Eco-Friendly Silver Nanoparticles Synthesized from a Soybean By-Product with Nematicidal Efficacy against Pratylenchus brachyurus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soybean Leaf Extract
2.2. Synthesis and Characterization of Nanoparticles
2.3. P. brachyurus Culture
2.4. Direct Exposure of P. brachyurus to 3.12AgNP and 6.25AgNP
2.5. AgNP Effects on Soybean Grown and Nematode Population in Soil System
2.6. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
3. Results
3.1. Optical and Physical-Chemical Characterization of Silver Nanoparticles
3.2. In Vitro and In Vivo Nematicidal Activity of AgNPs
3.3. 6.25AgNP Dry Size and Mode of Action on Phytonematodes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lordello, L.G.E. Nematóides Das Plantas Cultivadas; Nobel: São Paulo, SP, Brazil, 1986. [Google Scholar]
- Ferraz, L.; Brown, D. Nematologia de Plantas: Fundamentos e Importância; Norma Editora: Manaus, AM, Brazil, 2016; Volume 1, ISBN 978-85-99031-26-1. [Google Scholar]
- Castillo, P.; Vovlas, N. Pratylenchus, Nematoda: Pratylenchidae: Diagnosis, Biology, Pathogenicity and Management; Nematology monographs and perspectives; Brill: Leiden, UK, 2007; ISBN 978-90-04-15564-0. [Google Scholar]
- Goulart, A. Aspectos Gerais Sobre Nematóides das Lesões Radiculares (Gênero Pratylenchus). 2008. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CPAC-2010/30288/1/doc-219.pdf (accessed on 31 October 2023).
- Vieira, P.; Maier, T.R.; Eves-van Den Akker, S.; Howe, D.K.; Zasada, I.; Baum, T.J.; Eisenback, J.D.; Kamo, K. Identification of Candidate Effector Genes of Pratylenchus penetrans. Mol. Plant Pathol. 2018, 19, 1887–1907. [Google Scholar] [CrossRef]
- Vovlas, N.; Troccoli, A. Histopathology of broad bean roots infected by the lesion nematode Pratylenchus penetrans. Nematol. Medit. 1990, 18, 239–242. [Google Scholar]
- Thompson, J.P.; Owen, K.J.; Stirling, G.R.; Bell, M.J. Root-Lesion Nematodes (Pratylenchus thornei and P. neglectus): A Review of Recent Progress in Managing a Significant Pest of Grain Crops in Northern Australia. Australas. Plant Pathol. 2008, 37, 235. [Google Scholar] [CrossRef]
