The Role of Stabilizing Copolymer in Determining the Physicochemical Properties of Conjugated Polymer Nanoparticles and Their Nanomedical Applications
Abstract
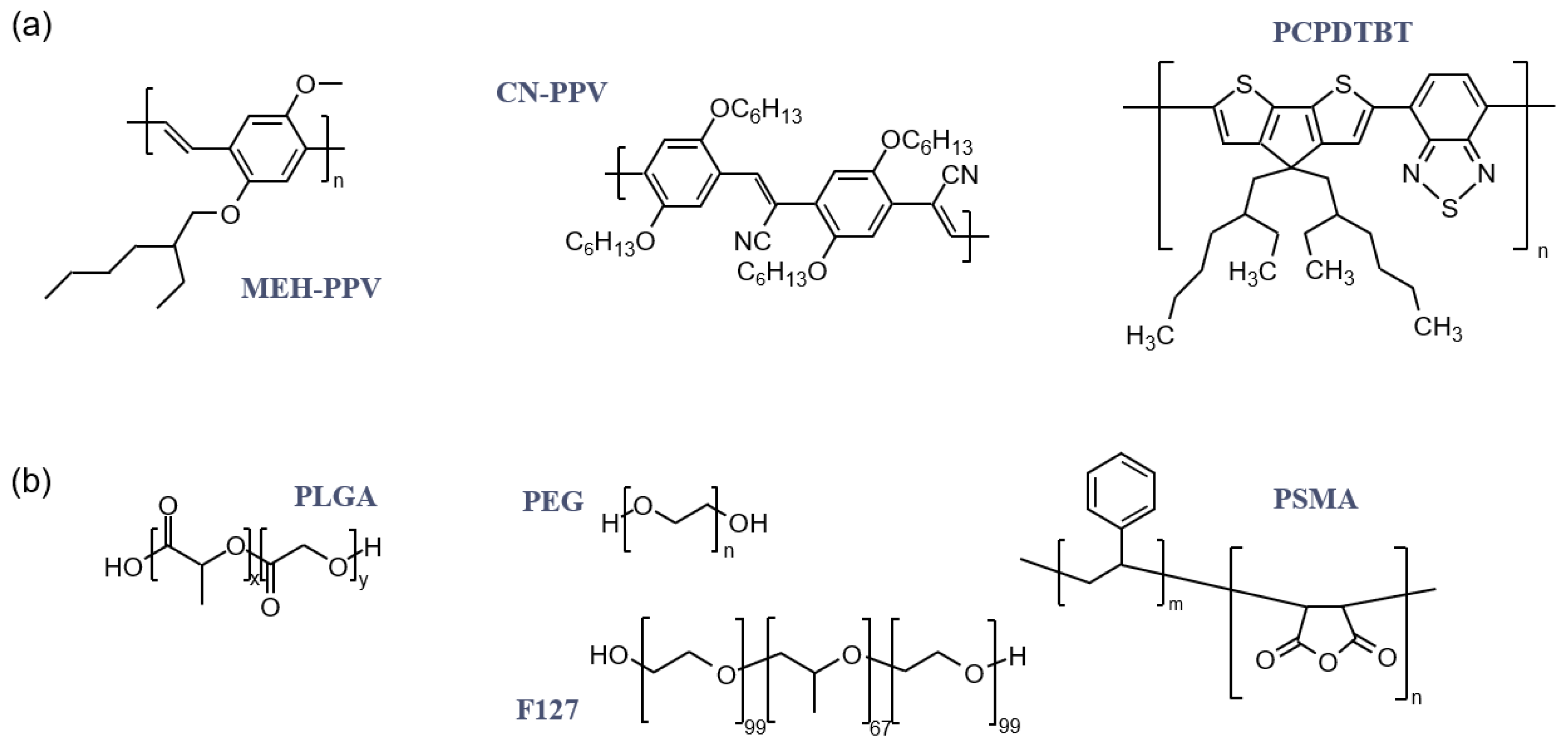
1. Introduction
2. Aqueous Solubility and Colloidal Stability of CPNs
2.1. Zeta Potential as Stability Indicator
| Core Material | Shell Material † | * (nm) | * (nm) | PLQY (%) | D (nm) | (mV) | Refs. |
|---|---|---|---|---|---|---|---|
| CN-PPV | sln (THF) | 450 | 550 | 52 | - | - | [45] |
| sln (THF) | 430 | 547 | 52 | - | - | [36] | |
| F127 + TMOS | 470 | 623 | 30 | 54 ± 3 | −12 | [45] | |
| PEG5K-PLGA55K | 430 | 635 | 35 | 75 | −8 to −11 | [36] | |
| CP1-4 | - | 750–816 | - | - | - | - | [50] |
| PSMA | - | - | - | 49 | - | [50] | |
| DPP-TT | DSPE-mPEG5K | 720 | 1100 | - | 90 | - | [51] |
| EBKCP | sln (THF) | 447 | 547 | 6 | - | - | [52] |
| PSMA | 442 | 563 | 15 | 65 | - | [52] | |
| F8BT | sln (THF) | 460 | 535 | 52–54 | - | - | [36] |
| - | 460 | 540 | 22 | 29 | −22 ± 6 | [53] | |
| PEG | 494 | 539 | 31 | 207 | - | [54] | |
| PEG5K-PLGA55K | 470 | 538 | 37 ± 1 | 105 | −4 to −10 | [36] | |
| PS-PEG-COOH | 470 | 560 | - | - | - | [55] | |
| PS-PEG-COOH | 460 | 540 | 30 | 15 | - | [30] | |
| HCPE | PEG(N3-PEG-NH2) | 355–361 | 409–415 | 30–40 | 10.8–13.5 | - | [56] |
| MEH-PPV | sln (THF) | 480 | 510 | 70 | - | - | [46] |
| sln (CHCl3) | 498 | 560 | 27 | - | - | [57] | |
| PSMA | - | 540 | 25 | 60–140 | −30 | [46] | |
| F127 | - | 495 | 35 | 40–80 | −10 | [46] | |
| F127 | 512 | 590 | 15 | 61 | 0 | [57] | |
| PLGA | - | 590 | - | 271 | −35 | [58] | |
| P2 | PEG(N3-PEG-NH2) | 375–505 | 640 | 1–12 | 130 | - | [29] |
| PBIBDF-BT | - | 811 | - | - | - | - | [59] |
| mPEG-b-PHEP | 811 | - | - | 50 | - | [59] | |
| PEG-PCL | 782 | - | - | 156 | - | [60] | |
| PBMC | PSMA | 417 | 558 | 2 | 44 | −57.7 | [15] |
| PBTB | - | 635 | - | - | - | - | [44] |
| F127 | 330–500 | 420–653 | - | 192 | −11 | [44] | |
| PCPDTBSe | - | 764 | - | - | 150 | −33.5 | [61] |
| F127 | 764 | - | - | 92 | 1.6 | [61] | |
| PCPDTBT | sln (THF) | 690 | 760 | 67.7 | - | - | [38] |
| PEG2K-PLGA4K | 670 | 850 | 2.3 | - | - | [38] | |
| PEG2K-PLGA15K | 650 | 850 | 7.5 | - | - | [38] | |
| PEG5K-PLGA55K | 640 | 850 | 1.1 | - | - | [38] | |
| PEG2K-DPPE | 650 | 850 | 7.5 | - | - | [33] | |
| PCPDTBT + PC70BM | PEG-b-PPG-b-PEG | 650 | 840 | - | 54 | - | [62] |
| pDA | DSPE-mPEG | 654 | 1047 | 2 | <6 | - | [63] |
| PDPP3T | - | 770 | - | - | - | - | [64] |
| F127 | 780 | - | - | 134.9 | - | [64] | |
| PDPP-DBT | DSPE-PEG-Mal | 750 | 822 | <0.1 | 100 | −34.4 ± 1.8 | [47] |
| DSPE-PEG-Mal-Tat | 750 | 822 | - | - | 23.1 ± 1.7 | [47] | |
| PFBD-N3 | sln (C6H5CH3) | 315,463 | 530 | - | - | - | [65] |
| sln (THF) | 315,463 | 537 | - | - | - | [65] | |
| sln (CHCl3) | 315,463 | 549 | - | - | - | [65] | |
| sln (CH2Cl2) | 315,463 | 557 | - | - | - | [65] | |
| PEG(N3-PEG-NH2) | 320,468 | 585 | 11–17 | - | - | [65] | |
| PFBO | PS-PEG-COOH | 550 | 603 | - | - | - | [55] |
| PFBT | PLGA | - | 560 | - | 243 | −33.4 | [58] |
| PF-DBT-COOtBut | - | 370,545 | - | 1.1 | - | −23.7 | [66] |
| F127 | 380,555 | 415,645 | 11.3 | 220 | −10.68 | [66] | |
| PFO | - | 385 | 419 | - | - | - | [67] |
| F127 | 380 | 439 | 63 | 105–142 | - | [67] | |
| PSMA | 375 | 430 | - | 10 | - | [68] | |
| PFODBT | - | 535 | 705 | 2.8 | 31 | −25 ± 5 | [53] |
| DPPC | 535 | 695 | 2.5 | 35 | −36 ± 7 | [53] | |
| PFP | PS-PEG-COOH | 375 | 425 | - | - | - | [55] |
| PFPE | - | 340 | 375,393 | - | - | - | [67] |
| F127 | 345 | 400,419 | 76 | 100–137 | - | [67] | |
| PFPtTFPP | PSMA | 375 | 651 | 3.3/9 | 21 | −33.4 | [43] |
| PFQ | PS-PEG-COOH | 400 | 500 | - | - | - | [55] |
| PFTBT5 | PSMA | 365 | 650 | - | 13 | - | [68] |
| PTB7 | - | 675 | 780 | 0.5 | 140 ± 50 | - | [69] |
| F127 | 682 | 775 | 76 | 190 ± 60 | - | [69] | |
| PSMA | 380 | 765 | 76 | 150 ± 40 | - | [69] | |
| PtTFPP + PFO | Poly-L-lysine | 440 | 650 | - | 110 | 45–53 | [70] |
| PFVBT | - | 365–502 | 612 | - | - | - | [71] |
| PSMA | 502 | 598 | - | 120 ± 11 | - | [72] | |
| PFVBT + PIDTTTQ | DSPE-PEG2K-Mal | 500 | 612 | 23 ± 1 | 34 ±0.9 | - | [71] |
| PIDTTTQ | - | 620–1100 | - | - | - | - | [71] |
| Poly[9,9-bis(2-ethylhexyl)fluorene] | - | 375 | 420 | 19 | - | - | [49] |
| PEI-PCL-PEG-FA | 375 | 420 | 33 | 100 | 30 | [49] | |
| PTPEDC | DSPE-PEG-Mal | 310 | 650 | 3 to 12 | 30 | −44.2 to −46.6 | [48] |
| DSPE-PEG-Mal-Tat | 310 | 650 | - | - | −2.5 to −6.6 | [48] | |
| SP2 | DSPE-mPEG2K | 635–748 | 835 | 0.1–10 | 46 | - | [73] |
