Long-Term Antibacterial Efficacy of Cetylpyridinium Chloride-Montmorillonite Containing PMMA Resin Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
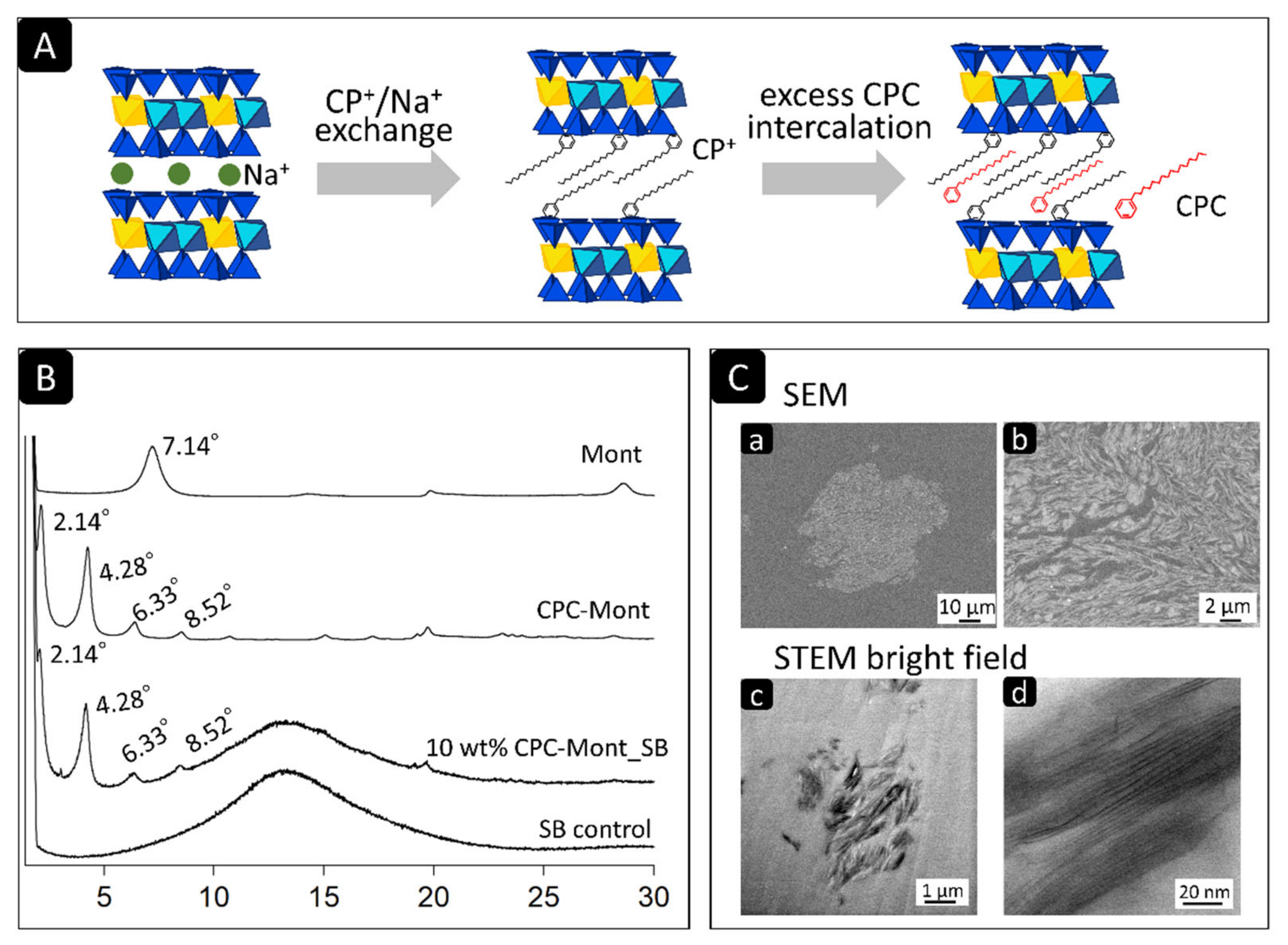
2.2. X-ray Diffraction (XRD)
2.3. Scanning (Transmission) Electron Microscopy (SEM/STEM) of CPC-Mont/SB Cross-Sections
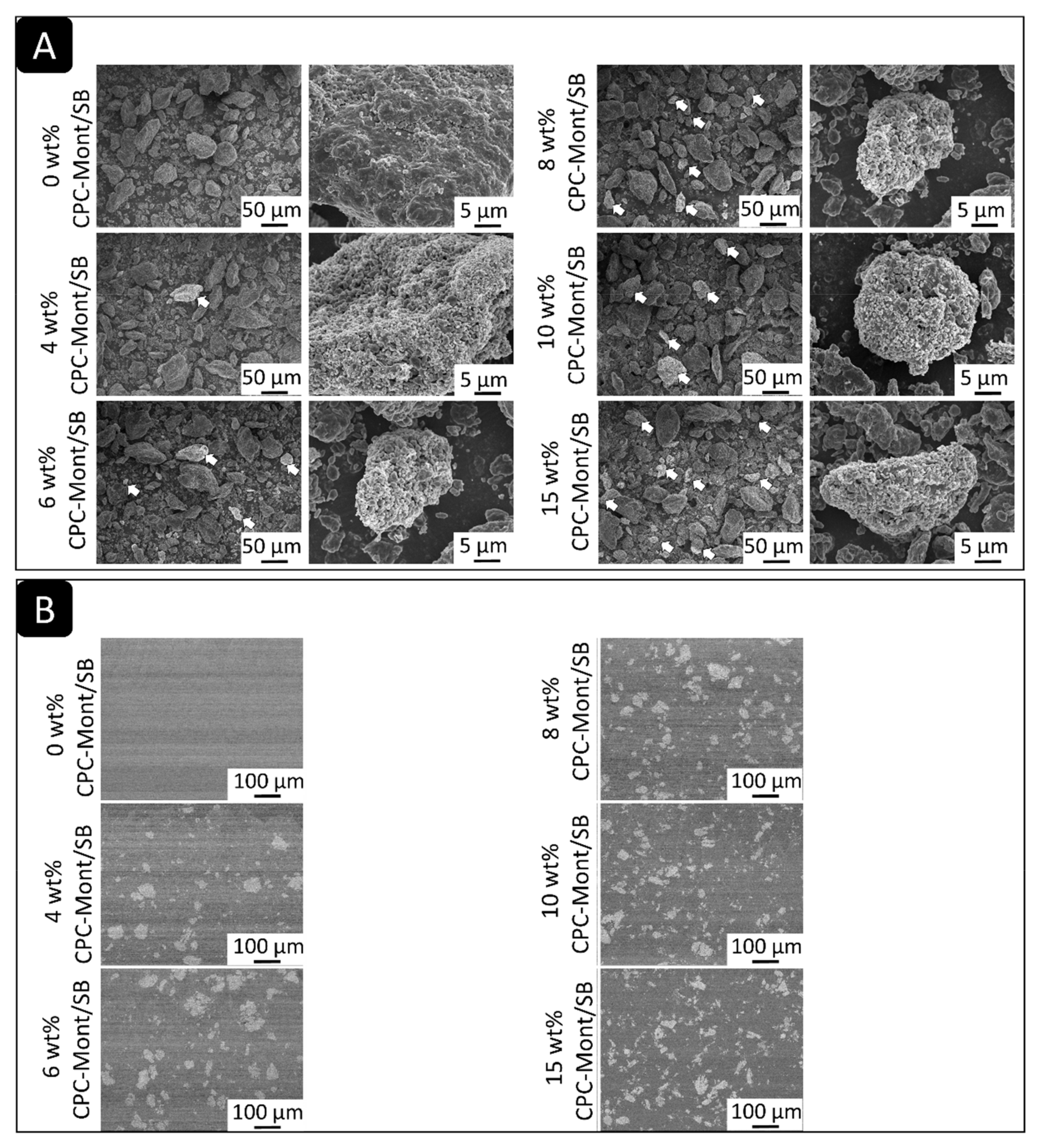
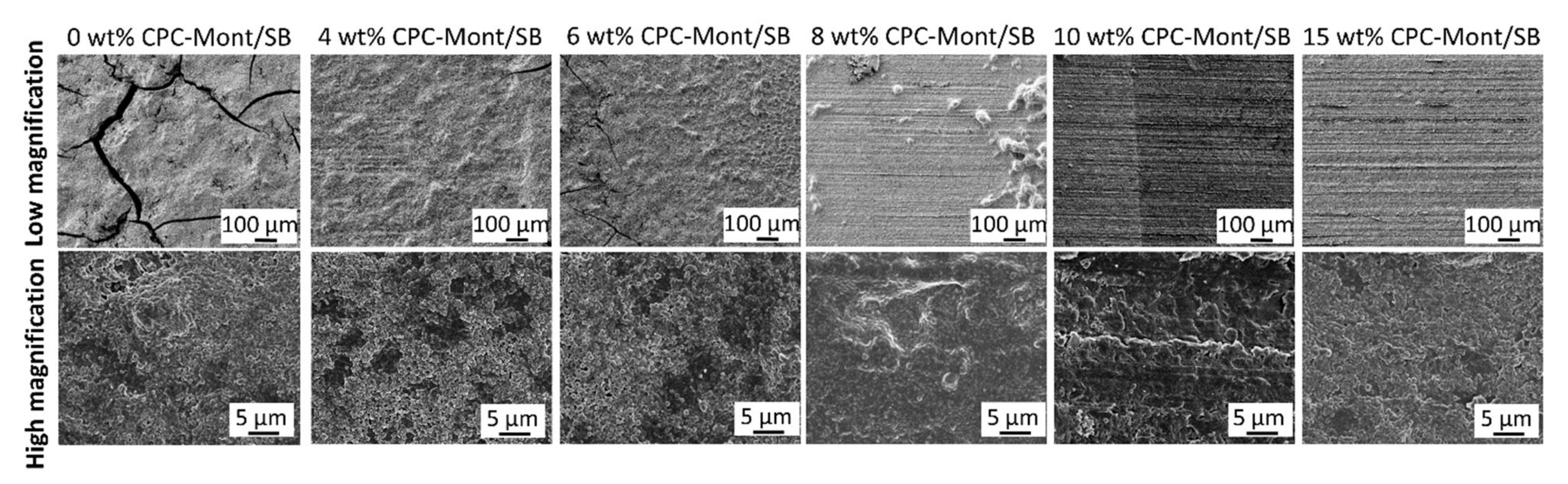
2.4. SEM of CPC-Mont/SB Powder and Cement Disks
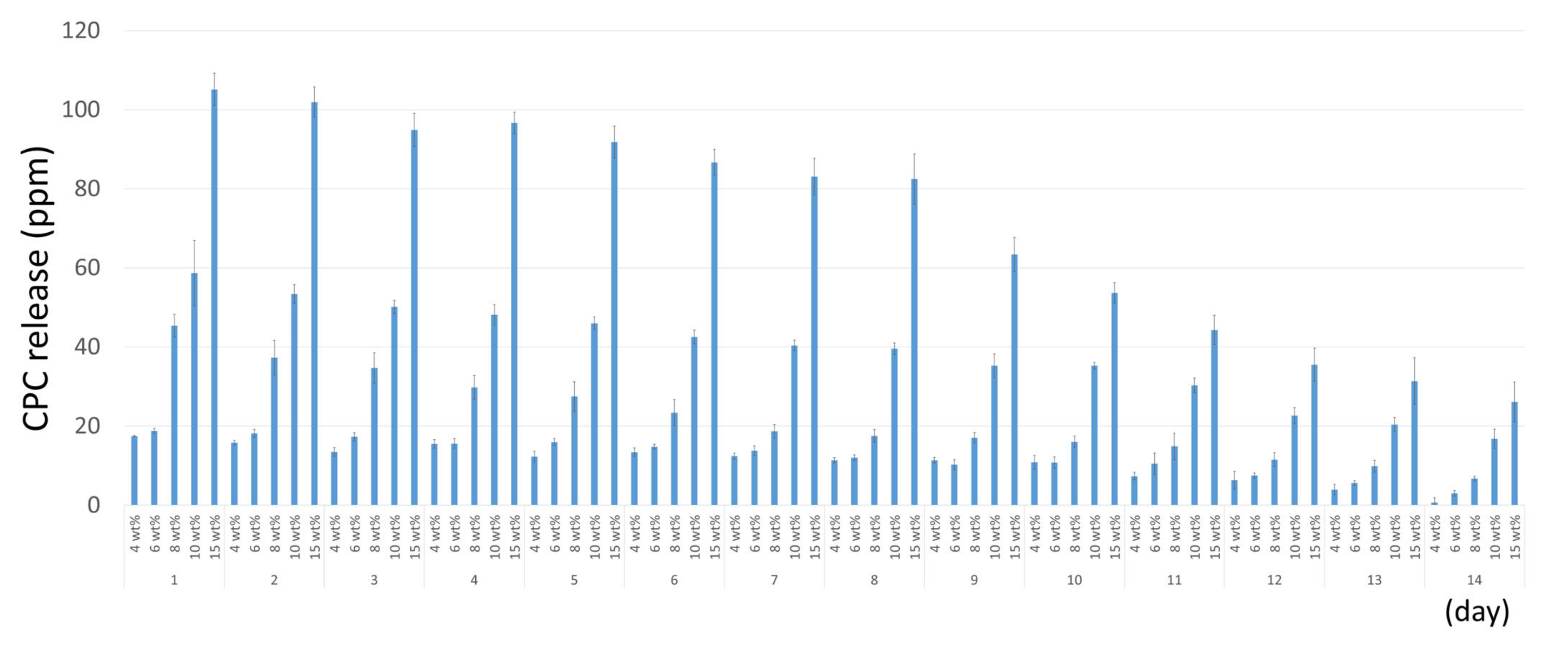
2.5. CPC Release
