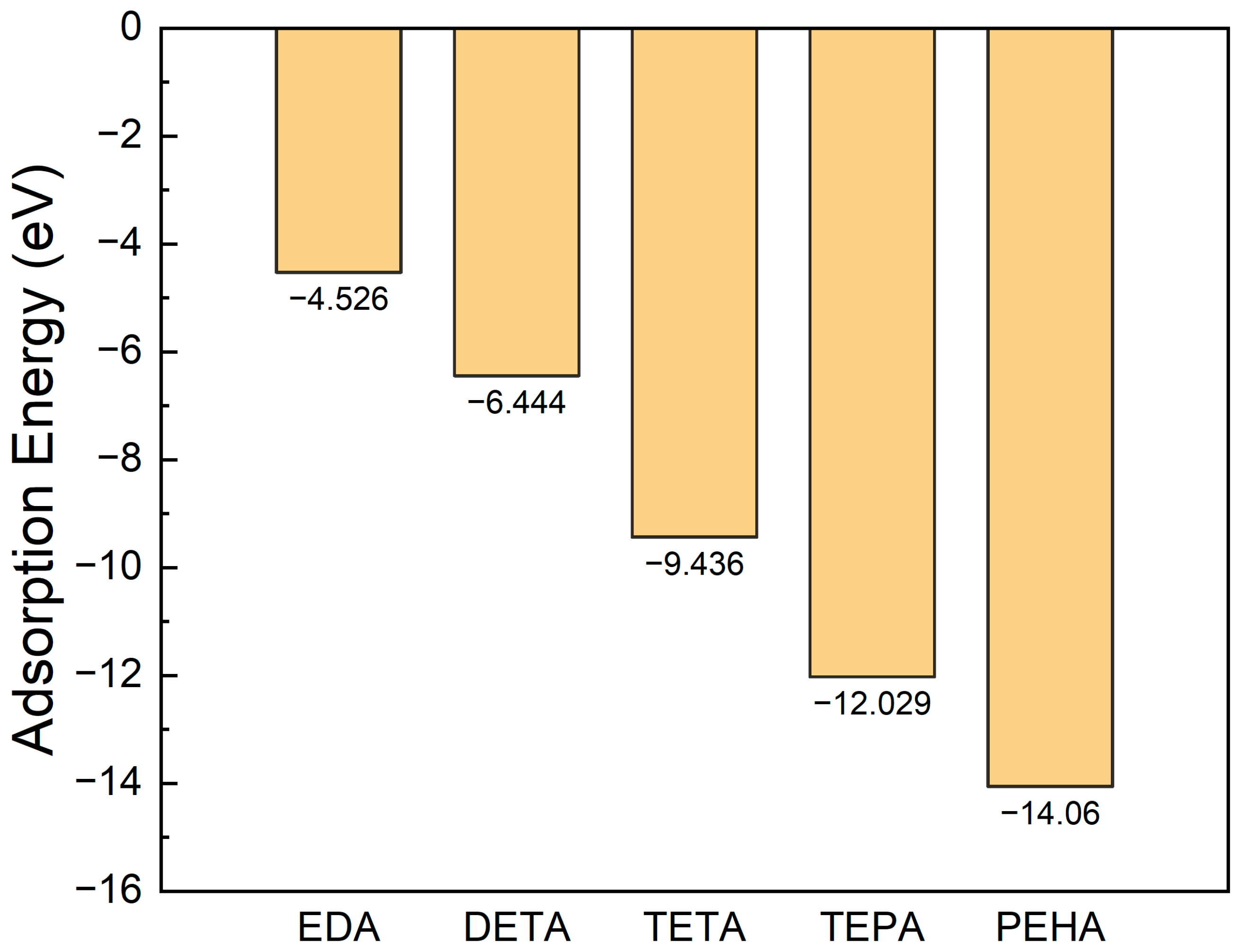
3.1. Unimolecular Adsorption of Polyamine
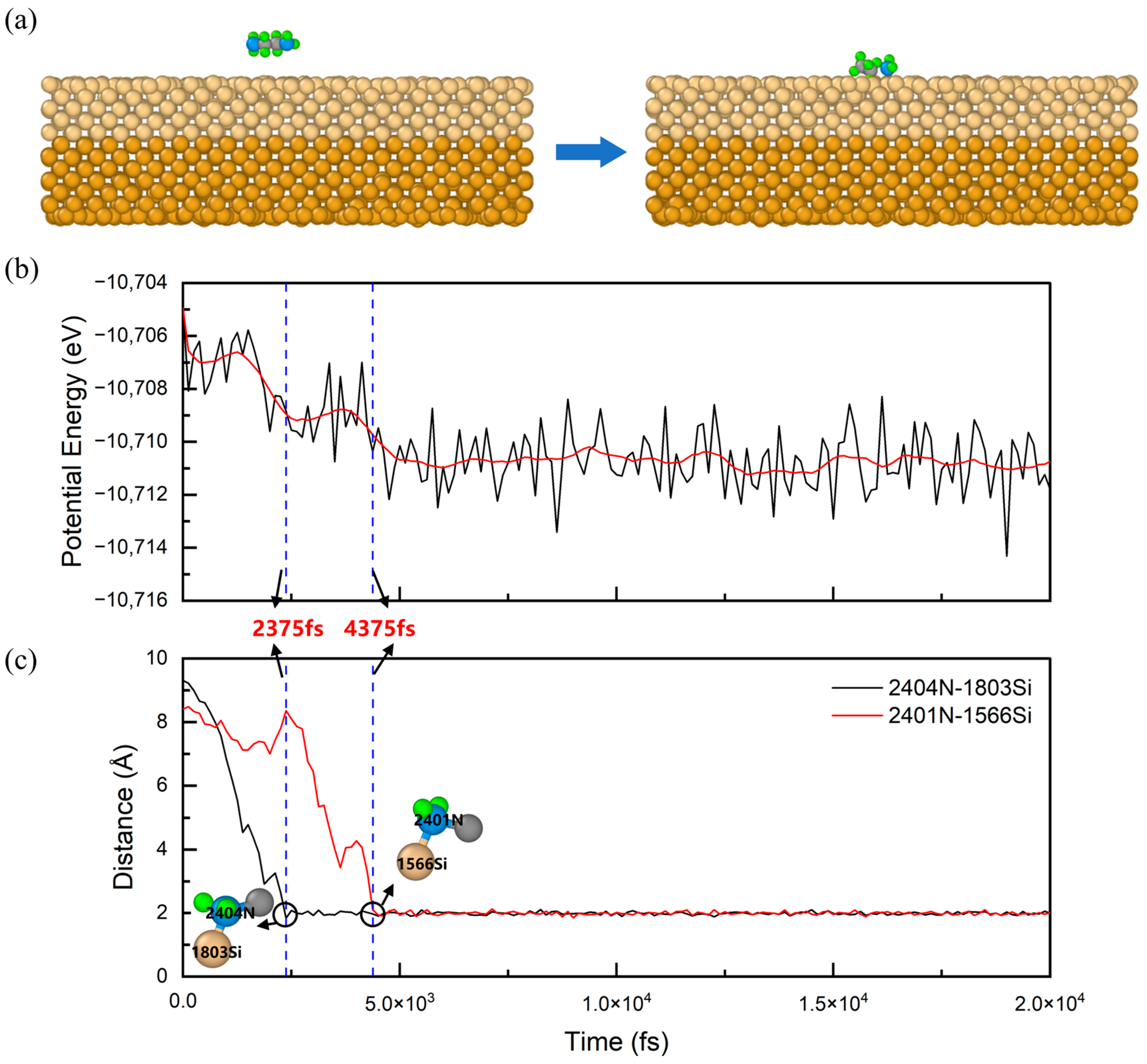
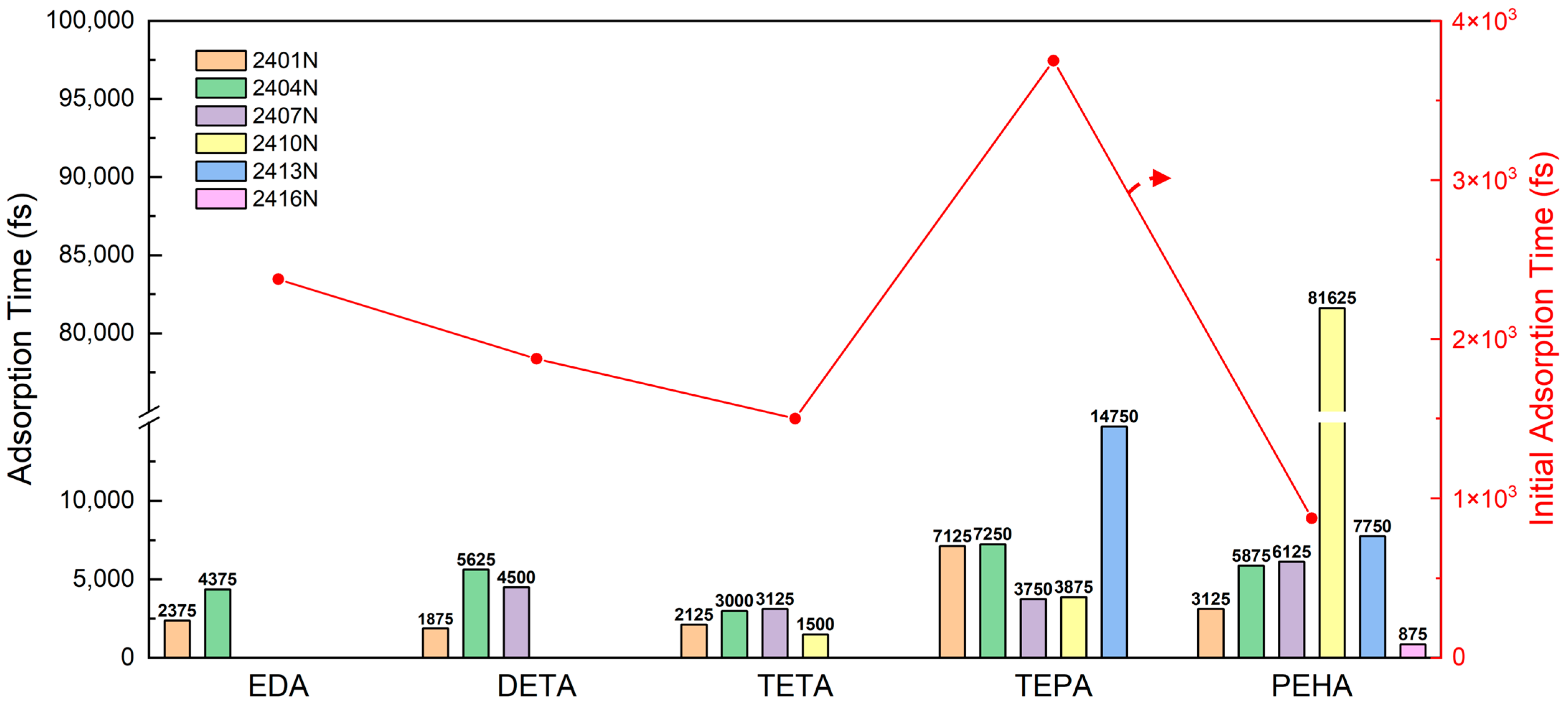
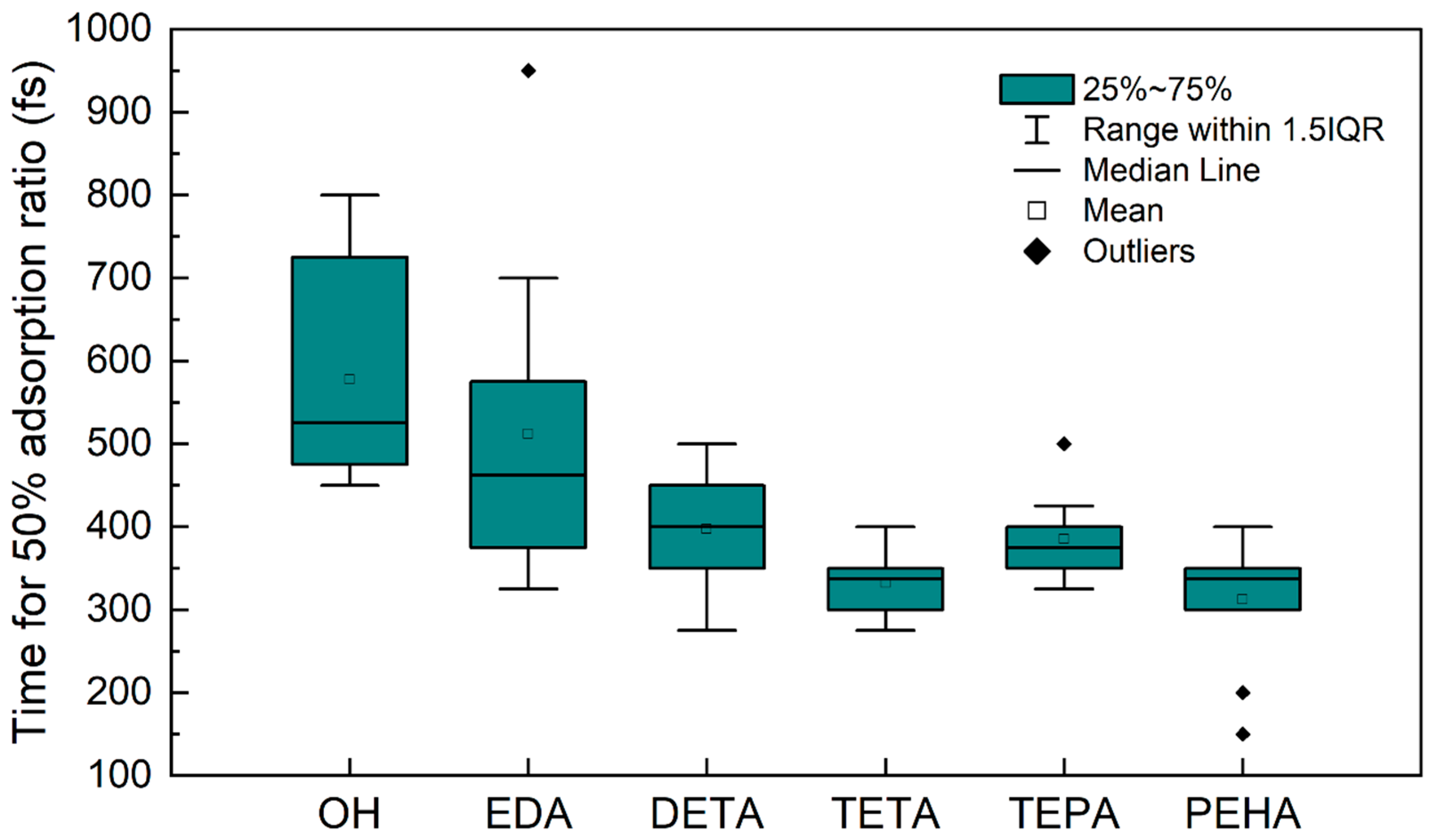
3.2. Multimolecular Adsorption of Polyamines
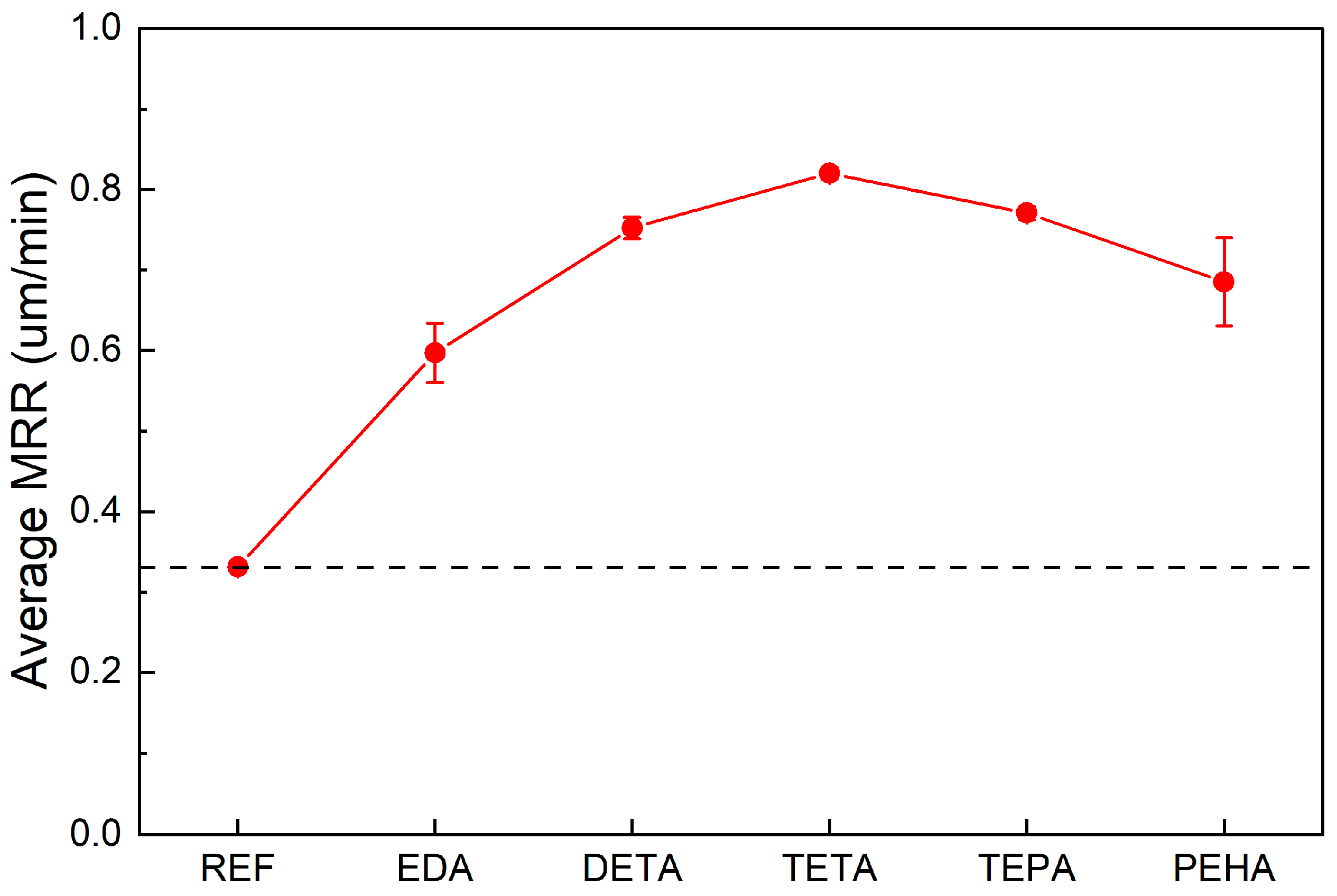
3.3. Effect of Polyamines on the MRR of Si CMP
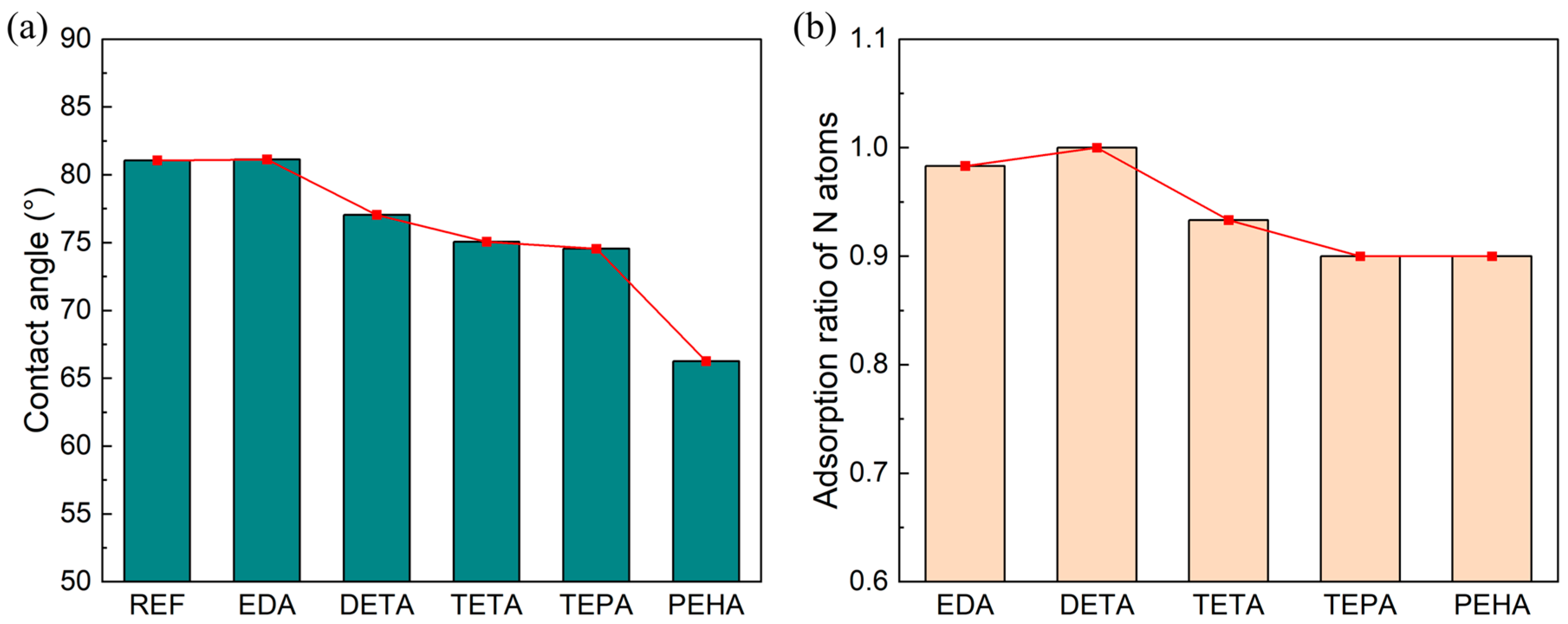
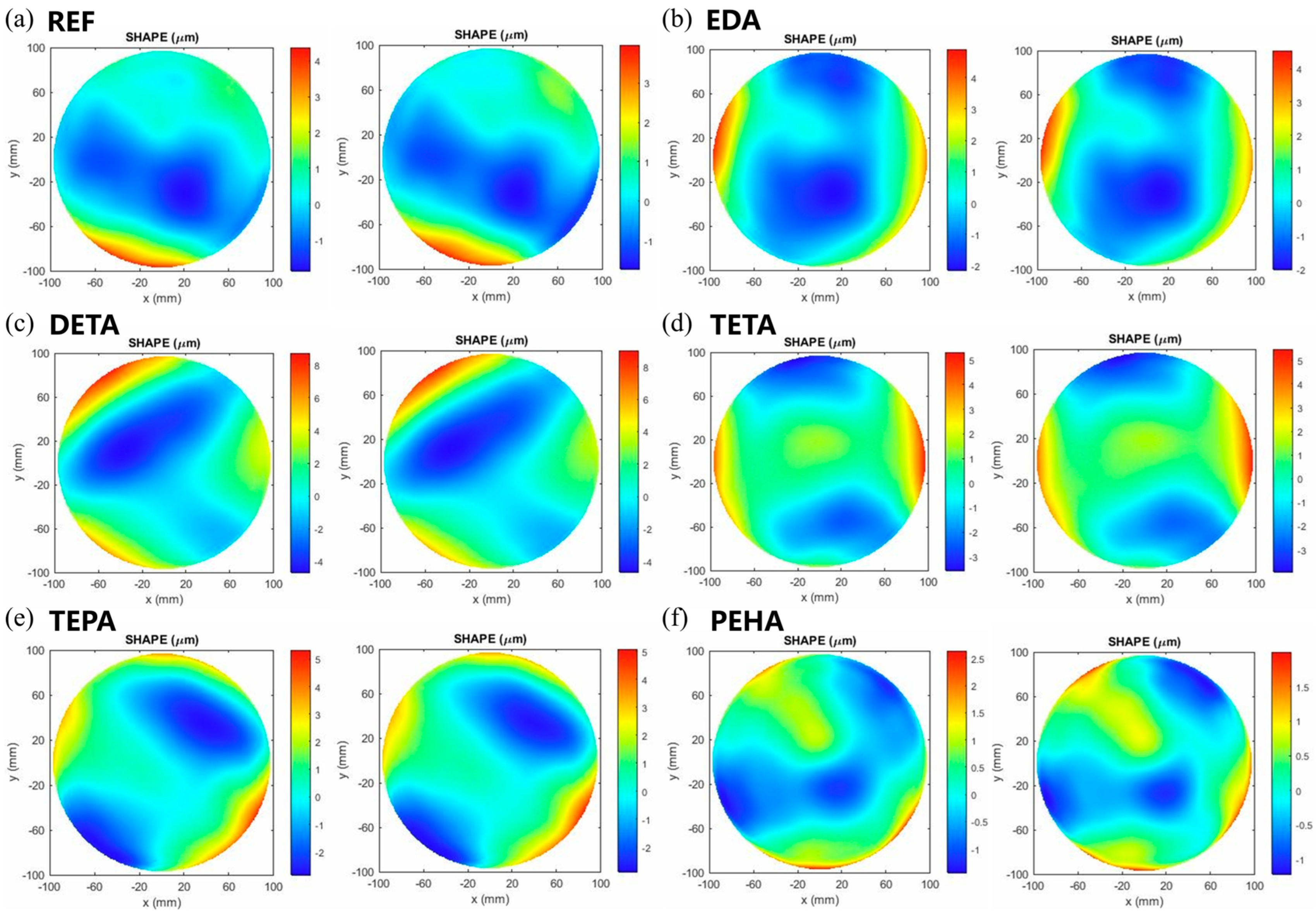
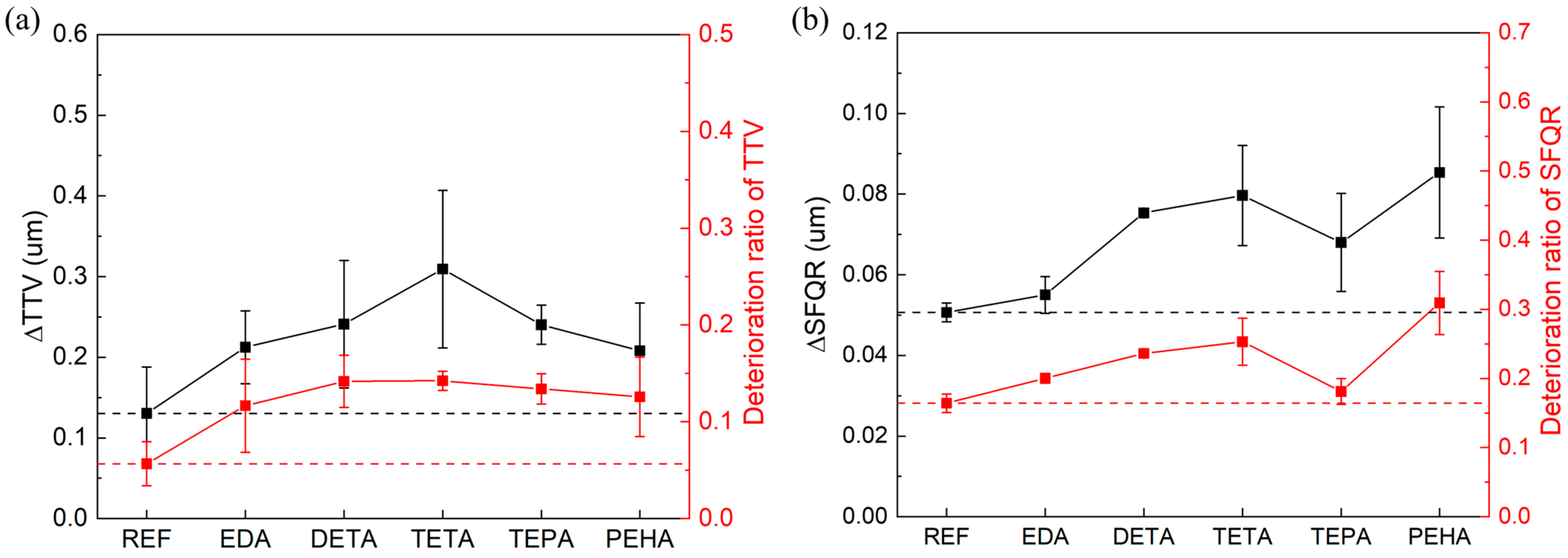
3.4. Effect of Polyamines on the Surface Quality of Si CMP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bu, Z.; Niu, F.; Chen, J.; Jiang, Z.; Wang, W.; Wang, X.; Wang, H.; Zhang, Z.; Zhu, Y.; Sun, T. Single crystal silicon wafer polishing by pretreating pad adsorbing SiO2 grains and abrasive-free slurries. Mater. Sci. Semicond. Proc. 2022, 141, 106418. [Google Scholar] [CrossRef]
- Wang, Y.; Ku, L.; Suo, S.; Dang, Y.; Ge, Z.; Yan, Z.; Zhou, Q. Finite element analysis on von Mises stress distributions of Si DSP. Mater. Sci. Semicond. Proc. 2013, 16, 165–170. [Google Scholar] [CrossRef]
- Pandey, K.; Sharma, A.; Sing, A.K. Silicon wafers; Its manufacturing processes and finishing techniques: An Overview. Silicon 2022, 14, 12031–12047. [Google Scholar] [CrossRef]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical mechanical planarization: Slurry chemistry, materials, and mechanisms. Chem. Rev. 2010, 