- Bergeson, G.B. Concepts of Nematode—Fungus Associations in Plant Disease Complexes: A Review. Exp. Parasitol. 1972, 32, 301–314. [Google Scholar] [CrossRef]
- Desaeger, J.; Wram, C.; Zasada, I. New Reduced-Risk Agricultural Nematicides—Rationale and Review. J. Nematol. 2020, 52, 1–16. [Google Scholar] [CrossRef]
- Ferris, J.M. Factors Influencing the Population Fluctuation of Pratylenchus penetrans in Soils of High Organic Content. I. Effect of Soil Fumigants and Different Crop Plants. J. Econ. Entomol. 1967, 60, 1708–1714. [Google Scholar] [CrossRef]
- Chowdhury, I.A.; Yan, G.; Kandel, H.; Plaisance, A. Population Development of the Root-Lesion Nematode Pratylenchus dakotaensis on Soybean Cultivars. Plant Dis. 2022, 106, 2117–2126. [Google Scholar] [CrossRef]
- Ferrari, E.; Ramos Junior, E.U.; Tavares, G.; Faleiro, V.D.O.; Shiomi, H.F.; Debiasi, H.; Franchini, J.C. Population Dynamics of the Nematode Pratylenchus brachyurus in Different Production Systems in MT. Sci. Elec. Arch. 2016, 9, 32–40. [Google Scholar]
- Bridge, J. Nematode Management in Sustainable and Subsistence Agriculture. Annu. Rev. Phytopathol. 1996, 34, 201–225. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Understanding Molecular Plant–Nematode Interactions to Develop Alternative Approaches for Nematode Control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef]
- Karuri, H. Root and Soil Health Management Approaches for Control of Plant-Parasitic Nematodes in Sub-Saharan Africa. Crop Prot. 2022, 152, 105841. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Hassan, M.E.; Zawam, H.S.; Nahas, S.E.E.; Desoukey, A.F. Comparison Study Between Silver Nanoparticles and Two Nematicides Against Meloidogyne incognita on Tomato Seedlings. Plant Pathol. J. 2016, 15, 144–151. [Google Scholar] [CrossRef]
- Hamed, S.M.; Hagag, E.S.; El-Raouf, N.A. Green Production of Silver Nanoparticles, Evaluation of Their Nematicidal Activity against Meloidogyne javanica and Their Impact on Growth of Faba Bean. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 9. [Google Scholar] [CrossRef]
