Green Production of Biomass-Derived Carbon Materials for High-Performance Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Fundamentals
2.1. Working Principles of LSBs
2.2. Obstacles for LSBs
3. Recent Progresses in Carbon Materials for LSBs
3.1. Structural Regulation of Carbons
3.2. Heteroatom-Doped Carbons for Cathode Materials
4. Biomass-Derived Carbon Materials for LSBs
4.1. Advantages of Biomass-Derived Carbon Materials
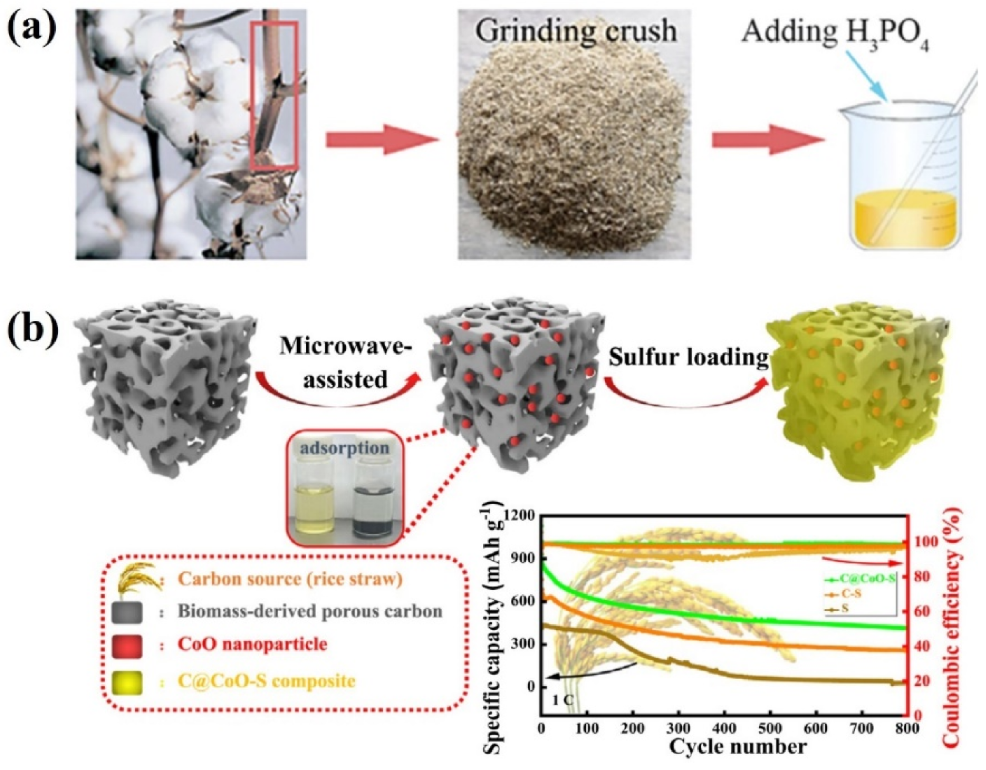
4.2. Biomass-Derived Carbons for the Cathode of LSBs
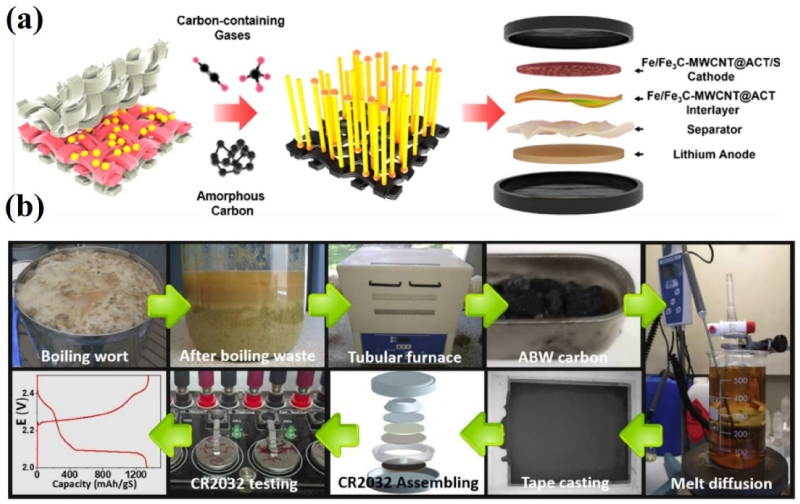
4.3. Biomass-Derived Carbons for the Interlayer of LSBs
4.4. Modified Biomass-Derived Carbons for LSBs
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.C.; Lu, J.; Guo, Z.P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on High-Loading and High-Energy Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 24, 1700260. [Google Scholar] [CrossRef]
- Zhang, W.C.; Lu, J.; Guo, Z.P. Challenges and future perspectives on sodium and potassium ion batteries for grid-scale energy storage. Mater. Today 2021, 50, 400–417. [Google Scholar] [CrossRef]
- Zhang, F.L.; Zhang, W.C.; David, W.; Guo, Z.P. Recent Progress and Future Advances on Aqueous Monovalent-Ion Batteries towards Safe and High-Power Energy Storage. Adv. Mater. 2022, 34, 2107965. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Feng, Y.; Liang, H.W.; Wu, Z.Y.; Yu, S.H. Macroscopic-Scale Three-Dimensional Carbon Nanofiber Architectures for Electrochemical Energy Storage Devices. Adv. Energy Mater. 2017, 7, 1700826. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.Q.; Feng, X.; Bai, Y.; Wu, C. Ether-based electrolytes for sodium ion batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef]
- Sen, X.; Lin, G.; Zhao, N.H. Smaller Sulfur Molecules Promise Better Lithium—Sulfur Batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.H.; Zhang, W.K.; Manthiram, A.; Yu, G.H. Nanostructured Host Materials for Trapping Sulfur in Rechargeable Li-S Batteries: Structure Design and Interfacial Chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Yang, D.X.; Ren, H.Y.; Wu, D.P.; Zhang, W.C.; Lou, X.D.; Wang, D.Q.; Cao, K.; Gao, Z.Y.; Xu, F.; Jiang, K. Bi-functional nitrogen-doped carbon protective layer on three-dimensional RGO/SnO2 composites with enhanced electron transport and structural stability for high-performance lithium-ion batteries. J. Colloid Interface Sci. 2019, 542, 81–90. [Google Scholar] [CrossRef]
- Wu, D.P.; Wang, Y.X.; Wang, F.J.; Wang, H.J.; An, Y.P.; Gao, Z.Y.; Xu, F.; Jiang, K. Oxygen-incorporated few-layer MoS2 vertically aligned on three-dimensional graphene matrix for enhanced catalytic performances in quantum dot sensitized solar cells. Carbon 2017, 123, 756–766. [Google Scholar] [CrossRef]
- Wang, L.; Jia, F.; Wu, D.P.; Wei, Q.X.; Liang, Y.; Hu, Y.S.; Li, R.F.; Yu, G.H.; Yuan, Q.P.; Wang, J.S. In-situ growth of graphene on carbon fibers for enhanced cell immobilization and xylitol fermentation. Appl. Surf. Sci. 2020, 527, 146793. [Google Scholar] [CrossRef]
- Chen, J.L.; Wu, D.P.; Wang, H.J.; Wang, F.J.; Wang, Y.X.; Gao, Z.Y.; Xu, F.; Jiang, K. In-situ synthesis of molybdenum sulfide/reduced graphene oxide porous film as robust counter electrode for dye-sensitized solar cells. J. Colloid Interface Sci. 2018, 524, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.X.; Wu, D.P.; Mu, Y.F.; Xing, L.Y.; Zhang, W.C.; Gao, Z.Y.; Xu, F.; Jiang, K. Boron and nitrogen Co-doped holey graphene aerogels with rich B-N motifs for flexible supercapacitors. Carbon 2020, 159, 94–101. [Google Scholar] [CrossRef]