110, 178–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zhang, L.C.; Biddut, A. Chemical effect on the material removal rate in the CMP of silicon wafers. Wear 2011, 270, 312–316. [Google Scholar] [CrossRef]
- Xiao, C.; Deng, C.; Zhang, P.; Qian, L.; Kim, S.H. Interplay between solution chemistry and mechanical activation in friction-induced material removal of silicon surface in aqueous solution. Tribol. Int. 2020, 148, 106319. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Li, W.; Cao, J.; Huang, J.; Zhu, L.; Guan, Z. Numerical analysis of the effect of retaining ring structure on the chemical mechanical polishing abrasive motion state. Materials 2023, 16, 62. [Google Scholar] [CrossRef]
- Bukkapatnam, S.; Rao, P.; Komanduri, R. Experimental dynamics characterization and monitoring of MRR in oxide chemical mechanical planarization (CMP) process. Int. J. Mach. Tools Manuf. 2008, 48, 1375–1386. [Google Scholar] [CrossRef]
- Zhong, Z.W.; Tian, Y.B.; Ng, J.H.; Ang, Y.J. Chemical mechanical polishing (CMP) processes for manufacturing optical silicon substrates with shortened polishing time. Mater. Manuf. Process. 2014, 29, 15–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, L. A micro-contact and wear model for chemical–mechanical polishing of silicon wafers. Wear 2002, 252, 220–226. [Google Scholar] [CrossRef]