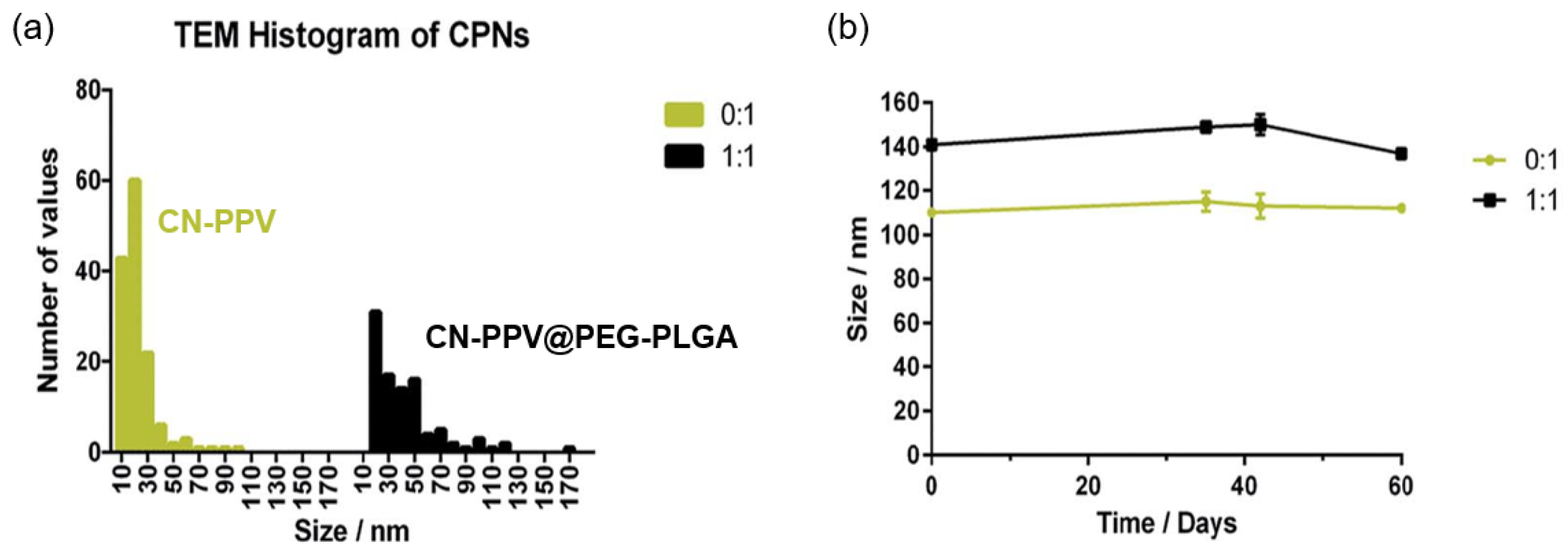
2.2. Improved Stability of Shelled CPNs
2.3. Stability of CPNs in Different Environments
2.4. Long Term Stability
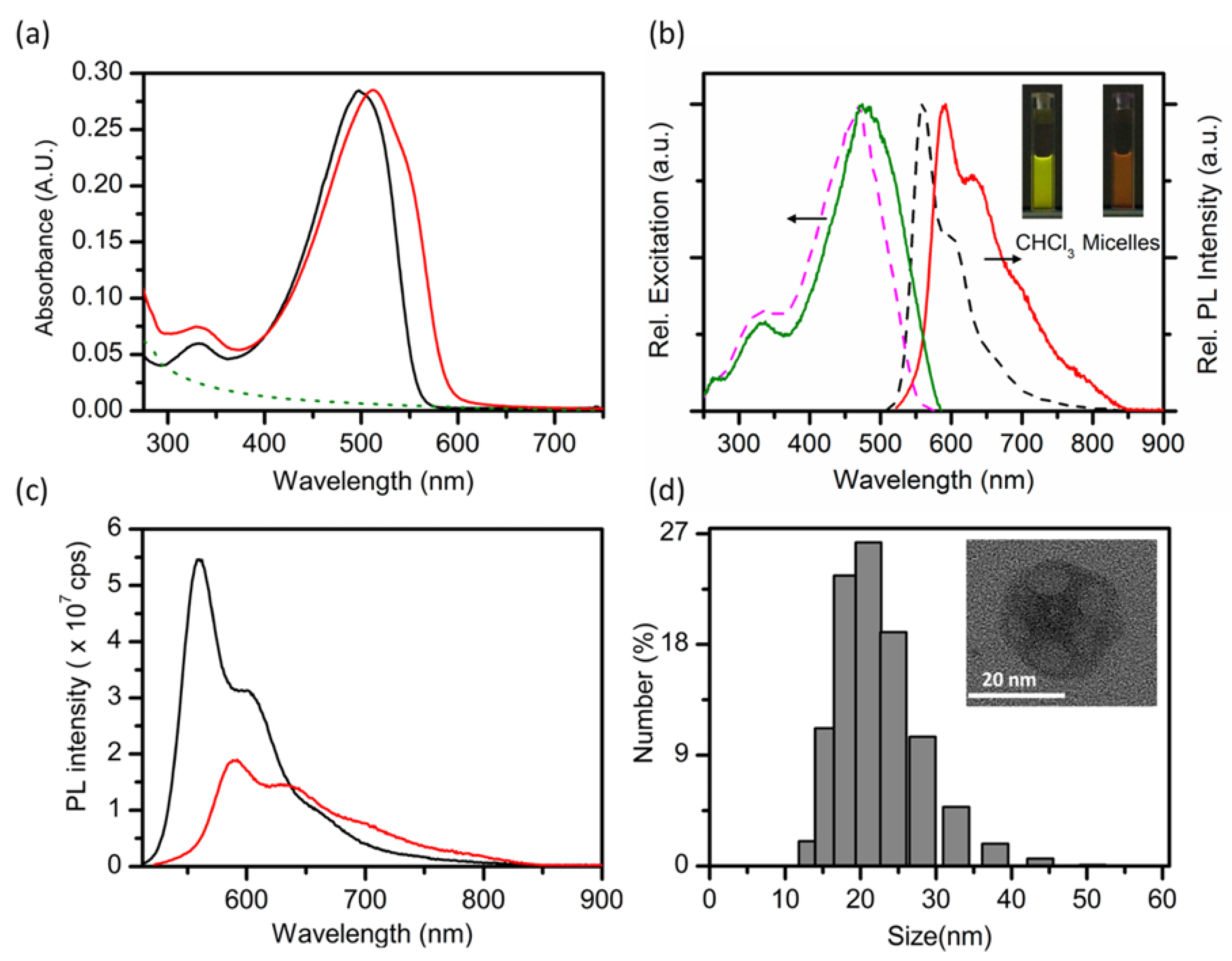
3. Optical Properties of CPNs
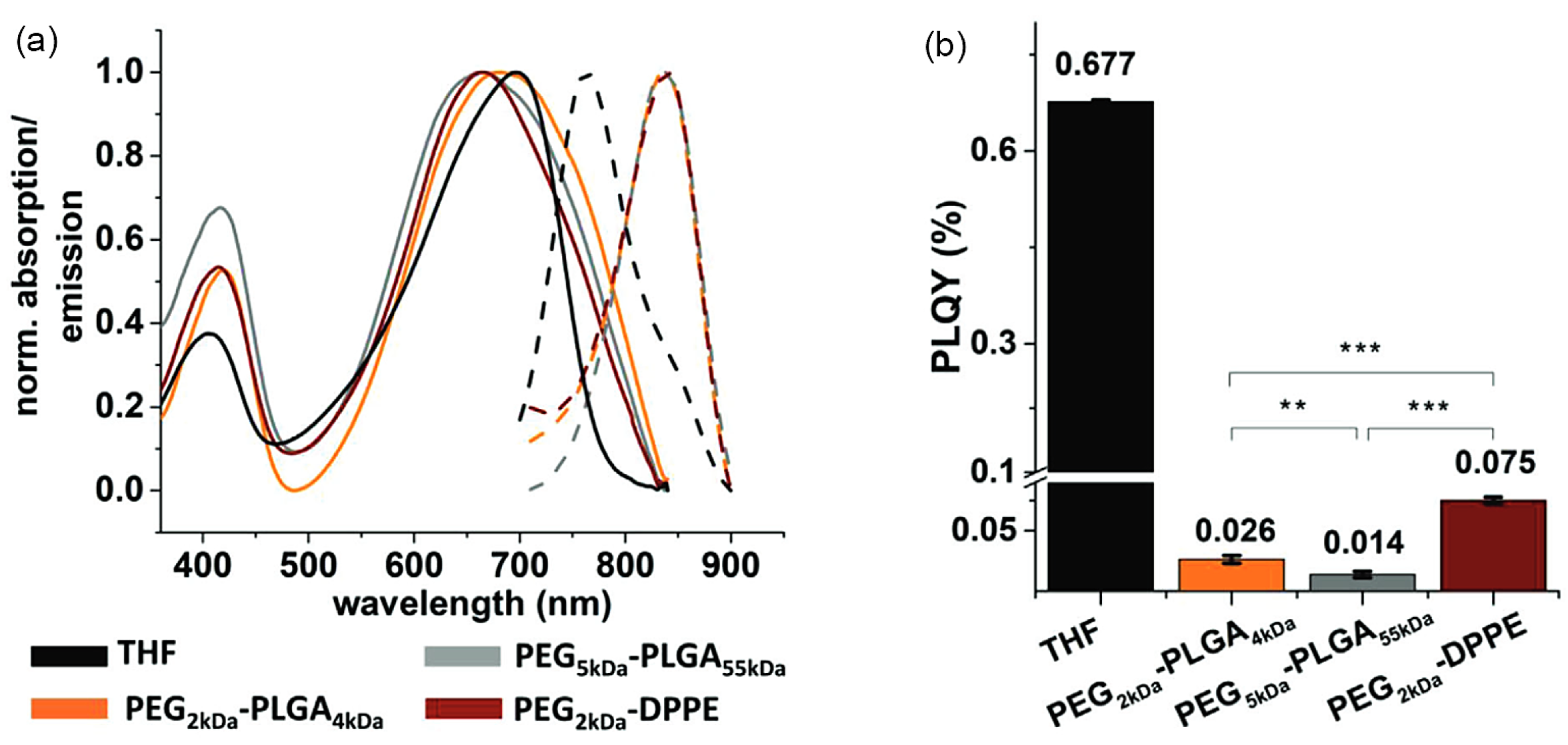
3.1. Shifts in Optical Spectra
3.2. Photoluminescence Quantum Yield Changes
3.3. Photostability Changes
4. Cell Targeting and Uptake
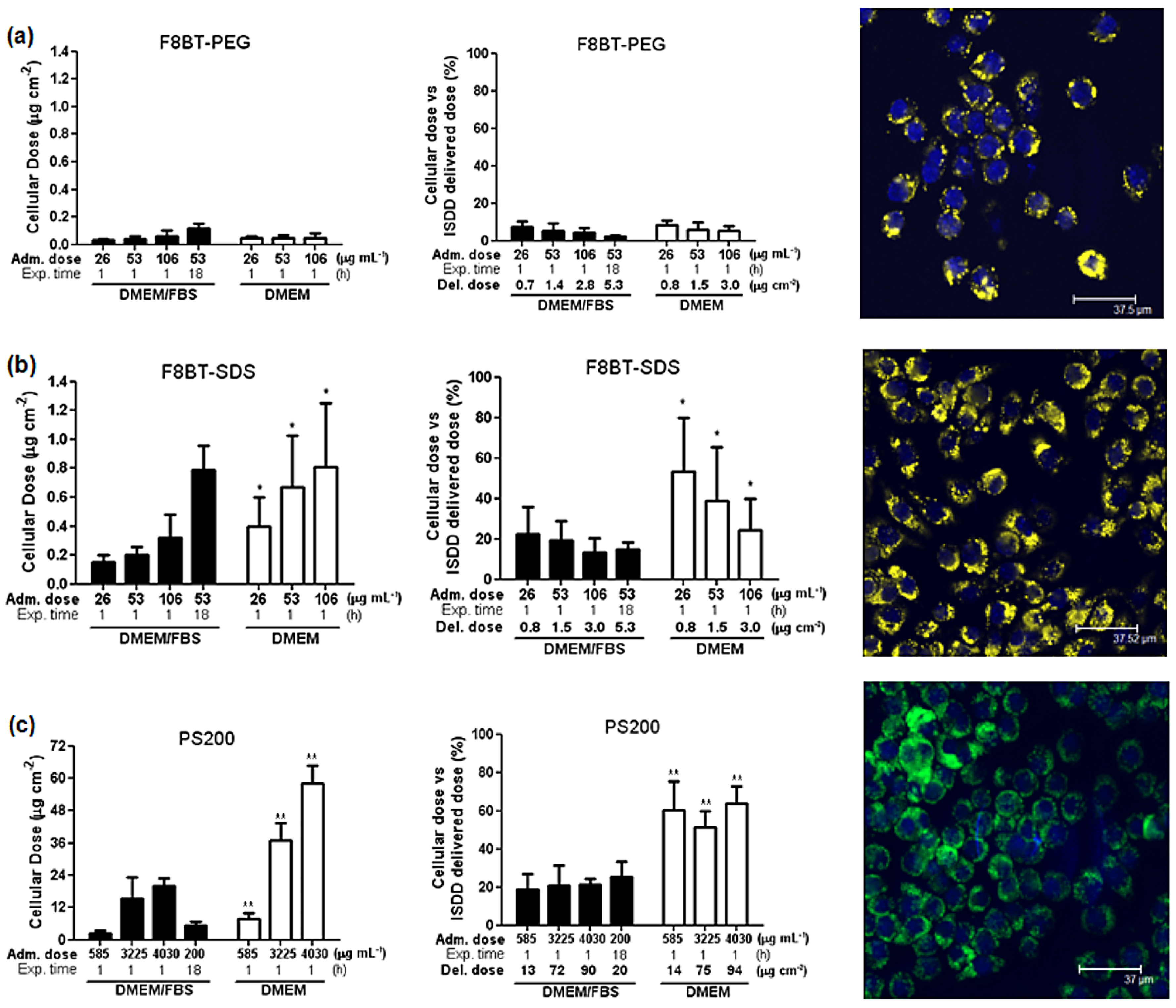
4.1. Cpns in Serum-Containing Environments
4.2. Effects of Zeta Potential and Size of CPNs
5. Multimodal CPNs-Based Probes
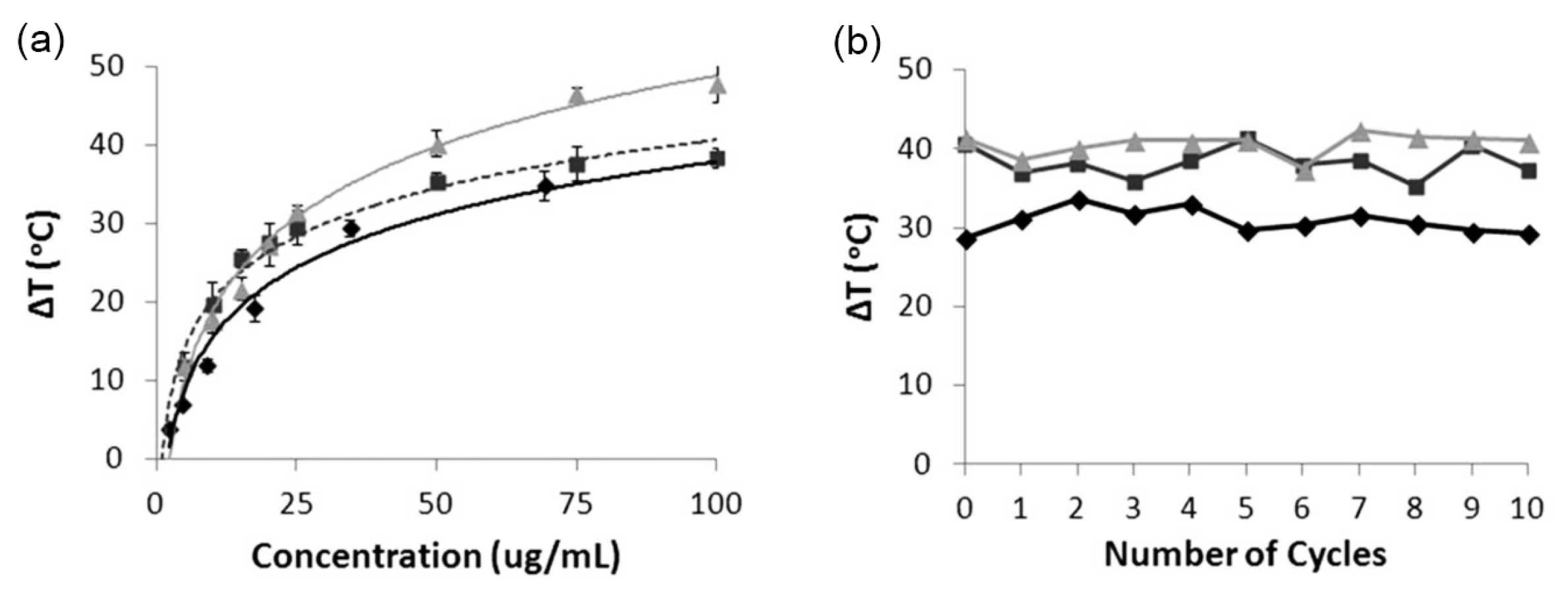
5.1. Photothermal Properties
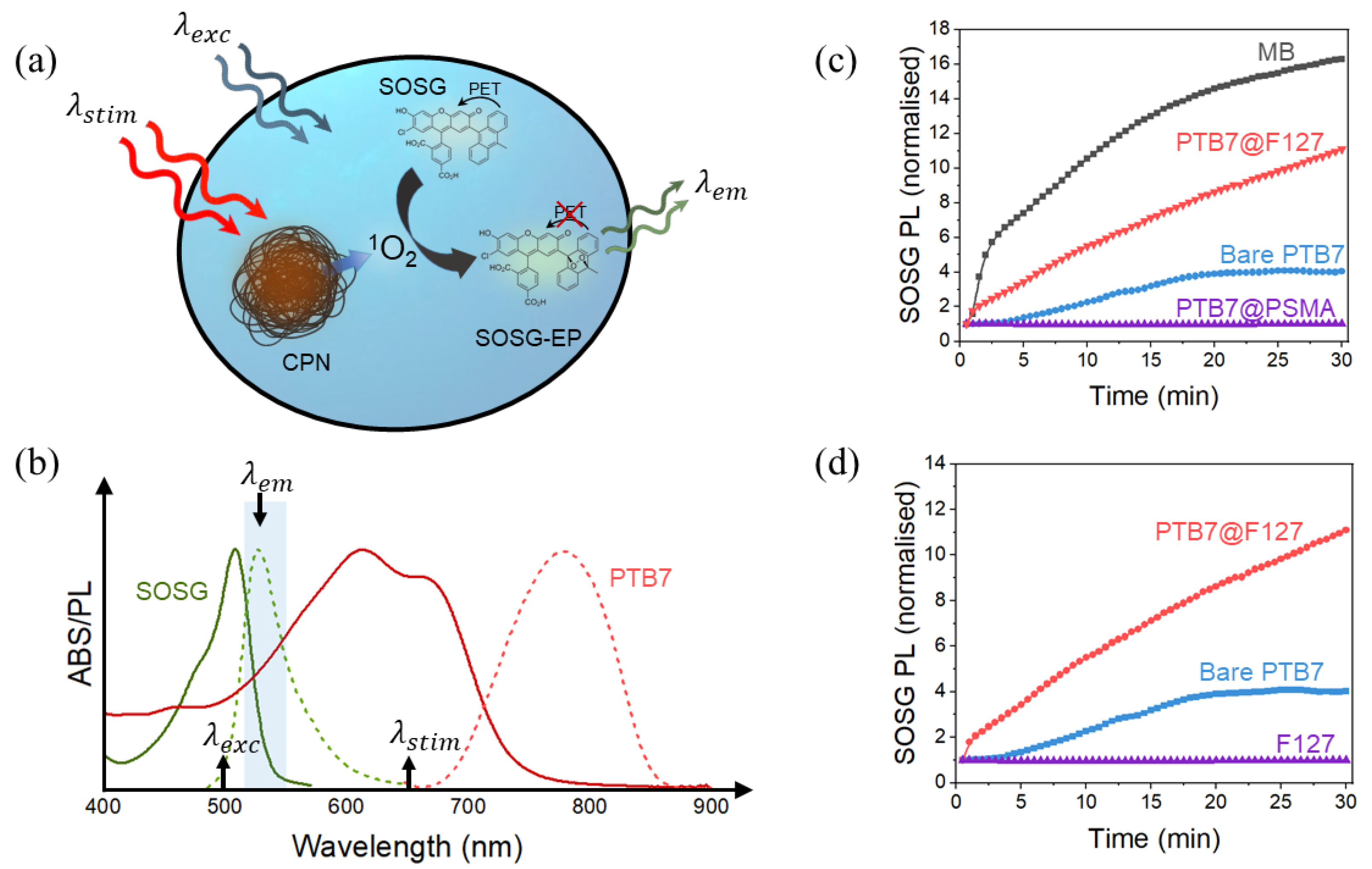
5.2. Photodynamic Properties
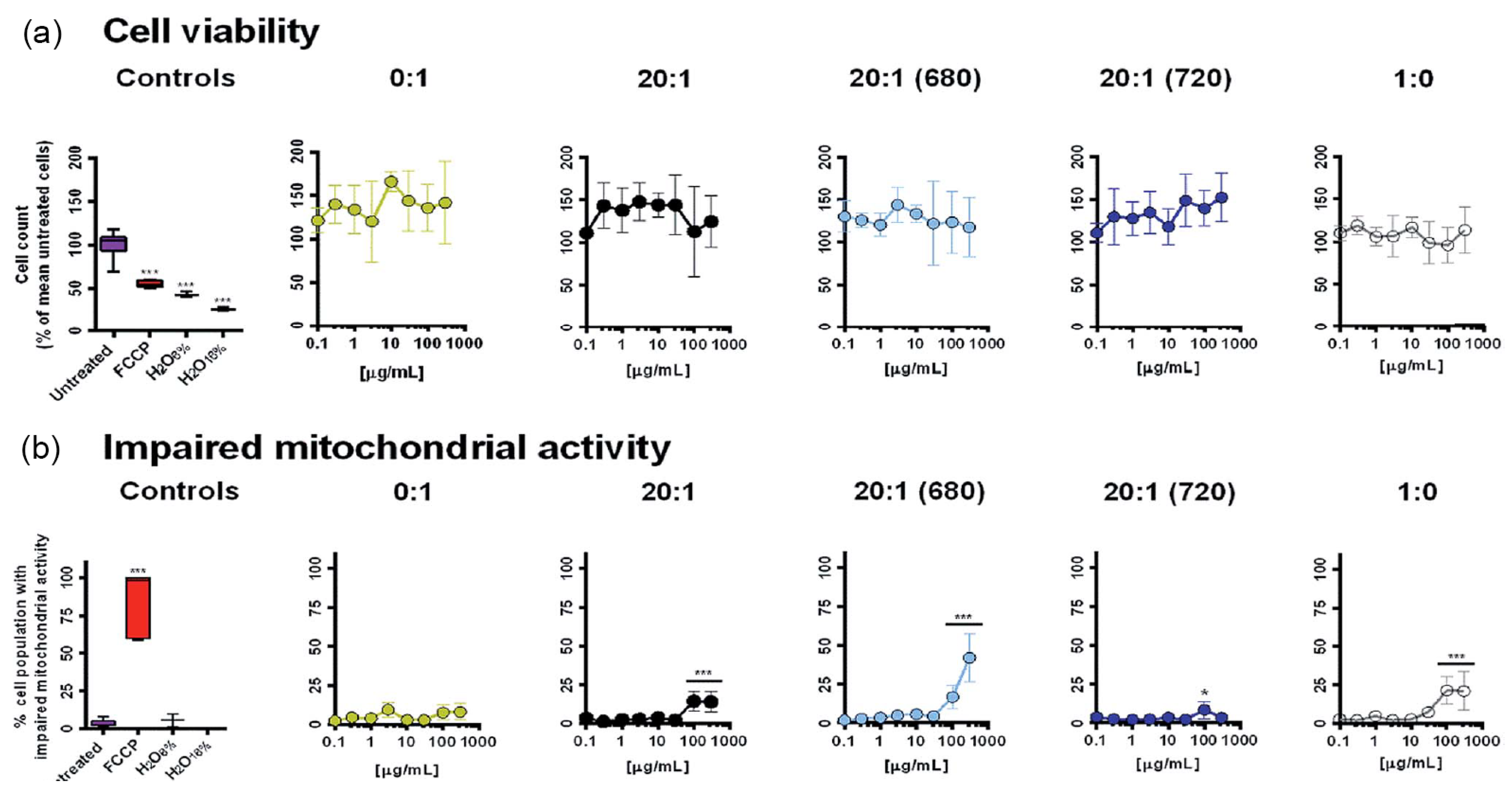
6. Biocompatibility and Cytotoxicity
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABDA | 9,10-Anthracenediyl-bis(methylene)dimalonic acid |
| ADMA | Assymetric dimethylarginine |
| BDT | Benzodithiophene |
| BBT | Benzobisthiadiazole |
| BT | Bithiophene |
| BIBDF | (3E,7E)-3,7-Bis(2-oxoindolin-3-ylidene)benzo-[1,2-b:4,5-b]-difuran-2,6(3H,7H)-dione |
| BIBDF | Bis(2- oxoindolin-3-ylidene)-benzodifuran-dione |
| BBT-EHT | Benzo[1,2-c;4,5-c0]bis[1,2,5]thiadiazole-4,7-bis(5-(2-ethylhexyl)thiophene) |
| BBT-2FT | Benzo[1,2-c;4,5-c0]bis[1,2,5]thiadiazole-4,7bis(9,9-dioctyl-9H-fluoren-2-yl)thiophene |
| CP(s) | Conjugated Polymer(s) |
| CPE | Conjugated polyelectrolyte |
| CPN(s) | Conjugated Polymer Nanoparticle(s) |
| CT | Cytotoxicity investigation |
| CN-PPV | Poly(2,5-di(hexyloxy)cyanoterephthalylidene) |
| DBT | 1,4-dithienylbenzothiadiazole |
| DCFDA | 2,7-Dichlorodihydrofluorescein diacetate |
| DPBF | 1,3-Diphenylisobenzofuran |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco modified essential medium |
| EPR | Enhanced permeability and retention |
| ESR | Electron spin resonance |
| FA | Folic acid |
| F8BT | Poly(9,9-dioctylfluorene-2,1,3-benzothiadiazole) |
| FDA | Food and drug administration |
| F127 | Pluronic poly(ethylene glycol)-block-poly(propylene glycol)-block- |
| -poly(ethylene glycol) diacrylate | |
| IMG | Confocal imaging |
| NP(s) | Nanoparticle(s) |
| Mal | Maleimide |
| MEH-PPV | Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] |
| MR | Magnetic resonance |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MW | Molecular weight |
| HCPE | Hyperbranched conjugated polyelectrolyte |
| HER2 | Human epidermal growth factor receptor 2 |
| P1 | Poly[9,9-bis(6 -(N, N -dimethylamino)hexyl))fluorenyldivinylene-alt-4,7- |
| (2,1,3,-benzothiadiazole) dibro-mide] | |
| P2 | poly[9,9-bis(N-(but-3-ynyl)-N,N-dimethylamino)hexyl))fluorenyldivinylene-alt- |
| 4,7-(2,1,3,-benzothiadiazole) dibromide] | |
| PA | Photoacoustic imaging |
| PBMC | poly[3-2-[2,5-Bis-(2-ethyl-hexyloxy)-4-propenyl-phenyl]-vinyl-9-butyl-6-methyl- |
| 9H-carbazole] | |
| PBS | Phosphate-buffered saline |
| PC | Protein corona |
| PC70BM | (6,6)-phenyl-C71-butyric acid methyl ester |
| PCBM | (6,6)-phenyl-C61-butyric acid methyl ester |
| PCPDTBT | Poly[2,6-(4,4-bis(2-ethylhexyl)-4H-cyclopenta- [2,1-b;3,4-b]dithiophene)-alt- |
| 4,7-(2,1,3-benzothiadiazole)] | |
| PDA | Poly(benzo[1,2-b:3,4-b]difuran-alt-fluorothieno-[3,4-b]thiophene) |
| PCPDTBSe | poly[4,4-bis(2-ethylhexyl)-cyclo-penta[2,1-b;3,4-b]dithiophene-2,6-diyl-alt22,1,3- |
| benzoselena-diazole-4,7-diyl] | |
| PDHF | Poly(9,9-dihexylfluorene) |
| PDPP3T | Poly((2,5-diyl-2,3,5,6- tetrahydro-3,6-dioxo-pyrrolo(3,4-c)pyrrole-1,4-diyl)- |
| alt-(2,2:5,2-terthiophene-5,5-diyl)) | |
| PDPP-DBT | Poly[2,6-4,8-di(5-ethylhexylthienyl)benzo[1,2-b;3,4-b]dithiophene-alt-5- |
| dibutyloctyl-3,6-bis(5-bromothiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4-dione] | |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PET | Photoinduced electron transfer |
| PEO | Poly(oxyethylene) |
| PF | Poly[9,9-bis(2-ethylhexyl)fluorene] |
| PF-2 | Poly[9,9-dihexylfluorene-alt-9,9-bis(2-(2-(2-methoxyethoxy)ethoxy)ethyl)fluorine] |
| PFBD-N | Poly(9,9-bis(6-azidohexyl)fluorene-alt-2,1,3-benzoxadiazole) |
| PFBO | 2,1,3-Benzooxadiazole-alt-fluorene |
| PFBT | Poly[9,9-bis(2-(2-(2-methox-yethoxy)ethoxy)ethyl)fluorene-alt-4,7- |
| (2,1,3-benzothiadiazol)] | |
| PFO | Poly(9,9-dioctylfluorenyl-2,7-diyl) |
| PFODBT | Poly[2,7-(9,9-dioctylfluorene)-alt-4,7-bis(thiophen-2-yl)benzothiadiazole] |
| PFPE | Poly(2,7-(9,9-hexylfluorene)-alt-4,4-phenylether) |
| PFPtTFPP | Poly((2,7-dibromo-9,9-dioctyl-9H-fluorene)(9,9-dioctyl-9H-fluorene-2,7-diboronic acid |