- Li, R.G.; Wu, D.P.; Song, J.K.; He, Y.C.; Zhu, W.Y.; Wang, X.P.; Wang, L.X.; Dube, N.M.; Jiang, K. In situ generation of reduced graphene oxide on 3D Cu-Ni foam as high-performance electrodes for capacitive deionization. Desalination 2022, 540, 115990. [Google Scholar] [CrossRef]
- Tabani, H.; Khodaei, K.; Moghaddam, A.Z. Introduction of graphene-periodic mesoporous silica as a new sorbent for removal: Experiment and simulation. Chem. Intermed. 2019, 45, 1795–1813. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.L.; Zhang, S.B.; Wu, D.P.; Lv, Y.Y.; Hu, Y.S.; Wei, Q.X.; Yuan, Q.P.; Wang, J.S. A rapid microwave-assisted phosphoric-acid treatment on carbon fiber surface for enhanced cell immobilization in xylitol fermentation. Colloids Surf. B 2019, 175, 697–702. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Zhang, Y.X.; Wei, Q.X.; Liang, Y.; Tian, H.L.; Wu, D.P.; Yuan, X.Q.; Yuan, Q.P.; Wang, J.S. A novel surface treatment of carbon fiber with Fenton reagent oxidization for improved cells immobilization and xylitol fermentation. Microporous Mesoporous Mater. 2021, 325, 111318. [Google Scholar] [CrossRef]
- Wang, L.; Liu, N.; Guo, Z.; Wu, D.P.; Chen, W.W.; Chang, Z.; Yuan, Q.P.; Hui, M.; Wang, J.S. Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials 2016, 9, 206. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.J.; Zhou, Y.; Gao, Z.Y.; Han, Y.; Jiang, K.; Zhang, W.C.; Wu, D.P. Green synthesis of N-doped porous carbon/carbon dot composites as metal-free catalytic electrode materials for iodide-mediated quasi-solid flexible supercapacitors. Inorg. Chem. Front. 2022, 9, 2530–2543. [Google Scholar] [CrossRef]
- Ma, Z.J.; Wu, D.P.; Han, X.Y.; Wang, H.J.; Zhang, L.M.; Gao, Z.Y.; Xu, F.; Jiang, K. Ultrasonic assisted synthesis of Zn-Ni bi-metal MOFs for interconnected Ni-N-C materials with enhanced electrochemical reduction of CO2. J. CO2 Util. 2019, 32, 251–258. [Google Scholar] [CrossRef]
- Ma, Z.J.; Zhang, X.L.; Wu, D.P.; Han, X.X.; Zhang, L.M.; Wang, H.J.; Xu, F.; Gao, Z.Y.; Jiang, K. Ni and nitrogen-codoped ultrathin carbon nanosheets with strong bonding sites for efficient CO2 electrochemical reduction. J. Colloid Interface Sci. 2020, 570, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Wang, Y.X.; Chen, H.Q.; Niu, B.X.; Zhang, W.C.; Wu, D.P. Synergistic mediation of polysulfide immobilization and conversion by a catalytic and dual-adsorptive system for high performance lithium-sulfur batteries. Chem. Eng. J. 2021, 406, 126802. [Google Scholar] [CrossRef]
- Zhang, M.M.; Huang, K.X.; Ding, Y.; Wang, X.Y.; Gao, Y.L.; Li, P.F.; Zhou, Y.; Gao, Z.; Zhang, Y.; Wu, D.P. N, S Co-Doped Carbons Derived from Enteromorpha prolifera by a Molten Salt Approach: Antibiotics Removal Performance and Techno-Economic Analysis. Nanomaterials 2022, 12, 4289. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wu, D.P.; Wang, H.J.; Gao, Z.Y.; Xu, F.; Jiang, K. Nitrogen-doped two-dimensional porous carbon sheets derived from clover biomass for high performance supercapacitors. J. Power Sources 2017, 363, 375–383. [Google Scholar] [CrossRef]