- Qin, K.; Moudgil, B.; Park, C.W. A chemical mechanical polishing model incorporating both the chemical and mechanical effects. Thin Solid Films 2004, 446, 277–286. [Google Scholar] [CrossRef]
- Su, J.; Chen, X.; Zhang, X.; Du, J.; Guo, D. Study on characteristic of material removal rate in chemical mechanical polishing of silicon wafer. J. Comput. Theor. Nanosci. 2008, 5, 1656–1660. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, P.; Yan, Y.; Zhang, B.; Kang, R.; Guo, D. Chemical–mechanical wear of monocrystalline silicon by a single pad asperity. Int. J. Mach. Tools Manuf. 2017, 120, 61–71. [Google Scholar] [CrossRef]
- Seo, J. A review on chemical and mechanical phenomena at the wafer interface during chemical mechanical planarization. J. Mater. Res. 2001, 36, 235–257. [Google Scholar] [CrossRef]
- Song, X.; Yang, H.; Zhang, X.; Liu, H.; Qiu, G.; Tang, M. Effects of H2O2 on electrochemical characteristics of silicon wafers during chemical mechanical polishing. J. Electrochem. Soc. 2008, 155, C530. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Chen, X.; Yu, S.; Shi, C.; Zhou, H.; Wen, W. Effect of cations on the improvement of material removal rate of silicon wafer in chemical mechanical polishing. Colloids Surf. A Physicochem. Eng. Asp. 2023, 67, 131576. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Wang, L.; Cui, X.; Yu, S.; Su, H.; Wang, S. Chemical mechanical polishing of silicon wafers using developed uniformly dispersed colloidal silica in slurry. J. Manuf. Process. 