| bis(pinacol)ester)(platinum(II) 5,15-bis(pentafluorophenyl)-10,20-bis(4-bromophenyl) | |
| porphyrin)) | |
| PFPV | Poly[9,9-dioctyl-2,7-divinylene-fluorenylene-alt-co-(2-methoxy-5-(2- ethylhexyloxy)- |
| 1,4-phenylene)] | |
| PFTBT5 | Poly(9,9-dioctylfluorene)-co-(4,7-di-2-thienyl-2,1,3-benzothiadiazole) |
| PFV | poly[9,9-bis(2-(2-(2-methoxyethoxy)ethoxy)ethyl)fluorenyldivinylene-alt-9,9-bis |
| (3-t-butylpropanoate)fluorene] | |
| PFVBT | Poly[9,9-bis(N-but-3-ynyl-N,N-dimethylaminohexyl)fluorenyldivinylene-alt- |
| 4,7-(2,1,3,-benzothiadiazole) dibromide] | |
| PI | Propidium iodide |
| PIDTTTQ | Poly[(4,4,9,9-tetrakis(4-(octyloxy)phenyl)-4,9-dihydro-s-indacenol-dithiophene-2,7- |
| diyl)-alt-co-4,9-bis(thiophen-2-yl)-6,7-bis(4-(hexyloxy)phenyl)- thiadiazolo- | |
| quinoxaline] | |
| PLGA | Poly(D, L-lactide-co-glycolide) |
| PLQY | Photoluminescence quantum yield |
| PPO | Poly(oxypropylene) |
| PSMA | Poly (styrene-co-maleic anhydride) |
| PTB7 | Poly(4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b]dithiophene-2,6-diyl(3-fluoro-2- |
| [(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl)) | |
| PTO | polythiophene |
| PTT | Photothermal therapy |
| PtTFPP | Platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)-porphyrin |
| QY | Quantum yield |
| RNO | p-Nitrosodimethylaniline |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulfate |
| SOSG | Singlet oxygen sensor green |
| SP1 | Poly(diketopyrrolopyrrole-altthiophene) |
| SP2 | Poly(diketopyrrolopyrrole-altthiadiazoloquinoxaline) |
| Tat | TAT peptide (GRKKRRQRRRPQ) |
| THF | Tetrahydrofuran |
References
- Qian, C.; Chen, Y.; Feng, P.; Xiao, X.; Dong, M.; Hu, Q.; Shen, Q.; Gu, Z. Conjugated polymer nanomaterials for theranostics. Acta Pharmacol. Sin. 2017, 38, 764–781. [Google Scholar] [CrossRef]
- Li, K.; Liu, B. Polymer encapsulated conjugated polymer nanoparticles for fluorescence bioimaging. J. Mater. Chem. 2012, 22, 1257–1264. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.; Liu, L.; Ma, Y.; Wang, S. Strategies to design conjugated polymer based materials for biological sensing and imaging. Coord. Chem. Rev. 2018, 354, 135–154. [Google Scholar] [CrossRef]
- Sun, H.; Schanze, S.K. Functionalization of Water-Soluble Conjugated Polymers for Bioapplications. ACS Appl. Mater. Interfaces 2022, 14, 20506–20519. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Bergstedt, T.; Shi, X.; Rininsland, F.; Kushon, S.; Xia, W.; Ley, K.; Achyuthan, K.; McBranch, D.; Whitten, D. Fluorescent-conjugated polymer superquenching facilitates highly sensitive detection of proteases. Proc. Natl. Acad. Sci. USA 2004, 101, 7511–7515. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification–diffusion methods: Critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles - A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Ye, X.; Zhang, J.; Chen, H.; Wang, X.; Huang, F. Fluorescent nanomicelles for selective detection of Sudan dye in pluronic F127 aqueous media. ACS Appl. Mater. Interfaces 2014, 6, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Abelha, T.; Dreiss, C.A.; Green, M.A.; Dailey, L.A.; Abelha, T.F. Conjugated polymers as nanoparticle probes for fluorescence and photoacousting imaging. J. Mater. Chem. B 2020, 8, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Rong, Y.; Kuo, C.T.; Zhou, X.H.; Chiu, D.T. Recent advances in the development of highly luminescent semiconducting polymer dots and nanoparticle for biological imaging and medicine. Anal. Chem. 2017, 89, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hussain, S.; Abbas, A.; Hao, Y.; Malik, A.H.; Tian, X.; Song, H.; Gao, R. Conjugated polymer nanoparticles and their nanohybrids as smart photoluminescent and photoresponsive material for biosensing, imaging and theranostics. Microchim. Acta 2022, 189, 83. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kim, J.; Jang, E.S. Recent progress in multifunctional conjugated polymer nanomaterial-based synergistic combination phototherapy for microbial infection diagnostics. Coord. Chem. Rev. 2022, 470, 214701. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, X.; Xing, S.; Lu, Q.; Yao, J.; Liu, Q.; Qiao, C.; Xie, R.; Ding, B. Conjugated polymer nanoparticles based on carbazole for detecting ferric ion (III) with a large Stokes shift and high sensitivity and the application in cell imaging. Dye Pigment 2019, 168, 68–76. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic®block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug. Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Jung, Y.; Hickey, R.J.; Park, S.J. Encapsulating light-emitting polymers in block copolymer micelles. Langmuir 2010, 26, 7540–7543. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jin, Y.; Schneider, T.; Burnham, D.R.; Smith, P.B.; Chiu, D.T. Ultrabright and bioorthogonal labeling of cellular targets using semiconducting polymer dots and click chemistry. Angew. Chemie -Int. Ed. 2010, 49, 9436–9440. [Google Scholar] [CrossRef]