- Chen, C.; Han, H.J.; Liu, X.P.; Chen, Y.; Wu, D.P.; Gao, Z.Y.; Gao, S.Y.; Jiang, K. Nitrogen, phosphorus, sulfur tri-doped porous carbon derived from covalent polymer with versatile performances in supercapacitor, oxygen reduction reaction and electro-fenton degradation. Microporous Mesoporous Mater. 2021, 325, 111335. [Google Scholar] [CrossRef]
- Wang, C.J.; Wu, D.P.; Wang, H.J.; Gao, Z.Y.; Xu, F.; Jiang, K. Biomass derived nitrogen-doped hierarchical porous carbon sheets for supercapacitors with high performance. J. Colloid Interface Sci. 2018, 523, 133–143. [Google Scholar] [CrossRef]
- Wu, D.P.; Chen, J.L.; Zhang, W.C.; Liu, W.D.; Li, J.Z.; Cao, K.; Gao, Z.Y.; Xu, F.; Jiang, K. Sealed pre-carbonization to regulate the porosity and heteroatom sites of biomass derived carbons for lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 579, 667–679. [Google Scholar] [CrossRef]
- Wang, C.J.; Wu, D.P.; Wang, H.J.; Gao, Z.Y.; Xu, F.; Jiang, K. A green and scalable route to yield porous carbon sheets from biomass for supercapacitors with high capacity. J. Mater. Chem. A 2018, 6, 1244–1254. [Google Scholar] [CrossRef]
- Chen, C.; Tian, M.; Han, H.J.; Wu, D.P.; Chen, Y.; Gao, Z.Y.; Gao, S.Y.; Jiang, K. N, P-dual doped carbonaceous catalysts derived from bifunctional-salt activation for effective electro-Fenton degradation on waterborne organic pollutions. Electrochim. Acta 2021, 389, 138732. [Google Scholar] [CrossRef]
- Bai, X.G.; Ying, L.Y.; Liu, N.; Ma, N.N.; Huang, K.X.; Wu, D.P.; Yin, M.M.; Jiang, K. Humulus scandens-Derived Biochars for the Effective Removal of Heavy Metal Ions: Isotherm/Kinetic Study, Column Adsorption and Mechanism Investigation. Nanomaterials 2021, 11, 3255. [Google Scholar] [CrossRef]
- Yin, M.M.; Bai, X.G.; Wu, D.P.; Li, F.B.; Jiang, K.; Ma, N.N.; Chen, Z.H.; Zhang, X.; Fang, L.P. Sulfur-functional group tunning on biochar through sodium thiosulfate modified molten salt process for efficient heavy metal adsorption. Chem. Eng. J. 2022, 433, 134441. [Google Scholar] [CrossRef]
- Bai, X.G.; Zhang, M.M.; Niu, B.X.; Zhang, W.L.; Wang, X.P.; Wang, J.S.; Wu, D.P.; Wang, L.; Jiang, K. Rotten sugarcane bagasse derived biochars with rich mineral residues for effective Pb (II) removal in wastewater and the tech-economic analysis. J Taiwan Inst. Chem. Eng. 2022, 132, 104231. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Long, C.; Chen, X.; Jiang, L.; Zhi, L.; Fan, Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, L.; Wei, X. A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Crudenb, A.; Carterb, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Lai, C.; Qiu, J.; Li, S.; Hou, Y.; Martens, W.; Mahmood, N.; Zhang, S. Microporous bamboo biochar for lithium-sulfur batteries. Nano Res. 2014, 8, 129–139. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Lia, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.L.; Yang, Y.L.; Suo, G.Q.; Zhang, L.; Lu, S.Y.; Chen, Z.G. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Kown, K. A review on biomass-derived N-doped carbons as electrocatalysts in electrochemical energy applications. Chem. Eng. J. 2022, 446, 137116. [Google Scholar] [CrossRef]
- Yuan, H.D.; Liu, T.F.; Liu, Y.J.; Nai, J.W.; Wang, Y.; Zhang, W.K.; Tao, X.Y. A review of biomass materials for advanced lithium–sulfur batteries. Chem. Sci. 2019, 10, 7484. [Google Scholar] [CrossRef]