2.6. Biofilm Inhibition
2.7. Flexural Strength
2.8. Shear Bond Strength to Dentin
2.9. Biofilm Formation on the Fractured Surface of Shear-Bond-Strength Specimens
3. Results
3.1. XRD
3.2. SEM and STEM of CPC-Mont/SB Cement-Disk Cross-Sections
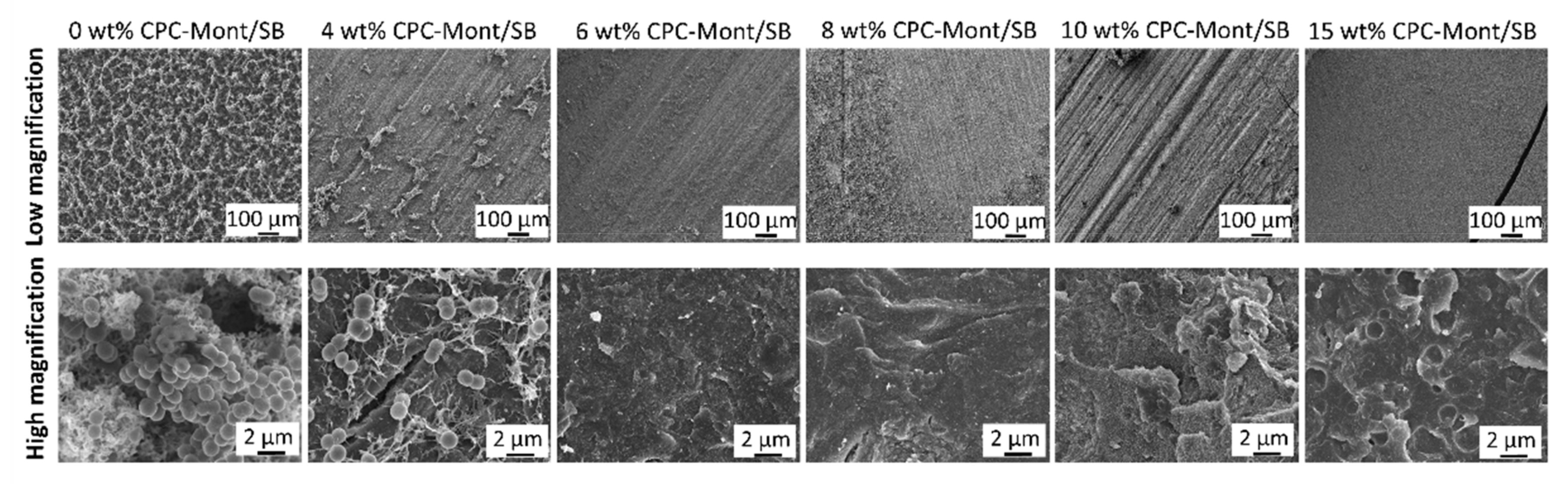
3.3. SEM of CPC-Mont/SB Powder and Disks
3.4. CPC Release
3.5. Biofilm Inhibition
3.6. Flexural Strength
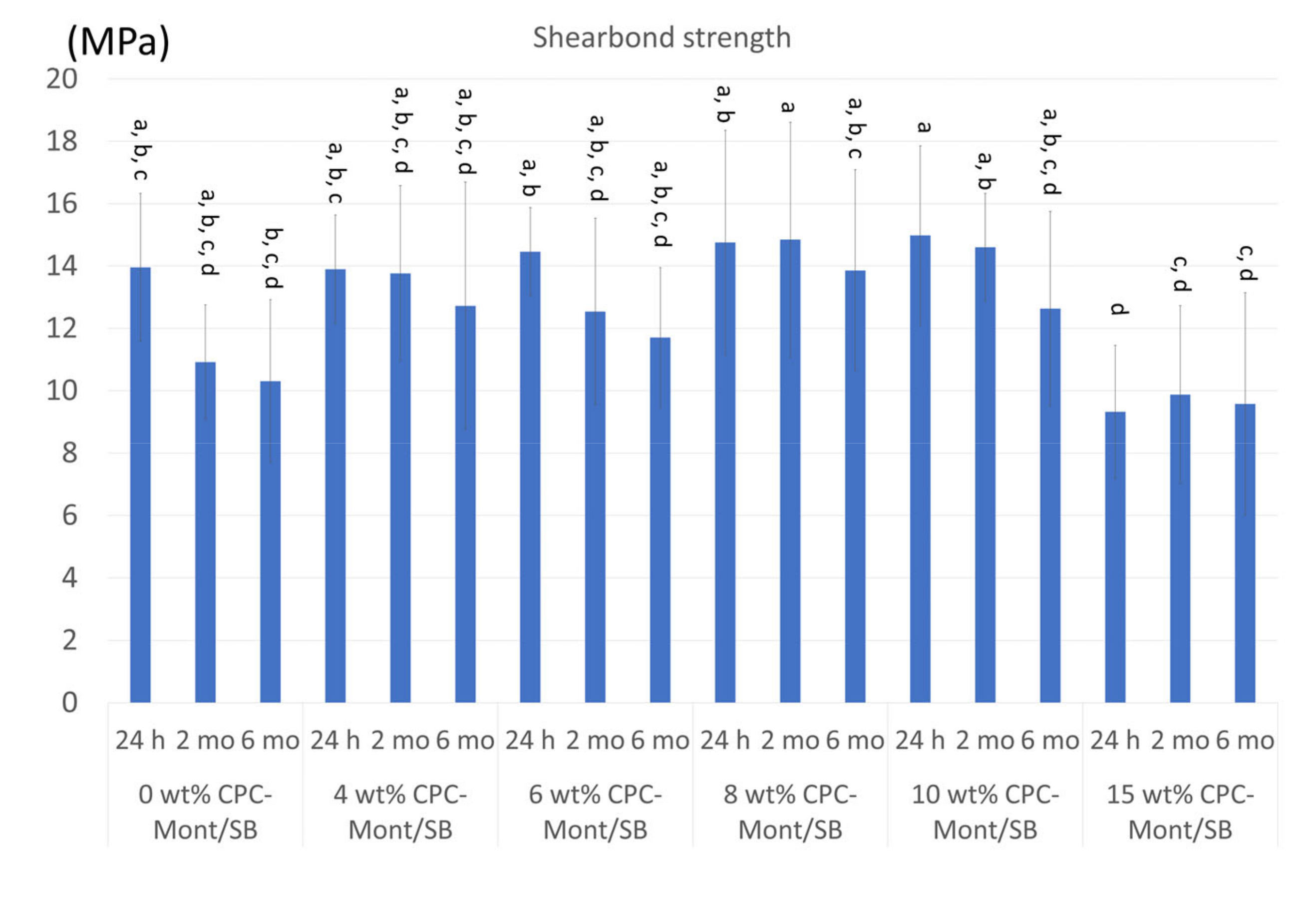
3.7. Shear Bond Strength to Dentin