- Alexandar, S.; de Vos, W.M.; Castle, T.C.; Cosgrove, T.; Prescott, S.W. Growth and shrinkage of pluronic micelles by uptake and release of flurbiprofen: Variation of pH. Langmuir 2012, 28, 6539–6545. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Zacharia, N.S.; Verploegen, E.; Hammond, P.T. Extended release antibacterial layer-by-layer films incorporating linear-dendritic block copolymer micelles. Chem. Mater. 2007, 19, 5524–5530. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, Y.; Park, T.G. Oil-encapsulating PEO-PPO-PEO/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules 2007, 8, 650–656. [Google Scholar] [CrossRef]
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.J.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Parray, Z.A.; Hassan, M.I.; Ahmad, F.; Islam, A. Amphiphilic nature of polyethylene glycols and their role in medical research. Polem. Test. 2020, 82, 106316. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, C.; Lin, W.; Hu, R.; Wang, Q.; Chen, H.; Li, L.; Chen, S.; Zheng, J. Binding characteristics between polyethylene glycol (PEF) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef]
- Zalipsky, S.; Menon-Rudolph, S. Hydrazide derivatives of poly(ethylene glycol) and their bioconjugates. In Poly(ethylene glycol): Chemistry and Biological Applications; Zalipsky, S., Harris, M.J., Eds.; American Chemical Society: Washington, DC, USA, 1997; pp. 21–318. [Google Scholar]
- Locatelli, E.; Comes Franchini, M. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanoparticle Res. 2012, 14, 1316. [Google Scholar] [CrossRef]
- Pu, K.Y.; Li, K.; Liu, B. A molecular brush approach to enhance quantum yeild and suppress nonspecific interactions of conjugated polyelectrolyte for targeted far-red/near-infrared fluorescence cell imaging. Adv. Funct. Mater. 2010, 20, 2770–2777. [Google Scholar] [CrossRef]
- Wu, C.; Schneider, T.; Zeigler, M.; Yu, J.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugated of ultrabright semiconducting polymer dots for specific cellular targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Muthu, M.S. Nanoparticles based on PLGA and its co-polymer: An overview. Asian J. Pharm. 2009, 3, 266–273. [Google Scholar] [CrossRef]
- Neumann, P.R.; Erdmann, F.; Holthof, J.; Hädrich, G.; Green, M.A.; Rao, J.; Dailey, L.A. Different PEG-PLGA matrices influence in vivo optical/photoacoustic imaging performance and biodistribution of NIR-emitting π-conjugated polymer constrast agents. Adv. Healthc. Mater. 2021, 10, 2001089. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, K.; Pu, K.Y.; Fing, D.; Liu, B. Conjugated polymer and gold nanoparticle co-loaded PLGA nanocomposites with eccentric internal nanostructure for dual-modal targeted cellular imaging. Small 2012, 8, 2421–2429. [Google Scholar] [CrossRef]
- Modicano, P.; Neumann, P.R.; Schüller, M.; Holthof, J.; Kyrilis, F.L.; Hamdi, F.; Kastritis, P.L.; Mäder, K.; Dailey, L.A. Enhanced optical imaging properties of lipid nanocapsules as vehicles for fluorescent conjugated polymers. Eur. J. Phram. Biopharm. 2020, 154, 297–308. [Google Scholar] [CrossRef]
- Abelha, T.F.; Phillips, T.W.; Bannock, J.H.; Nightingale, A.M.; Dreiss, C.A.; Kemal, E.; Urbano, L.; deMello, D.C.; Green, M.; Dailey, L.A. Bright conjugated polymer nanoparticles containing a biodegradable shell produced at high yields and with tuneable optical properties by a scalable microfluidic device. Nanoscale 2017, 9, 2009–2019. [Google Scholar] [CrossRef]
- Kemal, E.; Abelha, T.F.; Urbano, L.; Peters, R.; Owen, D.M.; Howes, P.; Green, M.A.; Dailey, M.G. Bright, near infrared emitting PLGA-PEG dye-doped CN-PPV nanoparticles for imaging applications. RSC Adv. 2017, 7, 15255–15264. [Google Scholar] [CrossRef]