- Liu, P.T.; Wang, Y.Y.; Liu, J.H. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.P.; Wang, Y.; Chen, Y.X.; Guo, X.D.; Wu, Z.G.; Zhong, B.H. Review of the application of biomass-derived porous carbon in lithium-sulfur batteries. Ionics 2020, 26, 4765–4781. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, J.; Xu, Y.; Wang, S.; An, W.; Chai, Q.; Prova, U.; Wang, C.; Huang, G. Biomass derived diverse carbon nanostructure for electrocatalysis, energy conversion and storage. Carbon 2023, 211, 118105. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Su, Z.; Chen, H.L.; Yi, S.; Zhang, W.Y.; Niu, B.; Zhang, Y.Y.; Long, D.H. Renewable biomass-derived carbon-based hosts for lithium–sulfur batteries. Sustain. Energy Fuels 2022, 6, 5211. [Google Scholar] [CrossRef]
- Tian, X.H.; Yan, C.Z.; Kang, J.B.; Yang, X.Y.; Li, Q.X.; Yan, J.; Deng, N.P.; Cheng, B.; Kang, W.M. Working Mechanisms and Structure Engineering of Renewable Biomass-Derived Materials for Advanced Lithium-Sulfur Batteries: A Review. ChemElectroChem 2022, 9, e202100995. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Huang, X.X.; Zhang, S.Q.; Hou, Y.L. Sulfur Hosts against the Shuttle Effect. Small Methods 2018, 2, 1700345. [Google Scholar] [CrossRef]
- Walle, M.D. Environmental Solid Waste-derived Carbon for Advanced Rechargeable Lithium-Sulfur Batteries: A Review. ChemistrySelect 2022, 7, e202200511. [Google Scholar] [CrossRef]
- Benitez, A.; Amaro-Gahete, J.; Chien, Y.C.; Caballero, A.; Morales, J.; Brandell, D. Recent advances in lithium-sulfur batteries using biomass-derived carbons as sulfur host. Renew. Renew. Sustain. Energy Rev. 2022, 154, 111783. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Liang, C.D.; Dudney, N.J.; Howe, J.Y. Hierarchically Structured Sulfur/Carbon Nanocomposite Material for High-Energy Lithium Battery Chemistry of Materials. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Lee, J.H.; Koo, H.Y.; Shakoor, R.A.; Kahraman, R.; Jo, Y.N.; Park, M.S.; Choi, J.W. Hierarchical porous carbon by ultrasonic spray pyrolysis yields stable cycling in lithium-sulfur battery. Nano Lett. 2014, 14, 4418–4425. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Yuan, L.X.; Yi, Z.Q.; Wu, C.; Liu, Y.; Strasser, P.; Huang, Y.H. A highly ordered meso@microporous carbon-supported sulfur@smaller sulfur core-shell structured cathode for Li-S batteries. ACS Nano 2014, 8, 9295–9303. [Google Scholar] [CrossRef]
- Chen, C.G.; Li, D.J.; Gao, L.; Harks, P.R.M.L.; Eichel, R.A.; Notten, P.H.L. Carbon-coated core–shell Li2S@C nanocomposites as high performance cathode materials for lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 1428–1433. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.L.; Wang, D.H.; Li, S.H.; Xie, D.; Zhang, X.Q.; Xia, X.H.; Tu, J.P. Facile and scalable synthesis of nanosized core–shell Li2S@C composite for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 16653–16660. [Google Scholar] [CrossRef]
- Sun, Q.; He, B.; Zhang, X.Q.; Lu, A.H. Engineering of Hollow Core-Shell Interlinked Carbon Spheres for Highly Stable Lithium-Sulfur Batteries. ACS Nano 2015, 9, 8504–8513. [Google Scholar] [CrossRef]