3.8. Biofilm Formation on the Fractured Surface of Shear-Bond-Strength Specimens
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Askar, H.; Krois, J.; Göstemeyer, G.; Bottenberg, P.; Zero, D.; Banerjee, A.; Schwendicke, F. Secondary caries: What is it and how it can be controlled detected and managed? Clin. Oral. Investig. 2020, 24, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Chisini, L.A.; Collares, K.; Cademartori, M.G.; de Oliveira, L.J.C.; Conde, M.C.M.; Demarco, F.F.; Corrêa, M.B. Restorations in primary teeth: A systematic review on survival and reasons for failures. Int. J. Paediatr. Dent. 2018, 28, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.D.; Hale, R.; Chestnutt, I.G.; Wilson, N.H.F. Reasons for placement and replacement of crowns in general dental practice. Br. Dent. J. 2018, 10, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 15, 6B–12B. [Google Scholar]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Fujimura, Y.; Weerasinghe, D.; Kawashima, M. Development of an antibacterial bioactive dental adhesive: Simplicity and innovation. Am. J. Dent. 2018, 15, 13B–16B. [Google Scholar]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Torii, M.; Russell, R.R.; McCabe, J.F. Incorporation of antibacterial monomer MDPB into dentin primer. J. Dent. Res. 1997, 76, 768–772. [Google Scholar] [CrossRef]
- CLEARFIL SE PROTECT Brochure. Available online: https://www.kuraraynoritake.jp/product/adhesives/pdf/megabondfa_catalog.pdf (accessed on 1 January 2023). (In Japanese).