- Abelha, T.F.; Neumann, P.R.; Holthof, J.; Dreiss, C.A.; Alexander, C.; Green, M.A.; Dailey, L.A. Low molecular weight PEG-PLGA polymers provide a superior matrix for conjugated polymer nanoparticle in terms of physicochemical properties, biocompatibility and optical/photoacoustic performance. J. Mater. Chem. B 2019, 7, 5115–5124. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Zyuzin, M.V.; Honold, T.; Carregal-Romero, S.; Kantner, K.; Karg, M.; Parak, W.J. Nanoparticle agglomeration: Influence of temperature on the colloidal stability of polymer-coated gold nanoparticles in cell culture media. Small 2016, 12, 1723–1731. [Google Scholar] [CrossRef]
- Segets, D.; Marczak, R.; Schäfer, S.; Paula, C.; Gnichwitz, J.-F.; Hirsch, A.; Peukert, W. Experimental and theoretical dtudies of the colloidal stability of nanoparticles – a general interpretation based on stability maps. ACS Nano 2011, 5, 4658–4669. [Google Scholar] [CrossRef] [PubMed]
- Pochapski, D.J.; Carvalho Dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Fang, X.; Ju, B.; Liu, Z.; Wang, F.; Xi, G.; Sun, Z.; Chen, D.; Sui, C.; Wang, M.; Wu, C. Compact conjugated polymer dots with covalently incorporated metalloporphyrins for hypoxia bioimaging. ChemBioChem 2019, 20, 521–525. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.Y.; Lim, Y.T.; Lee, T.S. Synthesis of conjugated polymer nanoparticles with core-shell structure for cell imaging and photodynamic cancer therapy. Macromol. Res. 2017, 25, 527–577. [Google Scholar] [CrossRef]
- Bourke, S.; Urbano, L.; Olona, A.; Valderrama, F.; Dailey, L.A.; Green, M.A. Silica passivated conjugated polymer nanoparticles for biological imaging applications. In Proceedings of the SPIE 10079, Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications IX: 30-31, San Francisco, CA, USA, 30–31 January 2017; Volume 10079, p. 100790A. [Google Scholar]
- Bourke, S.; Gonzalez, Y.T.; Donà, F.; Panamarova, K.; Suhling, K.; Eggert, U.; Dailey, L.A.; Zammit, P.; Green, M.A. Cellular imaging using emission-tuneable conjugated polymer nanoparticles. RSC Adv. 2019, 9, 37971–37976. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhang, P.; Bai, H.; Feng, L.; Lv, F.; Liu, L.; Wang, S. Photothermal-responsive conjugated polymer nanoparticles for remote control of gene expression in living cells. Adv. Mater. 2018, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-enhanced two-photon photosensitization for precise photodynamic therapy. ACS Nano 2019, 13, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Patra, A.; Scherf, U.; Kissel, T. Polyfluorene nanoparticles coated with folate-functionalized triblock copolymer: Effective agents for targeted cell imaging. Macromol. Biosci. 2012, 23, 1384–1390. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Hu, R.; Chen, H.; Li, M.; Wang, J.; Wang, Y.; Liu, L.; Lv, F.; Liang, X.-J.; et al. Near-infrared (NIR)-absorbing conjugated polymer dots as highly effective photothermal materials for in vivo cancer therapy. Chem. Mater. 2016, 28, 8669–8675. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Li, J.; Xia, B.; Xu, J.; Xie, C.; Fan, Q.; Huang, W. Single nanoparticles as versatile phototheranostics for tri-modal imaging-guided photothermal therapy. Biomater. Sci. 2019, 7, 3609–3613. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yang, D.; Hu, Y.; Deng, Y.; Yang, X.; Liu, Z. A novel boron ketoiminate-based conjugated polymer with large Stokes shift: AIEE feature and cell imaging application. N. J. Chem. 2021, 45, 4071–4076. [Google Scholar] [CrossRef]
- Pu, K.; Shuhendler, A.J.; Valta, M.P.; Cui, L.; Saar, M.; Peehl, D.M.; Rao, J. Phosphorylcholine-coated semiconducting polymer nanoparticles as rapid and efficient labeling agents for in vivo cell tracking. Adv. Healthc. Mater. 2014, 3, 1292–1298. [Google Scholar] [CrossRef]
- Ahmad Khanbeigi, R.; Abelha, T.F.; Woods, A.; Rastoin, O.; Harvey, R.D.; Jones, M.C.; Forbes, B.; Green, M.A.; Collins, H.; Dailey, L.A. Surface chemistry of photoluminescent F8BT conjugated polymer nanoparticles determines protein corona formation and internalization by phagocytic cells. Biomacromolecules 2015, 16, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, J.; Li, Y.; Feng, L. A high brightness probe of polymer nanoparticles for biological imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.Y.; Li, K.; Shi, J.; Liu, B. Fluorescent single-molecular core-shell nanospheres of hyperbranched conjugated polyelectrolyte for live-cell imaging. Chem. Mater. 2009, 21, 3816–3822. [Google Scholar] [CrossRef]
- Wang, S.; Ryan, J.W.; Singh, A.; Beirne, J.G.; Palomares, E.; Redmond, G. Encapsulation of MEH-PPV:PCBM Hybrids in the cores of block copolymer micellar assemblies: Photoinduced electron transfer in a nanoscale donor-acceptor system. Langmuir 2016, 32, 329–337. [Google Scholar] [CrossRef]