- Mahmoud, W.M.; Abdelmoneim, T.S.; Elazzazy, A.M. The Impact of Silver Nanoparticles Produced by Bacillus pumilus as Antimicrobial and Nematicide. Front. Microbiol. 2016, 7, 1746. [Google Scholar] [CrossRef]
- Oluwatoyin, F.; Gabriel, O.; Olubunmi, A.; Olanike, O. Preparation of Bio-Nematicidal Nanoparticles of Eucalyptus officinalis for the Control of Cyst Nematode (Heterodera sacchari). J. Anim. Plant Sci. 2020, 30, 1172–1177. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Razavi, M.; Salahinejad, E.; Fahmy, M.; Yazdimamaghani, M.; Vashaee, D.; Tayebi, L. Green Chemical and Biological Synthesis of Nanoparticles and Their Biomedical Applications. In Green Processes for Nanotechnology; Basiuk, V.A., Basiuk, E.V., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 207–235. ISBN 978-3-319-15460-2. [Google Scholar]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef]
- Thummaneni, C.; Surya Prakash, D.V.; Golli, R.; Vangalapati, M. Green Synthesis of Silver Nanoparticles and Characterization of Caffeic Acid from Myristica fragrans (Nutmeg) against Antibacterial Activity. Mater. Today Proc. 2022, 62, 4001–4005. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Abdel-Rasoul, M.A.; Nassar, A.M.K.; Soliman, B.S.M. Nematicidal Activity of Silver Nanoparticles of Botanical Products against Root-Knot Nematode, Meloidogyne incognita. Arch. Phytopathol. Plant Prot. 2017, 50, 909–926. [Google Scholar] [CrossRef]
- Danish, M.; Altaf, M.; Robab, M.I.; Shahid, M.; Manoharadas, S.; Hussain, S.A.; Shaikh, H. Green Synthesized Silver Nanoparticles Mitigate Biotic Stress Induced by Meloidogyne incognita in Trachyspermum ammi (L.) by Improving Growth, Biochemical, and Antioxidant Enzyme Activities. ACS Omega 2021, 6, 11389–11403. [Google Scholar] [CrossRef]
- Duraisamy, K.; Palanisamy, S.; Premasudha, P.; Hafez, S. Nematicidal Activity of Green Synthesized Silver Nanoparticles Using Plant Extracts against Root-Knot Nematode Meloidogyne incognita. Sci. Rep. 2017, 27, 81–94. [Google Scholar]