- Zhou, G.M.; Yin, L.C.; Wang, D.W.; Li, L.; Pei, S.F.; Gentle, I.R.; Li, F.; Cheng, H.M. Fibrous hybrid of graphene and sulfur nanocrystals for high-performance lithium-sulfur batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.M.; Li, L.; Ma, C.Q.; Wang, S.G.; Shi, Y.; Koratkar, N.; Ren, W.C.; Li, F.; Cheng, H.M. A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries. Nano Energy 2015, 11, 356–365. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, H.J.; Liang, J.Y.; Huang, J.Q.; Chen, C.M.; Guo, X.F.; Zhu, W.C.; Li, P.; Zhang, Q. Interconnected carbon nanotube/graphene nanosphere scaffolds as free-standing paper electrode for high-rate and ultra-stable lithium–sulfur batteries. Nano Energy 2015, 11, 746–755. [Google Scholar] [CrossRef]
- Song, J.X.; Gordin, M.L.; Xu, T.; Chen, S.R.; Yu, Z.X.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.H.; Wang, D.H. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes. Angew. Chem. Int. Ed. Engl. 2015, 54, 4325–4329. [Google Scholar] [CrossRef]
- Yang, C.P.; Yin, Y.X.; Ye, H.; Jiang, K.C.; Zhang, J.; Guo, Y.G. Insight into the effect of boron doping on sulfur/carbon cathode in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2014, 6, 8789–8795. [Google Scholar] [CrossRef]
- Wang, N.N.; Xu, Z.F.; Xu, X.; Liao, T.; Tang, B.; Bai, Z.C.; Dou, S.X. Synergistically Enhanced Interfacial Interaction to Polysulfide via N,O Dual-Doped Highly Porous Carbon Microrods for Advanced Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13573–13580. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.R.; Zhou, H.H.; Fu, C.P.; Wang, G.C.; Yang, L.M.; Xu, C.X.; Chen, Z.X.; Yang, W.J.; Kuang, Y.F. Hydrothermal preparation of nitrogen, boron co-doped curved graphene nanoribbons with high dopant amounts for high-performance lithium sulfur battery cathodes. J. Mater. Chem. A 2017, 5, 7403–7415. [Google Scholar] [CrossRef]
- Xu, J.; Su, D.W.; Zhang, W.X.; Bao, W.Z.; Wang, G.X. A nitrogen–sulfur co-doped porous graphene matrix as a sulfur immobilizer for high performance lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 17381–17393. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J.; Jeon, Y.; Piao, Y.Z. Multi-Heteroatom-Doped Hollow Carbon Attached on Graphene Using LiFePO4 Nanoparticles as Hard Templates for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26485–26493. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Doua, H.; Zhang, X. Biomass derived carbon for energy storage devices. J. Mater. Chem. A 2017, 5, 2411–2428. [Google Scholar] [CrossRef]
- Wu, D.P.; Liu, J.Y.; Chen, J.L.; Li, H.H.; Cao, R.G.; Zhang, W.C.; Gao, Z.Y.; Jiang, K. Promoting sulphur conversion chemistry with tri-modal porous N, O-codoped carbon for stable Li–S batteries. J. Mater. Chem. A 2021, 9, 5497. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.L.; Li, S.l.; Meng, F.C.; Chen, Y.Z.; Wu, H.T.; Liu, J.H. From purple sweet potato to sustainable lithium-sulfur batteries. Mater. Lett. 2022, 325, 132893. [Google Scholar] [CrossRef]
- Rojas, M.D.; Lobos, M.L.N.; Para, M.L.; María Quijón, E.G.; Cámara, O.; Barraco, D.; Moyano, E.L.; Luque, G.L. Activated carbon from pyrolysis of peanut shells as cathode for lithium-sulfur batteries. Biomass Bioenergy 2021, 146, 105971. [Google Scholar] [CrossRef]
- Chen, H.W.; Xia, P.T.; Lei, W.X.; Pan, Y.; Zou, Y.L.; Ma, Z.S. Preparation of activated carbon derived from biomass and its application in lithium–sulfur batteries. J. Porous Mater. 2019, 26, 1325–1333. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, Y.J.; Kim, J.K.; Lee, S.J.; Bae, J.S.; Jeong, E.D. Biomass-garlic-peel-derived porous carbon framework as a sulfur host for lithium-sulfur batteries. Ind. Eng. Chem. Res. 2021, 94, 272–281. [Google Scholar] [CrossRef]