- Li, K.; Pan, J.; Feng, S.; Wu, A.W.; Pu, K.; Liu, Y.; Liu, B. Generic strategy of preparing fluorescent conjugated-polymer-loaded poly(DL-lactide-co-Glycolide) nanoparticles for targeted cell imaging. Adv. Funct. Mater. 2009, 19, 3535–3542. [Google Scholar] [CrossRef]
- Li, D.D.; Wang, J.X.; Ma, Y.; Qian, H.S.; Wang, D.; Wang, L.; Zhang, G.; Qiu, L.; Wang, Y.-C.; Yang, X.Z. A donor-acceptor conjugated polymer with alternating isoindigo derivative and bithiophene units for near-infrared modulated cancer thermo-chemotherapy. ACS Appl. Mater. Interfaces 2016, 8, 19312–19320. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L.; Deng, Y.; Guo, Z.; Zhang, G.; Ge, Z.; Ke, H.; Chen, H. Ultrastable near-infrared conjugated-polymer nanoparticles for dually photoactive tumor inhibition. Adv. Mater. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- MacNeill, C.M.; Graham, E.G.; Levi-Polyachenko, N.H. Soft template synthesis of donor-acceptor conjugated polymer nanoparticles: Structural effects, stability, and photothermal studies. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1622–1623. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.; Zhen, X.; Ding, D.; Pu, K. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y.; et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Sun, T.; Chen, X.; Wang, X.; Liu, J.; Xie, Z. Enhanced efficacy of photothermal therapy by combining a semiconducting polymer with an inhibitor of a heat shock protein. Mater. Chem. Front. 2019, 3, 127–136. [Google Scholar] [CrossRef]
- Liu, J.; Ding, D.; Geng, J.; Liu, B. PEGylated conjugated polyelectrolytes containing 2,1,3-benzoxadiazole units for targeted cell imaging. Polym. Chem. 2012, 3, 1567–1575. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Chen, X.; Hao, Y.; Zhang, P.; Gao, R. Fabrication of conjugated polymer encapsulated fluorescent hybrid micelles for augmented, highly selective and step-wise detection of nitroaromatic pollutants and hepatobiliary biomarker. Sens. Actuators B Chem. 2023, 377, 133081. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, H.; Wang, X.; Sun, R. F127/conjugated polymers fluorescent micelles for trace detection of nitroaromatic explosives. Dye. Pigment 2016, 125, 367–374. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Li, R.; Shan, C.; Zeng, Z.; Xue, B.; Yuan, W.; Mo, C.; Xi, P.; Wu, C.; et al. Multicolor super-resolution fluorescence microscopy with blue and carmine small photoblinking polymer dots. ACS Nano 2017, 11, 8084–8091. [Google Scholar] [CrossRef]
- Zhao, M.; Leggett, E.; Bourke, S.; Poursanidou, S.; Carter-Searjeant, S.; Po, S.; do Carmo, M.P.; Dailey, L.A.; Manning, P.; Ryan, S.G.; et al. Theranostic Near-Infrared-Active Conjugated Polymer Nanoparticles. ACS Nano 2021, 15, 8790–8802. [Google Scholar] [CrossRef]
- Wang, X.H.; Peng, H.; Yang, W.; Ren, Z.; Liu, X.; Liu, Y. Indocyanine green-platinum porphyrins integrated conjugated polymer hybrid nanoparticles for near-infrared-triggered photothermal and two-photon photodynamic therapy. J. Mater. Chem. B 2017, 5, 1856–1862. [Google Scholar] [CrossRef]
- Feng, G.; Fang, Y.; Liu, J.; Geng, J.; Ding, D.; Liu, B. Multifunctional conjugated polymer nanoparticles for image-guided photodynamic and photothermal therapy. Small 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Liu, B. Conjugated-polyelectrolyte-based polyprodrug: Targeted and image-guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source. Angew. Chem. -Int. Ed. 2014, 53, 7163–7168. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Mei, J.; Jokerst, J.V.; Hong, G.; Antaris, A.L.; Chattopadhyay, N.; Shuhendler, A.J.; Kurosawa, T.; Zhou, Y.; Gambhir, S.S.; et al. Diketopyrrolopyrrole-based semiconducting polymer nanoparticles for in vivo photoacoustic imaging. Adv. Mater. 2015, 27, 5184–5190. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissu: Potential exploitation for the treatment of cancer. Canc. Res. 1996, 56, 1194–1198. [Google Scholar]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef]
- Liu, P.; Li, S.; Jin, Y.; Qian, L.; Gao, N.; Yao, S.Q.; Huang, F.; Xu, Q.; Cao, Y. Red-emitting DPSB-based conjugated polymer nanoparticles with high two-photon brightness for cell membrane imaging. ACS Appl. Mater. Interfaces 2015, 7, 6754–6763. [Google Scholar] [CrossRef]