- Matsuo, K.; Yoshihara, K.; Nagaoka, N.; Makita, Y.; Obika, H.; Okihara, T.; Matsukawa, A.; Yoshida, Y.; van Meerbeek, B. Rechargeable anti-microbial adhesive formulation containing cetylpyridinium chloride montmorillonite. Acta Biomater. 2019, 100, 388–397. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Hirotsu, T.; Sonoda, A. Montmorillonite modified with hexadecylpyridinium chloride as highly efficient anion exchanger for perchlorate ion. Chem. Eng. J. 2012, 191, 141–146. [Google Scholar] [CrossRef]
- Olad, A.; Hagh, H.B.K.; Mirmohseni, A.; Azhar, F.F. Graphene oxide and montmorillonite enriched natural polymeric scaffold for bone tissue engineering. Ceram. Int. 2019, 45, 15609–15619. [Google Scholar] [CrossRef]
- Namba, N.; Yoshida, Y.; Nagaoka, N.; Takashima, S.; Matsuura-Yoshimoto, K.; Maeda, H.; van Meerbeek, B.; Suzuki, K.; Takashiba, S. Antibacterial effect of bactericide immobilized in resin matrix. Dent. Mater. 2009, 25, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Al-Musallam, T.A.; Evans, C.A.; Drummond, J.L.; Matasa, C.; Wu, C.D. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am. J. Orthod. Dentofacial. Orthop. 2006, 129, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Bailón-Sánchez, M.E.; Baca, P.; Ruiz-Linares, M.; Ferrer-Luque, C.M. Antibacterial and anti-biofilm activity of AH plus with chlorhexidine and cetrimide. J. Endod. 2014, 40, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Haider, J.; Camilleri, L.; Camilleri, J. Clinical relevance of antimicrobial testing results for dental restorative materials. J. Appl. Biomater. Funct. Mater. 2017, 26, e153–e161. [Google Scholar] [CrossRef] [PubMed]
- Anumula, L.; Kumar, S.; Kumar, V.S.; Sekhar, C.; Krishna, M.; Pathapati, R.M.; Venkata-Sarath, P.; Vadaganadam, Y.; Manne, R.K.; Mudlapudi, S. An Assessment of Antibacterial Activity of Four Endodontic Sealers on Enterococcus faecalis by a Direct Contact Test: An In Vitro Study. ISRN Dent. 2012, 2012, 989781. [Google Scholar] [CrossRef]
- Türkün, L.S.; Türkün, M.; Ertuğrul, F.; Ateş, M.; Brugger, S. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J. Esthet. Restor. Dent. 2008, 20, 29–44. [Google Scholar] [CrossRef]
- Marti, L.M.; Becci, A.C.; Spolidorio, D.M.; Brighenti, F.L.; Giro, E.M.; Zuanon, A.C. Incorporation of chlorhexidine gluconate or diacetate into a glass-ionomer cement: Porosity, surface roughness, and anti-biofilm activity. Am. J. Dent. 2014, 27, 318–322. [Google Scholar]
- Bellis, C.A.; Nobbs, A.H.; O’Sullivan, D.J.; Holder, J.A.; Barbour, M.E. Glass ionomer cements functionalised with a concentrated paste of chlorhexidine hexametaphosphate provides dose-dependent chlorhexidine release over at least 14 months. J. Dent. 2016, 45, 53–58. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental materials with antibiofilm properties. Dent. Mater. 2014, 30, e1–e16. [Google Scholar] [CrossRef]
- Zhang., K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhou, C.C.; Weir, M.D.; Zhou, X.D.; Xu, H.H. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral. Sci. 2016, 29, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Maruo, Y.; Sano, H.; Yoshida, Y.; van Meerbeek, B. Bacterial adhesion not inhibited by ion-releasing bioactive glass filler. Dent. Mater. 2017, 33, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yoshihara, K.; Nagaoka, N.; van Meerbeek, B.; Yoshida, Y. Novel composite cement containing the anti-microbial compound CPC-Montmorillonite. Dent. Mater. 2022, 38, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Soulestin, J.; Rashmi, B.J.; Bourbigot, S.; Lacrampe, M.F.; Macromol, K. Mechanical and Optical Properties of Polyamide 6/Clay Nanocomposite Cast Films: Influence of the Degree of Exfoliation. Mater. Eng. 2012, 297, 444–454. [Google Scholar] [CrossRef]