2023, 90, 196–203. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Yu, S.; Li, L.; Cui, X.; Gu, Q.; Wang, Z. High efficiency chemical mechanical polishing for silicon wafers using a developed slurry. Surf. Interfaces 2023, 38, 102833. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Wang, F.; Di, W. Investigation on the final polishing slurry and technique of silicon substrate in ULSI. Microelectron. Eng. 2003, 66, 438–444. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhou, J.; Liu, Z.; Liu, G.; Ma, H.; Li, Y. Effect and mechanism of dual-official group of ethanolamines on the chemical mechanical polishing of monocrystalline silicon. ECS J. Solid State Sci. Technol. 2022, 11, 093005. [Google Scholar] [CrossRef]
- Pan, T.M.; Lei, T.F.; Ko, F.H.; Chao, T.S.; Chiu, T.H.; Lee, Y.H.; Lu, C.P. Comparison of novel cleaning solutions with various chelating agents for post-CMP cleaning on poly-Si film. IEEE Trans. Semicond. Manuf. 2001, 14, 365–371. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G. Study of chelating agents in silicon wafer polishing slurry. Adv. Mater. Res. 2012, 581, 790–793. [Google Scholar] [CrossRef]
- Bae, J.Y.; Han, M.H.; Le, S.J.; Kim, E.S.; Lee, K.; Lee, G.S.; Park, J.H. Silicon wafer CMP slurry using a hydrolysis reaction accelerator with an amine functional group remarkably enhances polishing rate. Nanomaterials 2022, 12, 3893. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, X. Mechanism study on silicon CMP using ethylenediamine. China Surf. Eng. 2021, 34, 67–73. [Google Scholar] [CrossRef]
- Wang, H.; Hu, L.; Cao, G.; Xia, R.; Cao, J.; Zhang, J.; Pan, G. Experimental and Computational Studies on Octyl Hydroxamic Acid as an Environmentally Friendly Inhibitor of Cobalt Chemical Mechanical Polishing. ACS Appl. Mater. Interfaces 2022, 14, 28321–28336. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, T.; Yin, D.; Tan, B.; Yang, F.; Liu, M.; Gao, P.; Zhang, S.; Wang, Y.; He, Y. Adsorption mechanism of potassium oleate on cobalt surface based on cobalt interconnection CMP: A combined experimental and DFT investigation. ECS J. Solid State Sci. Technol. 2021, 10, 024003. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Wang, Y.; Xie, M.; Liu, S.; Liu, M.; Xian, W.; Cui, D.; Zhang, K. Effect of synergetic inhibition of nonionic surfactant and benzotriazone for molybdenum in chemical mechanical polishing. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131164. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Wang, H.; Kang, R.; Gao, S. Atomistic understanding of rough surface on the interfacial friction behavior during the chemical mechanical polishing process of diamond. Friction 2023, 1–14. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Fang, T.H. Molecular dynamics simulation of abrasive characteristics and interfaces in chemical mechanical polishing. Appl. Surf. Sci. 2020, 509, 144676. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Fang, T.H. Material removal and interactions between an abrasive and a SiC substrate: A molecular dynamics simulation study. Ceram. Int. 2020, 46, 5623–5633. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; Psofogiannakis, G.; Van Duin, A.C.T.; Chen, L.; Qian, L.; Hu, Y.; Lu, X. Atomic insight into tribochemical wear mechanism of silicon at the Si/SiO2 interface in aqueous environment: Molecular dynamics simulations using ReaxFF reactive force field. Appl. Surf. Sci. 2016, 390, 216–223. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; Van Duin, A.C.T.; Lu, X. Atomistic mechanisms of Si chemical mechanical polishing in aqueous H2O2: ReaxFF reactive molecular dynamics simulations. Comput. Mater. Sci. 2017, 131, 230–238. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; Van Duin, A.C.T.; Lu, X. Surface orientation and temperature effects on the interaction of silicon with water: Molecular dynamics simulations using ReaxFF reactive force field. J. Phys. Chem. A 2017, 121, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Guo, X.; Li, P.; Zhang, S.; Li, M.; Jin, Z.; Kang, R.; Guo, D.; Liu, F.; Zhang, L. Atomistic