- Elkobrosy, D.; Al-Askar, A.A.; El-Gendi, H.; Su, Y.; Nabil, R.; Abdelkhalek, A.; Behiry, S. Nematocidal and Bactericidal Activities of Green Synthesized Silver Nanoparticles Mediated by Ficus sycomorus Leaf Extract. Life 2023, 13, 1083. [Google Scholar] [CrossRef]
- Heflish, A.A.; Hanfy, A.E.; Ansari, M.J.; Dessoky, E.S.; Attia, A.O.; Elshaer, M.M.; Gaber, M.K.; Kordy, A.; Doma, A.S.; Abdelkhalek, A.; et al. Green Biosynthesized Silver Nanoparticles Using Acalypha wilkesiana Extract Control Root-Knot Nematode. J. King Saud Univ. -Sci. 2021, 33, 101516. [Google Scholar] [CrossRef]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Nivitha, S.; Sundararaj, P. Green Synthesis of Silver Nanoparticles Using Latex Extract of Euphorbia tirucalli: A Novel Approach for the Management of Root Knot Nematode, Meloidogyne incognita. Crop Prot. 2019, 117, 108–114. [Google Scholar] [CrossRef]
- Vivekanandhan, S. Biological Synthesis of Silver Nanoparticles Using Glycine max (Soybean) Leaf Extract: An Investigation on Different Soybean Varieties. J. Nanosci. Nanotechnol. 2009, 9, 6828–6833. [Google Scholar] [CrossRef]
- Hosamani, G.; Patil, R.R.; Benagi, V.I.; Chandrashekhar, S.S.; Nandihali, B.S. Synthesis of Green Silver Nanoparticles from Soybean Seed and Its Bioefficacy on Spodoptera litura (F.). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 610–618. [Google Scholar] [CrossRef]
- Coolen, W.A.; D’Herde, C.J. A Method for the Quantitative Extraction of Nematodes from Plant Tissue. In Ghent; State Agricultural Research Centre: Gembloux, Belgium, 1972. [Google Scholar]
- Machado, A.C.Z.; Ferraz, L.C.C.B.; de Oliveira, C.M.G. Development of a Species-Specific Reverse Primer for the Molecular Diagnostic of Pratylenchus brachyurus. Nematropica 2007, 37, 10. [Google Scholar]
- Flegg, J.J.M. Extraction of Xiphinema and Longidorus Species from Soil by a Modification of Cobb’s Decanting and Sieving Technique. Ann. Appl. Biol. 1967, 60, 429–437. [Google Scholar] [CrossRef]
- Oostenbrink, M. Major Characteristics of the Relation between Nematodes and Plants. In Proceedings of the 8th International Symposium of Nematology, Antibes, France, 8–14 September 1966; pp. 1–46. [Google Scholar]