- Chang, Y.G.; Ren, Y.M.; Zhu, L.K.; Li, Y.; Li, T.; Ren, B.Z. Preparation of macadamia nut shell porous carbon and its electrochemical performance as cathode material for lithium–sulfur batteries. Electrochim. Acta 2022, 420, 140454. [Google Scholar] [CrossRef]
- Deng, Y.X.; Lei, T.Y.; Feng, Y.Y.; Zhang, B.; Ding, H.Y.; Lu, Q.; Tian, R.; Mushtaq, M.; Guo, W.J.; Yao, M.M.; et al. Biomass fallen leaves derived porous carbon for high performance lithium sulfur batteries. Ionics 2023, 29, 1029–1038. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, R.; Fan, C.J.; Huang, Y.; Yan, Y.L.; Zou, Y.M.; Xu, Y.H. Eucommia leaf residue-derived hierarchical porous carbon by KCl and CaCl2 Co-auxiliary activation for lithium sulfur batteries. Mater. Charact. 2023, 195, 112522. [Google Scholar] [CrossRef]
- Zhang, L.M.; Zhao, W.Q.; Yuan, S.H.; Jiang, F.; Chen, X.Q.; Yang, Y.; Ge, P.; Sun, W.; Ji, X.B. Engineering the morphology/porosity of oxygen-doped carbon for sulfur host as lithium-sulfur batteries. J. Energy Chem. 2021, 60, 531–545. [Google Scholar] [CrossRef]
- Wen, X.Y.; Zhang, C.F.; Zhou, W.; Chen, H.; Xiang, K.X. Nitrogen/sulfur co-doping for biomass carbon foam as superior sulfur hosts for lithium-sulfur batteries. Int. J. Energy Res. 2022, 46, 10606–10619. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, Y.J.; Jeong, E.D.; Lee, S.J.; Kim, H.G.; Chung, J.M.; Kim, J.S.; Lee, S.Y.; Bae, J.S. Synthesis and Electrochemical Performance of Microporous Hollow Carbon from Milkweed Pappus as Cathode Material of Lithium–Sulfur Batteries. Nanomaterials 2022, 12, 3605. [Google Scholar] [CrossRef] [PubMed]
- Nurhilal, O.; Hidayat, S.; Sumiarsa, D.; Risdiana, R. Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries. Renew. Energy 2023, 15, 1039. [Google Scholar] [CrossRef]
- Xue, M.Z.; Xu, H.; Tan, Y.; Chen, C.; Li, B.; Zhang, C.M. A novel hierarchical porous carbon derived from durian shell as enhanced sulfur carrier for high performance Li-S batteries. J. Energy Chem. 2021, 893, 115306. [Google Scholar] [CrossRef]
- Xiao, Q.H.Q.; Li, G.R.; Li, M.J.; Liu, R.P.; Li, H.B.; Ren, P.F.; Dong, Y.; Feng, M.; Chen, Z.W. Biomass-derived nitrogen-doped hierarchical porous carbon as efficient sulfur host for lithium–sulfur batteries. J. Energy Chem. 2020, 44, 61–67. [Google Scholar] [CrossRef]
- Ma, Z.W.; Sui, W.H.; Liu, J.; Wang, W.J.; Li, S.M.; Chen, T.T.; Yang, G.L.; Zhu, K.X.; Li, Z.J. Pomelo peel-derived porous carbon as excellent LiPS anchor in lithium-sulfur batteries. J. Solid State Electrochem. 2022, 26, 973–984. [Google Scholar] [CrossRef]
- Wen, Y.T.; Wang, X.B.; Huang, J.Y.; Li, Y.; Li, T.; Ren, B.Z. Coffee grounds derived sulfur and nitrogen dual-doped porous carbon for the cathode material of lithium-sulfur batteries. Carbon Lett. 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, D.J.; Cho, K.K.; Zou, Y.M.; Ahn, H.J.; Ahn, J.H. Promoting long cycle life with honeycomb-like tri-modal porous carbon for stable lithium-sulfur polymer batteries. J. Alloys Compd. 2023, 932, 167704. [Google Scholar] [CrossRef]
- Salimi, P.; Venezia, E.; Taghavi, S.; Tieuli, S.; Carbone, L.; Prato, M.; Signoretto, M.; Qiu, J.F.; Zaccaria, R.P. Lithium-Metal Free Sulfur Battery Based on Waste Biomass Anode and Nano-Sized Li2S Cathode. Energy Environ. Mater. 2023, e12567. [Google Scholar] [CrossRef]
