Chitin-Derived Nitrogen-Doped Carbon Nanopaper with Subwavelength Nanoporous Structures for Solar Thermal Heating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
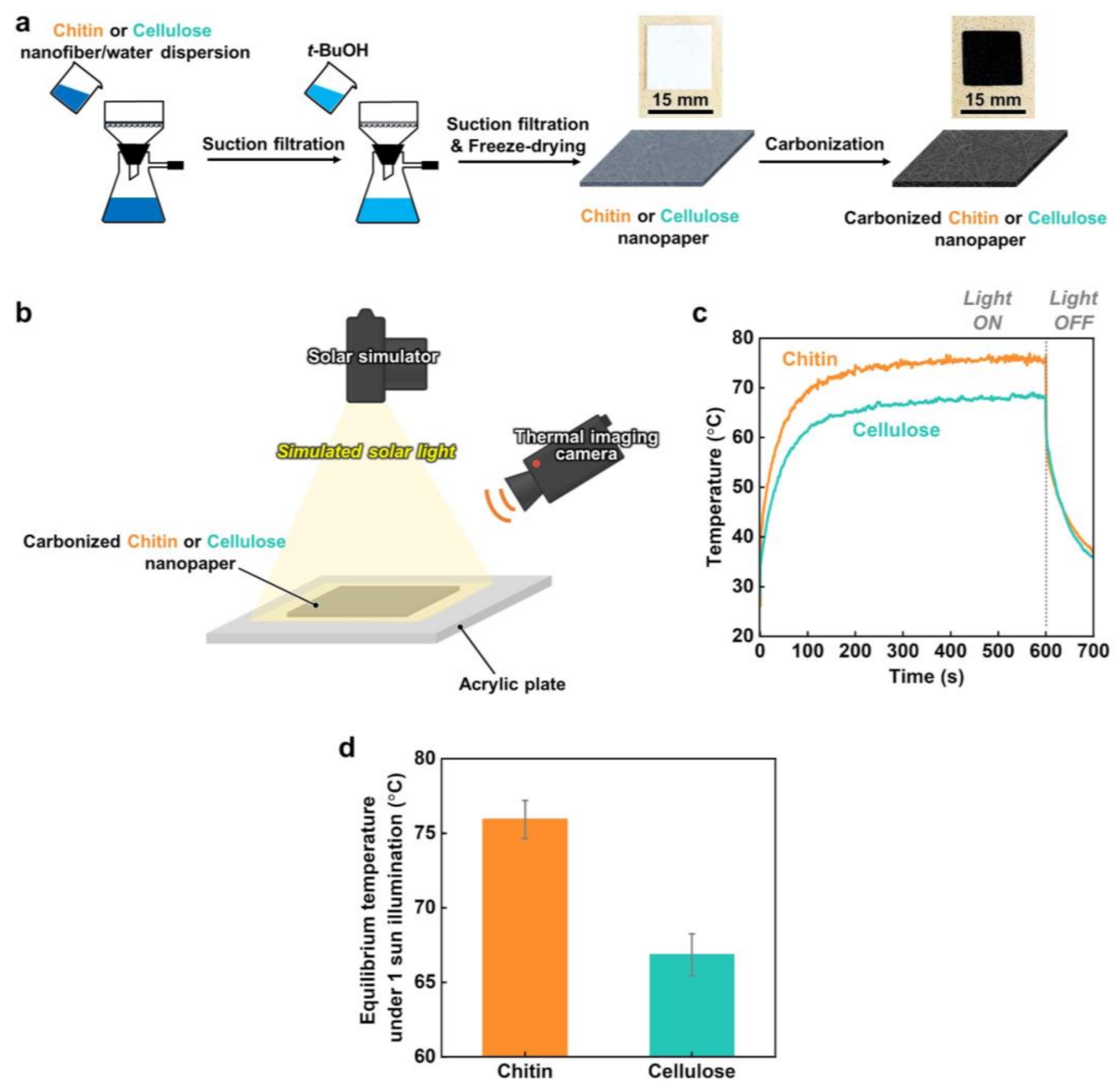
2.2. Preparation and Carbonization of Cellulose and Chitin Nanopapers
2.3. Solar Thermal Heating Performances
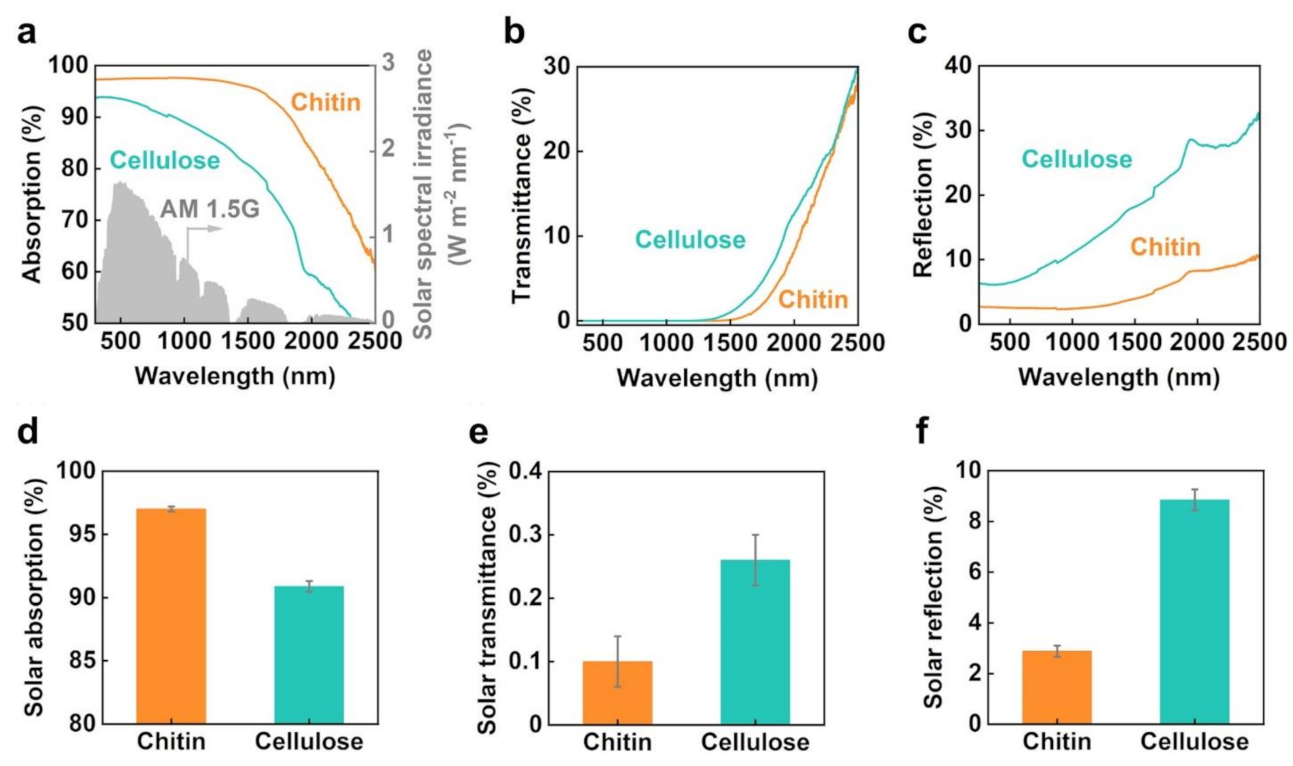
2.4. Optical Properties
2.5. Molecular Structures
2.6. Nanoporous Structures
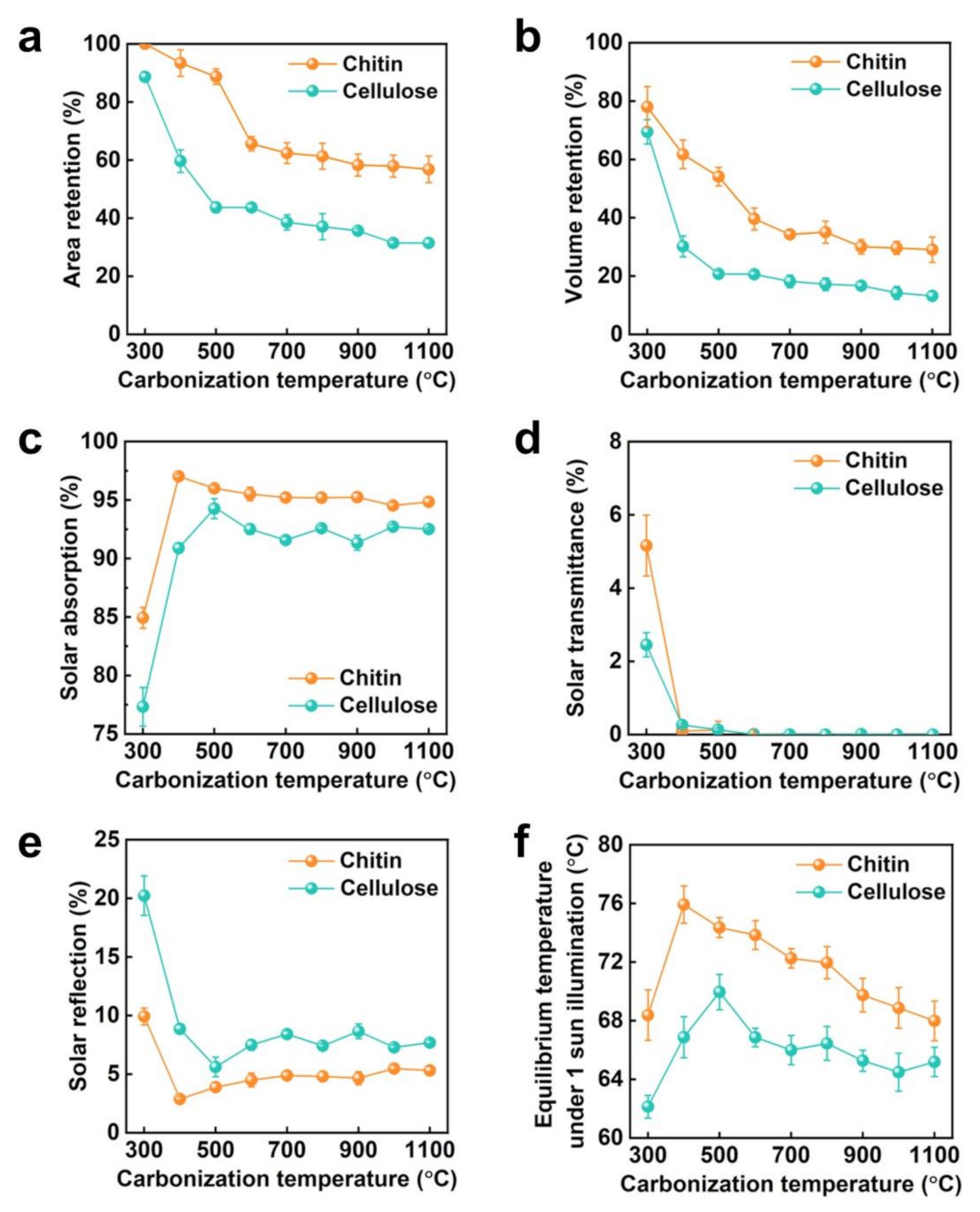
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guney, M.S. Solar power and application methods. Renew. Sustain. Energy Rev. 2016, 57, 776–785. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Liang, Y.; Koshelev, K.; Zhang, F.; Lin, H.; Lin, S.; Wu, J.; Jia, B.; Kivshar, Y. Bound States in the Continuum in Anisotropic Plasmonic Metasurfaces. Nano Lett. 2020, 20, 6351–6356. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.Y.Y.; Safari, M.; Mao, C.; Viasus, C.J.; Eleftheriades, G.V.; Ozin, G.A.; Kherani, N.P. Near-Perfect Absorbing Copper Metamaterial for Solar Fuel Generation. Nano Lett. 2021, 21, 9124–9130. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, Y.-L.; Chen, Q.-D.; Sun, H.-B. Carbon-based photothermal actuators. Adv. Funct. Mater. 2018, 28, 1802235. [Google Scholar] [CrossRef]
- ASTM G173-03; Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface. ASTM International: West Conshohocken, PA, USA, 2012.