- Karnovsky, M. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry, 3rd ed.; Harcourt College Publishers: Fort Worth, TX, USA, 2001; ISBN 978-0-03-031961-7. [Google Scholar]
- Santiago, T.R.; Bonatto, C.C.; Rossato, M.; Lopes, C.A.P.; Lopes, C.A.; G Mizubuti, E.S.; Silva, L.P. Green Synthesis of Silver Nanoparticles Using Tomato Leaf Extract and Their Entrapment in Chitosan Nanoparticles to Control Bacterial Wilt. J. Sci. Food Agric. 2019, 99, 4248–4259. [Google Scholar] [CrossRef]
- Roy, N.; Gaur, A.; Jain, A.; Bhattacharya, S.; Rani, V. Green Synthesis of Silver Nanoparticles: An Approach to Overcome Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 807–812. [Google Scholar] [CrossRef]
- Abboud, Z.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K. Leaf Extract Mediated Biogenic Process for the Decoration of Graphene with Silver Nanoparticles. Mater. Lett. 2016, 178, 115–119. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, G.; Yao, L.; Huang, L.; Wang, J.; Gao, W. Effects of Metal Nanoparticles and Other Preparative Materials in the Environment on Plants: From the Perspective of Improving Secondary Metabolites. J. Agric. Food Chem. 2022, 70, 916–933. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, H. A Review on Green Silver Nanoparticles Based on Plants: Synthesis, Potential Applications and Eco-Friendly Approach. Int. Food Res. J. 2016, 23, 446. [Google Scholar]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.-K.; Lee, H.-S.; Chung, N. Rod-Shaped Iron Oxide Nanoparticles Are More Toxic than Sphere-Shaped Nanoparticles to Murine Macrophage Cells: Toxicity of Rod and Sphere Iron Oxide Nanoparticles. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public. Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Kumar Petla, R.; Vivekanandhan, S.; Misra, M.; Kumar Mohanty, A.; Satyanarayana, N. Soybean (Glycine max) Leaf Extract Based Green Synthesis of Palladium Nanoparticles. J. Biomater. Nanobiotechnology 2012, 3, 14–19. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Gu, F.L.; Zhan, Q.; Palvannan, T.; Mohd Yusoff, A.R. Flavonoids Mediated ‘Green’ Nanomaterials: A Novel Nanomedicine System to Treat Various Diseases—Current Trends and Future Perspective. Mater. Lett. 2018, 210, 26–30. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and Phytochemicals: A Natural Reservoir for the Green Synthesis of Gold and Silver Nanoparticles. IET Nanobiotechnol. 2011, 5, 69. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Ma, Z.; Rao, Z.; Zheng, X.; Tang, K. Soluble Soybean Polysaccharide/Carrageenan Antibacterial Nanocomposite Films Containing Green Synthesized Silver Nanoparticles. ACS Appl. Polym. Mater. 2022, 4, 5608–5618. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Liu, Y.; Zheng, X.; Tang, K. Green Synthesis of Silver Nanoparticles Using Soluble Soybean Polysaccharide and Their Application in Antibacterial Coatings. Int. J. Biol. Macromol. 2021, 166, 567–577. [Google Scholar] [CrossRef]
- Cromwell, W.A.; Yang, J.; Starr, J.L.; Jo, Y.-K. Nematicidal Effects of Silver Nanoparticles on Root-Knot Nematode in Bermudagrass. J. Nematol. 2014, 46, 261–266. [Google Scholar]
- Ardakani, A.S. Toxicity of Silver, Titanium and Silicon Nanoparticles on the Root-Knot Nematode, Meloidogyne incognita, and Growth Parameters of Tomato. Nematology 2013, 15, 671–677. [Google Scholar] [CrossRef]
- Rani, K.; Devi, N.; Banakar, P.; Kharb, P.; Kaushik, P. Nematicidal Potential of Green Silver Nanoparticles Synthesized Using Aqueous Root Extract of Glycyrrhiza glabra. Nanomaterials 2022, 12, 2966. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Shams El-Din, N.G.E.-D.; Maghraby, D.M.E.; Ibrahim, D.S.S.; Abdel-Megeed, A.; Abdelsalam, N.R. Nematicidal Activity of Seaweed-Synthesized Silver Nanoparticles and Extracts against Meloidogyne incognita on Tomato Plants. Sci. Rep. 2022, 12, 3841. [Google Scholar] [CrossRef]
- Lallo Da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Javed, Z.; Dashora, K.; Mishra, M.; Fasake, V.D.; Srivastva, A. Effect of Accumulation of Nanoparticles in Soil Health—A Concern on Future. Front. Nanosci. Nanotechnol. 2019, 5, 1. [Google Scholar] [CrossRef]