- Choi, J.R.; Kim, E.; Park, B.I.; Choi, I.; Park, B.H.; Lee, S.B.; Lee, J.L.; Yu, S. Meringue-derived hierarchically porous carbon as an efficient polysulfide regulator for lithium-sulfur batteries. J. Ind. Eng. Chem. 2022, 115, 355–364. [Google Scholar] [CrossRef]
- Feng, L.J.; Lu, M.; Shen, W.N.; Qiu, X.Y. N/O dual-doped hierarchical porous carbon boosting cathode performance of lithium-sulfur batteries. Mater. Express 2022, 12, 337–346. [Google Scholar] [CrossRef]
- Cui, J.Q.; Liu, J.; Chen, X.; Meng, J.S.; Wei, S.Y.; Wu, T.; Wang, Y.; Xie, Y.M.; Lu, C.Z.; Zhang, X.C. Ganoderma Lucidum-derived erythrocyte-like sustainable materials. Carbon 2022, 196, 70–77. [Google Scholar] [CrossRef]
- Liang, J.F.; Xu, Y.Q.; Li, C.; Yan, C.; Wang, Z.W.; Xu, J.F.; Guo, L.L.; Li, Y.F.; Zhang, Y.G.; Liu, H.T.; et al. Traditional Chinese medicine residue-derived micropore-rich porous carbon frameworks as efficient sulfur hosts for high-performance lithium–sulfur batteries. Dalton Trans. 2022, 51, 129. [Google Scholar] [CrossRef]
- Li, X.; Cheng, X.; Gao, M.; Ren, D.; Liu, Y.; Guo, Z.; Shang, C.; Sun, L.; Pan, H. Amylose-Derived Macrohollow Core and Microporous Shell Carbon Spheres as Sulfur Host for Superior Lithium-Sulfur Battery Cathodes. ACS Appl. Mater. Interfaces 2017, 9, 10717–10729. [Google Scholar] [CrossRef]
- Li, J.; Qin, F.; Zhang, L.; Zhang, K.; Li, Q.; Lai, Y.; Zhang, Z.; Fang, J. Mesoporous carbon from biomass: One-pot synthesis and application for Li–S batteries. J. Mater. Chem. A 2014, 2, 13916. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, X.; Zhang, Q.; Tu, J. Popcorn Inspired Porous Macrocellular Carbon: Rapid Puffing Fabrication from Rice and Its Applications in Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1701110. [Google Scholar] [CrossRef]
- Ren, G.; Li, S.; Fan, Z.-X.; Warzywodac, J.; Fan, Z. Soybean-derived hierarchical porous carbon with large sulfur loading and sulfur content for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 16507–16515. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.P.; Yang, L.W.; Liu, Y.H.; Chen, Y.X.; Guo, X.D.; Wu, Z.G.; Zhong, B.H. N, O co-doped chlorella-based biomass carbon modified separator for lithium-sulfur battery with high capacity and long cycle performance. J. Colloid Interface Sci. 2021, 585, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, J.N.; Xie, H.B.; Shen, X.Q. Biomass-derived high value-added porous carbon as the interlayer material for advanced lithium–sulfur batteries. Ionics 2022, 28, 3207–3215. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.W.; Li, R.; Chen, Y.X.; Wu, Z.G.; Zhong, B.H.; Guo, X.D. Heteroatom-doped Ginkgo Folium porous carbon modified separator for high-capacity and long-cycle lithium-sulfur batteries. Appl. Surf. Sci. 2022, 602, 154342. [Google Scholar] [CrossRef]
- Guo, Y.C.; Chen, L.X.; Wu, Y.; Lian, J.L.; Tian, Y.; Zhao, Z.Y.; Shao, W.Y.; Ye, Z.Z.; Lu, J.G. Rotten albumen derived layered carbon modified separator for enhancing performance of Li-S batteries. J. Electroanal. Chem. 2021, 895, 115511. [Google Scholar] [CrossRef]
- Shao, H.; Ai, F.; Wang, W.; Zhang, H.; Wang, A.; Feng, W.; Huang, Y. Crab shell-derived nitrogen-doped micro-/mesoporous carbon as an effective separator coating for high energy lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 19892–19900. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C.; Liu, F.; Yang, W.; Hou, Y.; Zhang, S. A conductive interwoven bamboo carbon fiber membrane for Li–S batteries. J. Mater. Chem. A 2015, 3, 9502–9509. [Google Scholar] [CrossRef]