- Spano, F.C.; Silva, C. H- and J-aggregate behavior in polymeric semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 447–500. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.J.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Ho, C.J.H.; Li, K.; Driessen, W.; Dinish, U.S.; Wong, C.L.; Ntziachristos, V.; Liu, B.; Olivo, M. Molecular photoacoustic imaging of breast cancer using an actively targeted conjugated polymer. Int. J. Nanomed. 2015, 10, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Win, K.Y.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, L.; Lv, F.; Bazan, G.C.; Wang, S. Preparation and biofunctionalization of multicolor conjugated polymer nanoparticles for imaging and detection of tumor cells. Adv/ Mater. 2014, 26, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Kenry; Hu, D.; Lin, X.; Xu, S.; Liu, C.; Zheng, H.; Liu, B. Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumors in the second near-infrared window. Mater. Horizons 2017, 4, 1151–1156. [Google Scholar] [CrossRef]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Lyu, Y.; Zhang, L.; Xiong, Q.; Pramanik, M.; Pu, K. Broadband absorbing semiconducting polymer nanoparticles for photoacoustic imaging in second near-infrared window. Nano Lett. 2017, 17, 4964–4969. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-enhanced photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “stealthy” nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Guarnieri, D.; Guaccio, A.; Fusco, S.; Netti, P.A. Effect of serum proteins on polystyrene nanoparticle uptake and intracellular trafficking in endothelial cells. J. Nanoparticle Res. 2011, 13, 4295–4309. [Google Scholar] [CrossRef]
- Jeon, S.; Clavadetscher, J.; Lee, D.K.; Chankeshwara, S.V.; Bradley, M.; Cho, W.S. Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 2017, 353, 26–32. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Qiao, Y.; Pan, T.; Li, J.; Yang, C.; Wen, J.; Zhong, K.; Wu, S.; Su, F.; Tian, Y. Extracellular oxygen sensors based on PtTFPP and four-arm block copolymers. Appl. Sci. 2019, 9, 4404. [Google Scholar] [CrossRef]
- Moustaoui, H.; Saber, J.; Liu, Q.; Diallo, A.T.; Spadavecchia, J.; de la Chapelle, M.L.; Djaker, N. Shape and size effect on photothermal heat elevation of gold nanoparticles: Absorption coefficient experimental measurement of spherical and urchin-shaped gold nanoparticles. J. Phys. Chem. C 2019, 123, 17548–17554. [Google Scholar] [CrossRef]
- Huang, S.; Kannadorai, R.K.; Chen, Y.; Liu, Q.; Wang, M. A narrow-bandgap benzobisthiadiazole derivative with high near-infrared photothermalconversion efficiency and robust photostability for cancer therapy. ChemComm 2015, 51, 4223–4226. [Google Scholar]
- Huang, S.; Upputuri, P.K.; Liu, H.; Pramanik, M.; Wang, M. A dual-functional benzobisthiadiazole derivative as an effective theranostic agent for near-infrared photoacoustic imaging and photothermal therapy. J. Mater. Chem. 2016, 4, 1696–1703. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Zeng, Y.; Liao, N.; Cai, Z.; Liu, G.; Liu, X.; Liu, J. Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. J. Mater. Chem. B 2016, 4, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles–from the past to the future. Mater. Horizons 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
| PF | PFV 1 | PFBT | MEH-PPV | |
|---|---|---|---|---|
| Particle size 2 (nm) | ||||
| Polydispersity | ||||
| Zeta potential (mV) | ||||
| Encapsulation efficiency (%) | ||||
| Leakage of CPs in 5 days (%) |
| Cell Line | Core Material | Shell Material (Target Ligand) | Application * | Uptake # | Refs. |
|---|---|---|---|---|---|
| MCF-7 | PFPtTFPP | PSMA | IMG | unclear | [43] |
| P1-P4 | PSMA (anti-EpCAM) | IMG | membrane | [82] | |
| PFVBT + PIDTTTQ | DSPE-PEG2K-Mal (anti-HER2) | IMG | no | [71] | |
| PFVBT | N3-PEG-NH2 (cRGD) | CT | - | [72] | |
| CPE | N3-PEG-NH2 (FA) | IMG | uptake | [29] | |
| HCPE | N3-PEG-NH2 | IMG | yes | [56] | |
| PFBD-N3 | NH2-PEG-COOH (RGD) | IMG | no | [65] | |
| F8BT | PS-PEG-COOH (IgG) | IMG | membrane | [30] | |
| PFBT | PSMA (click reaction) | IMG | membrane | [20] | |
| PF/PFV/PFBT/MEH-PPV | PLGA (FA) | IMG | yes | [58] | |
| U87 Glioma | P1 (BDT + BBT) | DSPE-PEG2K | PA | unclear | [83] |
| pDA | DSPE-mPEG (erbitux) | IMG | no | [63] | |
| J774A.1 | F8BT | PEG | CT | - | [54] |
| PCPDTBT | PEG-PLGA | CT | - | [38] | |
| KB | Poly[9,9-bis(2-ethylhexyl)fluorene] | PEI-PCL-PEG (FA) | IMG / CT | unclear | [49] |
| A549 | PFP/PFQ/F8BT/PFBO | PS-PEG-COOH | CT | - | [55] |
| PTB7 | PSMA | IMG | unclear | [69] | |