understanding of interfacial processing mechanism of silicon in water environment: A ReaxFF molecular dynamics simulation. Front. Mech. Eng. 2021, 16, 570–579. [Google Scholar] [CrossRef]
- Tao, H.; Zeng, Q.; Liu, Y.; Zhao, D.; Lu, X. Effects of grinding-induced surface topography on the material removal mechanism of silicon chemical mechanical polishing. Appl. Surf. Sci. 2023, 631, 157509. [Google Scholar] [CrossRef]
- Brugnoli, L.; Miyatani, K.; Akaji, M.; Urata, S.; Pedone, A. New atomistic insights on the chemical mechanical polishing of silica glass with ceria nanoparticles. Langmuir 2023, 39, 5527–5541. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Aktulga, H.M.; Fogarty, J.C.; Pandit, S.A.; Grama, A.Y. Parallel reactive molecular dynamics: Numerical methods and algorithmic techniques. Parallel Comput. 2012, 38, 245–259. [Google Scholar] [CrossRef]
- Bhati, M.; Senftle, T.P. Identifying adhesion properties at Si/polymer interfaces with ReaxFF. J. Phys. Chem. C 2019, 123, 27036–27047. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The open visualization tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Nagendran, S.; Butcher, R.J. Synthesis and characterization of new organosilanediols with Si−N bonds. Organometallics 1999, 18, 4488–4492. [Google Scholar] [CrossRef]