- Ajiboye, T.T.; Ajiboye, T.O.; Babalola, O.O. Impacts of Binary Oxide Nanoparticles on the Soybean Plant and Its Rhizosphere, Associated Phytohormones, and Enzymes. Molecules 2023, 28, 1326. [Google Scholar] [CrossRef]
- Ballikaya, P.; Mateos, J.M.; Brunner, I.; Kaech, A.; Cherubini, P. Detection of Silver Nanoparticles inside Leaf of European Beech (Fagus sylvatica L.). Front. Environ. Sci. 2023, 10, 1107005. [Google Scholar] [CrossRef]
- Abdellatif, K.F.; Abdelfattah, R.H.; El-Ansary, M.S.M. Green Nanoparticles Engineering on Root-Knot Nematode Infecting Eggplants and Their Effect on Plant DNA Modification. Iran. J. Biotechnol. 2016, 14, 250–259. [Google Scholar] [CrossRef]
- Nazir, K.; Mukhtar, T.; Javed, H. In Vitro Effectiveness of Silver Nanoparticles against Root-Knot Nematode (Meloidogyne incognita). Pak. J. Zool. 2019, 51, 2077–2083. [Google Scholar] [CrossRef]
- Rossbach, L.M.; Oughton, D.H.; Maremonti, E.; Coutris, C.; Brede, D.A. In Vivo Assessment of Silver Nanoparticle Induced Reactive Oxygen Species Reveals Tissue Specific Effects on Cellular Redox Status in the Nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 721, 137665. [Google Scholar] [CrossRef]
- Tuncsoy, B. Nematicidal Activity of Silver Nanomaterials against Plant-Parasitic Nematodes. In Silver Nanomaterials for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–548. ISBN 978-0-12-823528-7. [Google Scholar]
- Dzięgielewska, M.; Skwiercz, A.; Wesołowska, A.; Kozacki, D.; Przewodowski, W.; Kulpa, D. Effects of Silver, Gold, and Platinum Nanoparticles on Selected Nematode Trophic Groups. Preprint, 2023; Version 1. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde. In Vigilância e Controle da Qualidade da Água para Consumo Humano; Ministério da Saúde: Brasília, Brazil, 2006; 212p, ISBN 978-85-334-1240-8. [Google Scholar]
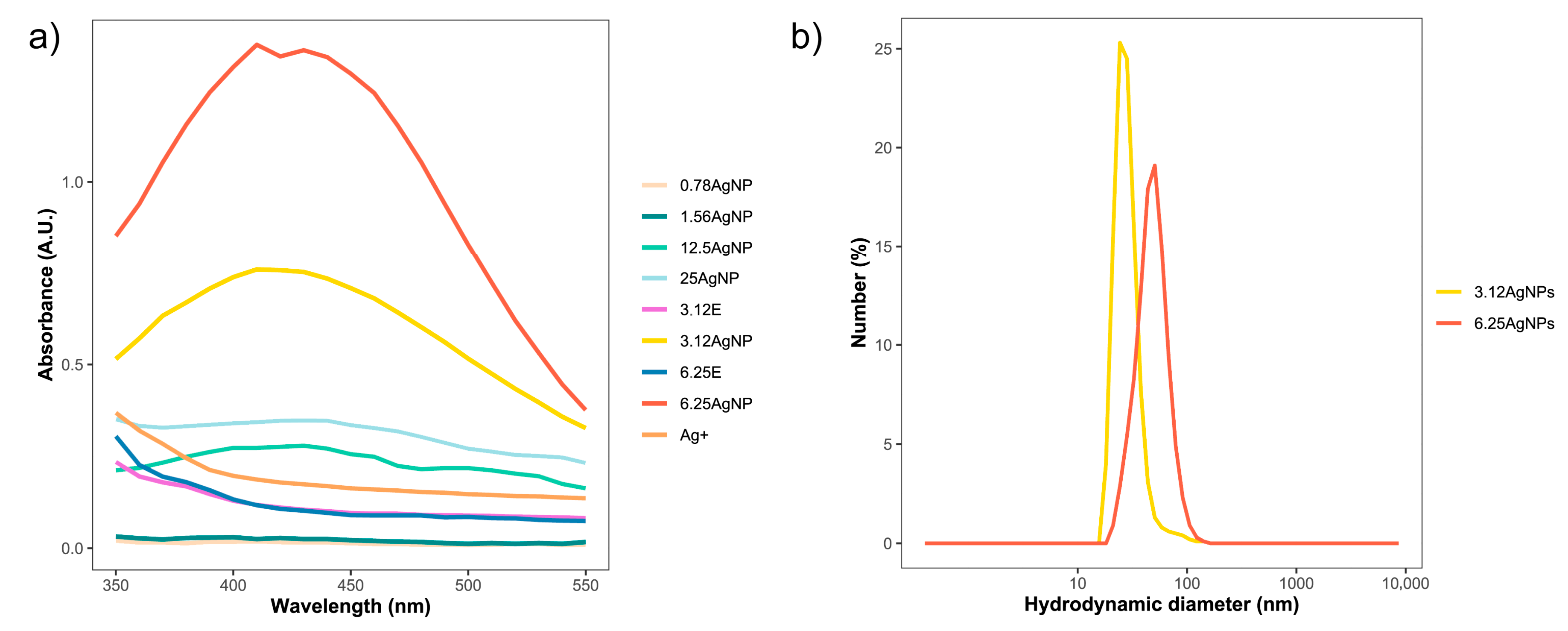
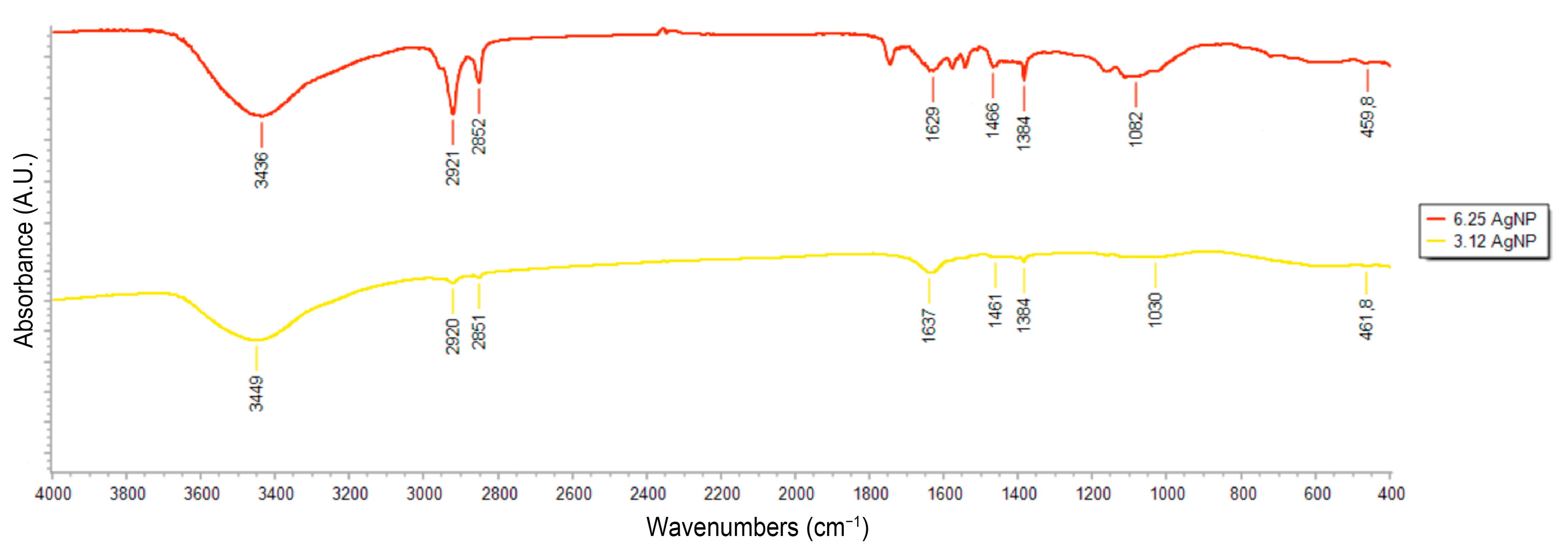

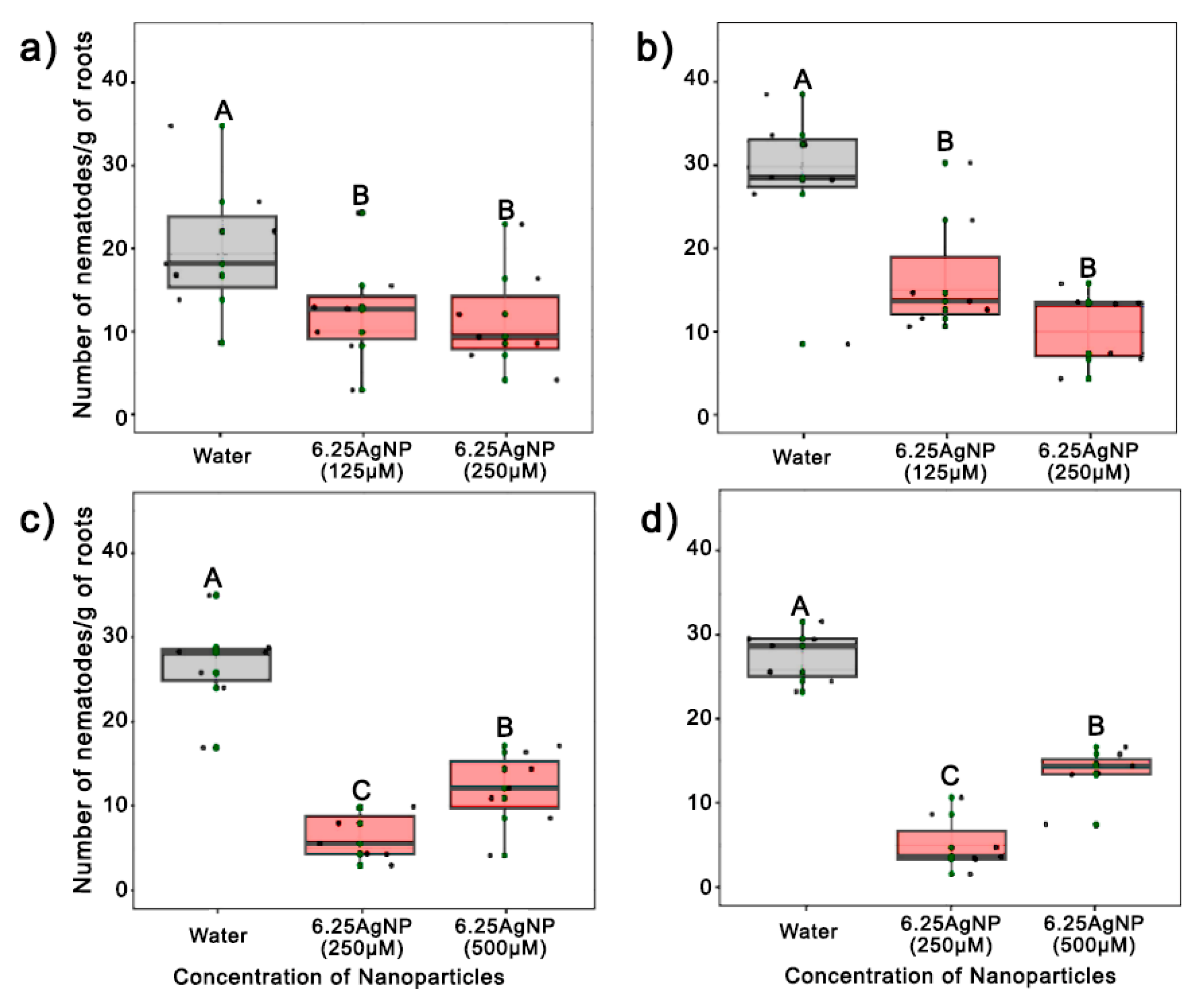
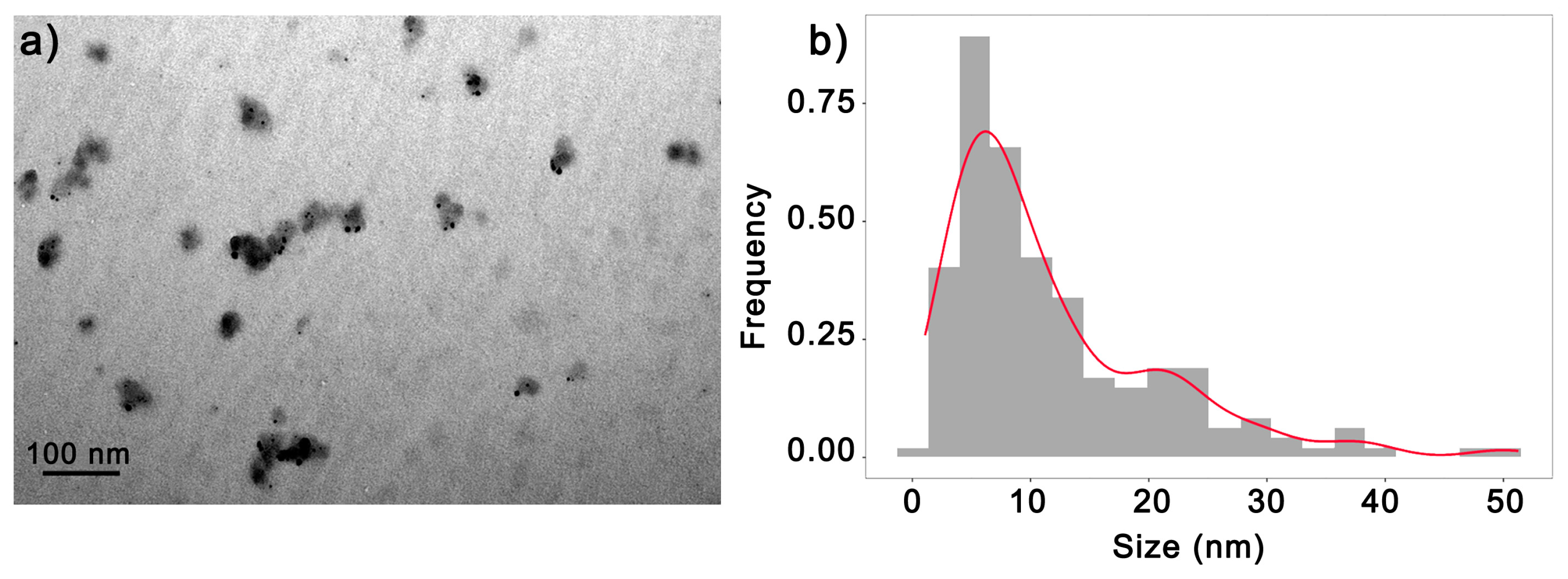

| First Synthesis | Second Synthesis | Third Synthesis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nanoparticles | Size (d·nm) | PdI | Zeta Potential (mV) | Size (d·nm) | PdI | Zeta Potential (mV) | Size (d·nm) | PdI | Zeta Potential (mV) |