- Parija, S.; Nayak, S.K.; Verma, S.K.; Tripathy, S.S. Studies on physico-mechanical properties and thermal characteristics of polypropylene/layered silicate nanocomposites. Polym. Compos. 2004, 25, 646. [Google Scholar] [CrossRef]
- Wang, X.; Su, Q.; Shan, J.; Zheng, J. The effect of clay modification on the mechanical properties of poly(methyl methacrylate)/organomodified montmorillonite nanocomposites prepared by in situ suspension polymerization. Polym. Compos. 2016, 37, 1705–1714. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; de Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, B. Long-term marginal adaptation and nanoleakage of class V cavity restored with organic filler filled 4-META/MMA-TBB resin. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2050–2056. [Google Scholar] [CrossRef]
- Kitasako, Y.; Burrow, M.F.; Nikaido, T.; Tagami, J. Long-term tensile bond durability of two different 4-META containing resin cements to dentin. Dent. Mater. 2002, 18, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Alexandros, K.; Nikolaidis, D.S.; Achilias, G.; Karayannidis, P. Effect of the type of organic modifier on the polymerization kinetics and the properties of poly(methyl methacrylate)/organomodified montmorillonite nanocomposites. Eur. Polym. J. 2012, 48, 240–251. [Google Scholar]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Maske, T.T.; Kuper, N.K.; Cenci, M.S.; Huysmans, M.D.N.J.M. Minimal Gap Size and Dentin Wall Lesion Development Next to Resin Composite in a Microcosm Biofilm Model. Caries Res. 2017, 51, 475–481. [Google Scholar] [CrossRef] [PubMed]

| (wt%) | 0 | 4 | 6 | 8 | 10 | 15 | |
|---|---|---|---|---|---|---|---|
| POWDER (wt%) | Superbond (SB) | 100 | 96 | 94 | 92 | 90 | 85 |
| CPC-Mont | 0 | 4 | 6 | 8 | 10 | 15 | |
| LIQUID | MMA, 4-META | 4 monomer drops mixed with 1 catalyst drop, and 0.2 mL powder | |||||
| TBB catalyst | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshihara, K.; Nagaoka, N.; Makita, Y.; Yoshida, Y.; Van Meerbeek, B. Long-Term Antibacterial Efficacy of Cetylpyridinium Chloride-Montmorillonite Containing PMMA Resin Cement. Nanomaterials 2023, 13, 1495. https://doi.org/10.3390/nano13091495
Yoshihara K, Nagaoka N, Makita Y, Yoshida Y, Van Meerbeek B. Long-Term Antibacterial Efficacy of Cetylpyridinium Chloride-Montmorillonite Containing PMMA Resin Cement. Nanomaterials. 2023; 13(9):1495. https://doi.org/10.3390/nano13091495
Chicago/Turabian StyleYoshihara, Kumiko, Noriyuki Nagaoka, Yoji Makita, Yasuhiro Yoshida, and Bart Van Meerbeek. 2023. "Long-Term Antibacterial Efficacy of Cetylpyridinium Chloride-Montmorillonite Containing PMMA Resin Cement" Nanomaterials 13, no. 9: 1495. https://doi.org/10.3390/nano13091495
APA StyleYoshihara, K., Nagaoka, N., Makita, Y., Yoshida, Y., & Van Meerbeek, B. (2023). Long-Term Antibacterial Efficacy of Cetylpyridinium Chloride-Montmorillonite Containing PMMA Resin Cement. Nanomaterials, 13(9), 1495. https://doi.org/10.3390/nano13091495