- Che, C.W.; Huang, C.C.; Lin, Y.Y.; Chen, L.C.; Chen, K.H. The affinity of Si–N and Si–C bonding in amorphous silicon carbon nitride (a-SiCN) thin film. Diam. Relat. Mater. 2005, 14, 1126–1130. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Zhu, J.; Huang, Q.; Zhu, L.; Li, W.; Yu, W. Mechanism Exploration of the Effect of Polyamines on the Polishing Rate of Silicon Chemical Mechanical Polishing: A Study Combining Simulations and Experiments. Nanomaterials 2024, 14, 127. https://doi.org/10.3390/nano14010127
Lin Z, Zhu J, Huang Q, Zhu L, Li W, Yu W. Mechanism Exploration of the Effect of Polyamines on the Polishing Rate of Silicon Chemical Mechanical Polishing: A Study Combining Simulations and Experiments. Nanomaterials. 2024; 14(1):127. https://doi.org/10.3390/nano14010127
Chicago/Turabian StyleLin, Ziwei, Junli Zhu, Qi Huang, Lei Zhu, Weimin Li, and Wenjie Yu. 2024. "Mechanism Exploration of the Effect of Polyamines on the Polishing Rate of Silicon Chemical Mechanical Polishing: A Study Combining Simulations and Experiments" Nanomaterials 14, no. 1: 127. https://doi.org/10.3390/nano14010127
APA StyleLin, Z., Zhu, J., Huang, Q., Zhu, L., Li, W., & Yu, W. (2024). Mechanism Exploration of the Effect of Polyamines on the Polishing Rate of Silicon Chemical Mechanical Polishing: A Study Combining Simulations and Experiments. Nanomaterials, 14(1), 127. https://doi.org/10.3390/nano14010127