- Fillet, R.; Nicolas, V.; Fierro, V.; Celzard, A. A review of natural materials for solar evaporation. Sol. Energy Mater. Sol. Cells 2021, 219, 110814. [Google Scholar] [CrossRef]
- Guan, W.; Guo, Y.; Yu, G. Carbon materials for solar water evaporation and desalination. Small 2021, 17, e2007176. [Google Scholar] [CrossRef]
- Saleque, A.M.; Nowshin, N.; Ivan, M.N.A.S.; Ahmed, S.; Tsang, Y.H. Natural porous materials for interfacial solar steam generation toward clean water production. Sol. RRL 2022, 6, 2100986. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, L.; Zhang, Y.; Sun, J.; Wen, J.; Yuan, Y. Upgrading earth-abundant biomass into three-dimensional carbon materials for energy and environmental applications. J. Mater. Chem. A 2019, 7, 4217–4229. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, G.Q.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L. Photonic nanostructures for solar energy conversion. Energy Environ. Sci. 2016, 9, 2511–2532. [Google Scholar] [CrossRef]
- Yeamsuksawat, T.; Morishita, Y.; Shirahama, J.; Huang, Y.; Kasuga, T.; Nogi, M.; Koga, H. Semicarbonized subwavelength-nanopore-structured nanocellulose paper for applications in solar thermal heating. Chem. Mater. 2022, 34, 7379–7388. [Google Scholar] [CrossRef]
- Koga, H.; Nagashima, K.; Suematsu, K.; Takahashi, T.; Zhu, L.; Fukushima, D.; Huang, Y.; Nakagawa, R.; Liu, J.; Uetani, K.; et al. Nanocellulose paper semiconductor with a 3D network structure and its nano–micro–macro trans-scale design. ACS Nano 2022, 16, 8630–8640. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in functional chitin materials: A review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions. Biomacromolecules 2008, 9, 1919–1923. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Shopsowitz, K.E.; MacLachlan, M.J. Mesoporous nitrogen-doped carbon from nanocrystalline chitin assemblies. J. Mater. Chem. A 2014, 2, 5915. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Zhang, J.; Yan, N. Chitin-derived mesoporous, nitrogen-containing carbon for heavy-metal removal and styrene epoxidation. ChemPlusChem 2015, 80, 1556–1564. [Google Scholar] [CrossRef]
- Ding, B.; Huang, S.; Pang, K.; Duan, Y.; Zhang, J. Nitrogen-enriched carbon nanofiber aerogels derived from marine chitin for energy storage and environmental remediation. ACS Sustain. Chem. Eng. 2018, 6, 177–185. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Y.; Morishita, Y.; Uetani, K.; Nogi, M.; Koga, H. Pyrolyzed chitin nanofiber paper as a three-dimensional porous and defective nanocarbon for photosensing and energy storage. J. Mater. Chem. C 2021, 9, 4444–4452. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Kasuga, T.; Nogi, M.; Koga, H. Chitin-derived-carbon nanofibrous aerogel with anisotropic porous channels and defective carbon structures for strong microwave absorption. Chem. Eng. J. 2022, 450, 137943. [Google Scholar] [CrossRef]
- Nogi, M.; Kurosaki, F.; Yano, H.; Takano, M. Preparation of nanofibrillar carbon from chitin nanofibers. Carbohydr. Polym. 2010, 81, 919–924. [Google Scholar] [CrossRef]
- Mandal, J.; Wang, D.; Overvig, A.C.; Shi, N.N.; Paley, D.; Zangiabadi, A.; Cheng, Q.; Barmak, K.; Yu, N.; Yang, Y. Scalable, “dip-and-dry” fabrication of a wide-angle plasmonic selective absorber for high-efficiency solar-thermal energy conversion. Adv. Mater. 2017, 29, 1702156. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi (B) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef]
- Yu, H.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.N.; Zhao, Y.; Zhou, C.; Wu, L.Z.; Tung, C.H.; Zhang, T. Nitrogen-doped porous carbon nanosheets templated from g-C3N4 as metal-free electrocatalysts for efficient oxygen reduction reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Zhou, X.; Guo, S.; Wu, N. Fingerprinting photoluminescence of functional groups in graphene oxide. J. Mater. Chem. 2012, 22, 23374. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Isayama, M.; Nomiyama, K.; Kunitake, T. Template synthesis of a large, self-supporting graphite film in montmorillonite. Adv. Mater. 1996, 8, 641–644. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeamsuksawat, T.; Zhu, L.; Kasuga, T.; Nogi, M.; Koga, H. Chitin-Derived Nitrogen-Doped Carbon Nanopaper with Subwavelength Nanoporous Structures for Solar Thermal Heating. Nanomaterials 2023, 13, 1480. https://doi.org/10.3390/nano13091480
Yeamsuksawat T, Zhu L, Kasuga T, Nogi M, Koga H. Chitin-Derived Nitrogen-Doped Carbon Nanopaper with Subwavelength Nanoporous Structures for Solar Thermal Heating. Nanomaterials. 2023; 13(9):1480. https://doi.org/10.3390/nano13091480
Chicago/Turabian StyleYeamsuksawat, Thanakorn, Luting Zhu, Takaaki Kasuga, Masaya Nogi, and Hirotaka Koga. 2023. "Chitin-Derived Nitrogen-Doped Carbon Nanopaper with Subwavelength Nanoporous Structures for Solar Thermal Heating" Nanomaterials 13, no. 9: 1480. https://doi.org/10.3390/nano13091480
APA StyleYeamsuksawat, T., Zhu, L., Kasuga, T., Nogi, M., & Koga, H. (2023). Chitin-Derived Nitrogen-Doped Carbon Nanopaper with Subwavelength Nanoporous Structures for Solar Thermal Heating. Nanomaterials, 13(9), 1480. https://doi.org/10.3390/nano13091480