| Ag+ | 64.7 ± 4.3 | 0.57 ± 0.04 | −19.1 ± 1.3 | - | - | - | - | - | - |
| 3.12AgNP | 104.3 ± 2.4 | 0.23 ± 0.01 | −25.1 ± 0.5 | 101.1 ± 3.4 | 0.19 ± 0.01 | −28.1 ± 0.5 | 113.3 ± 1.7 | 0.22 ± 0.02 | −20.2 ± 1.4 |
| 6.25AgNP | 73.5 ± 1.5 | 0.22 ± 0.02 | −23.3 ± 0.2 | 70.2 ± 2.2 | 0.20 ± 0.03 | −23.3 ± 0.2 | 79.1 ± 1.8 | 0.19 ± 0.05 | −19.7 ± 2.1 |
| Nanoparticles | Experiment_1 | Experiment_2 |
|---|---|---|
| 3.12AgNP_IC50 | 17.21 (8.65–28.77) | 25.18 (13.65–36.71) |
| 6.25AgNP_IC50 | 3.66 (0.85–8.19) | 9.6 (0.68–12.9) |
| 3.12AgNP_IC90 | 157.02 (33.66–316.7) | 506.45 (123.33–836.26) |
| 6.25AgNP_IC90 | 119.29 (6.93–205.52) | 140.09 (22.35–212.53) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, L.S.d.; Furtado, L.L.; Diniz, F.d.A.d.S.; Mendes, B.L.; Araújo, T.R.d.; Silva, L.P.; Santiago, T.R. Eco-Friendly Silver Nanoparticles Synthesized from a Soybean By-Product with Nematicidal Efficacy against Pratylenchus brachyurus. Nanomaterials 2024, 14, 101. https://doi.org/10.3390/nano14010101
Oliveira LSd, Furtado LL, Diniz FdAdS, Mendes BL, Araújo TRd, Silva LP, Santiago TR. Eco-Friendly Silver Nanoparticles Synthesized from a Soybean By-Product with Nematicidal Efficacy against Pratylenchus brachyurus. Nanomaterials. 2024; 14(1):101. https://doi.org/10.3390/nano14010101
Chicago/Turabian StyleOliveira, Letícia Santana de, Leila Lourenço Furtado, Francisco de Assis dos Santos Diniz, Bruno Leonardo Mendes, Thalisson Rosa de Araújo, Luciano Paulino Silva, and Thaís Ribeiro Santiago. 2024. "Eco-Friendly Silver Nanoparticles Synthesized from a Soybean By-Product with Nematicidal Efficacy against Pratylenchus brachyurus" Nanomaterials 14, no. 1: 101. https://doi.org/10.3390/nano14010101
APA StyleOliveira, L. S. d., Furtado, L. L., Diniz, F. d. A. d. S., Mendes, B. L., Araújo, T. R. d., Silva, L. P., & Santiago, T. R. (2024). Eco-Friendly Silver Nanoparticles Synthesized from a Soybean By-Product with Nematicidal Efficacy against Pratylenchus brachyurus. Nanomaterials, 14(1), 101. https://doi.org/10.3390/nano14010101