| SK-BR-3 | P1-P4 | PSMA (anti-EpCAM) | IMG | membrane | [82] |
| PFVBT + PIDTTTQ | DSPE-PEG2K-Mal (anti-HER2) | IMG | yes | [71] | |
| F8BT | PS-PEG-COOH (IgG) | IMG | membrane | [30] | |
| NIH 3T3 | SP2 | PEG-b-PPG-b-PEG | CT | - | [84] |
| PFVBT + PIDTTTQ | DSPE-PEG2K-Mal (anti-HER2) | IMG | no | [71] | |
| CPE | N3-PEG-NH2 (FA) | IMG | no | [29] | |
| HPCE | N3-PEG-NH2 | CT | - | [56] | |
| DPP-TT | DSPE-mPEG5K | CT | - | [51] | |
| PF/PFV/PFBT/MEH-PPV | PLGA (FA) | IMG | no | [58] | |
| BPSB unit (S2 and M2) | PSMA | IMG | membrane | [76] | |
| 4T1 | CP | PSMA | CT | - | [50] |
| BT-BIBDF | PEG-PCL | CT | - | [60] | |
| CP1-CP4 | DSPE-mPEG2K | CT | - | [85] | |
| MDA-MB-468 | pDA | DSPE-mPEG (erbitux) | IMG | membrane | [63] |
| MDA-MB-231 | PBIBDF-BT | mPEG-b-PHEP | IMG | uptake | [59] |
| PFVBT | N3-PEG-NH2 | IMG | yes | [72] | |
| HeLa | PTPEDC | DSPE-PEG-Mal (Tat) | IMG | unclear | [48] |
| PDPP-DBT | DSPE-PEG-Mal (Tat) | CT | - | [47] | |
| P1-P4 | PSMA (anti-EpCAM) | IMG | no | [82] | |
| PCPDTBT + PC70BM | PEG-b-PPG-b-PEG | CT | - | [62] | |
| PBTB | F127 | CT | - | [44] | |
| MEH-PPV | F127 | IMG/CT | unclear | [46] | |
| MEH-PPV | PSMA | IMG/CT | unclear | [46] | |
| CN-PPV | F127 + TMOS | IMG | yes | [45] | |
| CN-PPV | PEG-PLGA | IMG | unclear | [37] | |
| PBMC | PSMA | CT | - | [15] | |
| DPSB unit (S2 and M2) | PSMA | IMG | membrane | [76] | |
| DPP-TT | DSPE-mPEG5K | IMG | unclear | [51] | |
| PFTBT5 + PFO | PSMA | CT | - | [68] | |
| BS-C-1 | PFTBT5 + PFO | PSMA | IMG | yes | [68] |
| HepG-2 | PtTFPP + PFO | poly-L-lysine | IMG/CT | yes | [70] |
| DPSB unit (S2 and M2) | PSMA | IMG/CT | membrane | [76] | |
| HT 29 | PFBD-N3 | NH2-PEG-COOH (RGD) | IMG/CT | yes | [65] |
| HCE | MEH-PPV | PSMA | IMG/CT | unclear | [46] |
| MEH-PPV | F127 | IMG/CT | unclear | [46] | |
| HEK 293 | MEH-PPV | PSMA | CT | - | [46] |
| CN-PPV | F127 + TMOS | CT | - | [45] | |
| FHs 74 Int. | PCPDTBSe | F127 | CT | - | [61] |
| CT-26 | PCPDTBSe | F127 | IMG/CT | unclear | [61] |
| WPE1-NB26 | CN-PPV | F127 + TMOS | IMG | unclear | [45] |
| WPE1-NA22 | CN-PPV | F127 + TMOS | IMG | unclear | [45] |
| RWPE-1 | CN-PPV | F127 + TMOS | IMG | unclear | [45] |
| Core Material | Shell Material | ROS Detected † | Assay/Sensor Used # | Irradiation Conditions * | Refs. |
|---|---|---|---|---|---|
| PTB7 | F127 | singlet oxygen | SOSG | 635 nm CW, 4.5 mW | [69] |
| superoxide anion | chronoamperometry | UV lamp | [69] | ||
| intracellular ROS | DCFDA assay | 660–850 nm, 10 J.cm−2 | [69] | ||
| PSMA | no | SOSG | 635 nm CW, 4.5 mW | [69] | |
| superoxide anion | chronoamperometry | UV lamp | [69] | ||
| PTPEDC | DSPE-PEG-Mal | singlet oxygen | ABDA,DCFDA | 400–700 nm, 50 mW.cm−2 | [48] |
| PFVBT | DSPE-PEG-Mal | singlet oxygen | DCFH,ABDA | 60 s CW WL at 0.25 W.cm−2 | [71] |
| DCFDA | 30 s CW WL at 0.25 W.cm−2 | [71] | |||
| BT-BIBDF | PEG-PCL | singlet oxygen | DPBF, ESR | 60 s CW WL at 0.25 W.cm−2 | [65] |
| DCFDA | 30 s CW WL at 0.25 W.cm−2 | [65] | |||
| PBTB | F127 | singlet oxygen | RNO | 254 nm, 2 W.cm−2 | [44] |
| DCFDA | [44] | ||||
| PtTFPP + PFO | poly-L-lysine | singlet oxygen | ADMA, DPBF | 540 nm | [70] |
| MTT | 405 nm, 0.03 and 0.06 W.cm−2; 740 nm, 3.0 W.cm−2 | [70] | |||
| CP1-CP4 | DSPE-mPEG | singlet oxygen | ABDA | 400–700 nm, 60 mW.cm−2 | [85] |
| DCFDA, PI, MTT | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Uzunoff, A.; Green, M.; Rakovich, A. The Role of Stabilizing Copolymer in Determining the Physicochemical Properties of Conjugated Polymer Nanoparticles and Their Nanomedical Applications. Nanomaterials 2023, 13, 1543. https://doi.org/10.3390/nano13091543
Zhao M, Uzunoff A, Green M, Rakovich A. The Role of Stabilizing Copolymer in Determining the Physicochemical Properties of Conjugated Polymer Nanoparticles and Their Nanomedical Applications. Nanomaterials. 2023; 13(9):1543. https://doi.org/10.3390/nano13091543
Chicago/Turabian StyleZhao, Miao, Anton Uzunoff, Mark Green, and Aliaksandra Rakovich. 2023. "The Role of Stabilizing Copolymer in Determining the Physicochemical Properties of Conjugated Polymer Nanoparticles and Their Nanomedical Applications" Nanomaterials 13, no. 9: 1543. https://doi.org/10.3390/nano13091543
APA StyleZhao, M., Uzunoff, A., Green, M., & Rakovich, A. (2023). The Role of Stabilizing Copolymer in Determining the Physicochemical Properties of Conjugated Polymer Nanoparticles and Their Nanomedical Applications. Nanomaterials, 13(9), 1543. https://doi.org/10.3390/nano13091543