- Yang, J.; Chen, F.; Li, C.; Bai, T.; Longa, B.; Zhou, X. A free-standing sulfur-doped microporous carbon interlayer derived from luffa sponge for high performance lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 14324–14333. [Google Scholar] [CrossRef]
- Wei, Y.B.; Cheng, W.H.; Huang, Y.D.; Liu, Z.J.; Sheng, R.; Wang, X.C.; Jia, D.Z.; Tang, X.C. P-Doped Cotton Stalk Carbon for High-Performance Lithium-Ion Batteries and Lithium–Sulfur Batteries. Langmuir 2022, 38, 11610–11620. [Google Scholar] [CrossRef]
- Sabet, S.M.; Sapkota, N.; Chiluwal, S.; Zheng, T.; Clemos, C.M.; Rao, A.M.; Pilla, S. Sulfurized Polyacrylonitrile Impregnated Delignified Wood-Based 3D Carbon Framework for High-Performance Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2023, 11, 2314–2323. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.L.; Meng, F.C.; Jin, L.Y.; Li, S.L.; Cheng, S.; Jiang, S.D.; Zhou, R.L.; Liu, J.H. Natural nori-based porous carbon composite for sustainable lithium-sulfur batteries. Sci. China Technol. Sci. 2022, 65, 2380–2387. [Google Scholar] [CrossRef]
- Zhu, M.L.; Wu, J.; Li, S.Q. Flower-like Ni/NiO microspheres decorated by sericin-derived carbon for high-rate lithium-sulfur batteries. Ionics 2021, 27, 5137–5145. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, Á.; Morales, J. Improved performance of electrodes based on carbonized olive stones/S composites by impregnating with mesoporous TiO2 for advanced Li-S batteries. J Power Sources 2016, 313, 21–29. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed]
- Luna-Lama, F.; Hernández-Rentero, C.; Caballero, A. Biomass-derived carbon/g-MnO2 nanorods/S composites prepared by facile procedures with improved performance for Li/S batteries. Electrochim. Acta 2018, 292, 522–531. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.; Shen, L. CoO embedded porous biomass-derived carbon as dual-functional host material for lithium-sulfur batteries. J. Colloid Interface Sci. 2023, 640, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Lama, F.L.; Caballero, Á.; Morales, J. Synergistic effect between PPy: PSS copolymers and biomass-derived activated carbons: A simple strategy for designing sustainable highperformance Li–S batteries. Sustain. Energy Fuels 2022, 6, 1568–1586. [Google Scholar] [CrossRef]
- Chen, R.X.; Zhou, Y.C.; Li, X.D. Cotton-Derived Fe/Fe3C-Encapsulated Carbon Nanotubes for High-Performance Lithium–Sulfur Batteries. Nano Lett. 2022, 22, 1217–1224. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, J.C.; Shu, X.Q.; Guan, J.D.; Tong, G.S.; Ding, H.; Chen, L.Y.; Zhou, N.; Shuai, Y. N, P, O-codoped biochar from phytoremediation residues: A promising cathode material for Li–S batteries. Nanotechnology 2022, 33, 215403. [Google Scholar] [CrossRef]
- Tesio, A.Y.; Gómez-Camer, J.L.; Morales, J. Simple and sustainable preparation of non-activated porous carbon from brewing waste for high-performance lithium–sulfur batteries. ChemSusChem 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Benítez, A.; Márquez, P.M.; Martín, Á. Simple and Sustainable Preparation of Cathodes for Li–S Batteries: Regeneration of Granular Activated Carbon from the Odor Control System of a Wastewater Treatment Plant. ChemSusChem 2021, 14, 3915–3925. [Google Scholar] [CrossRef]
- Páez Jerez, A.L.; Mori, M.F.; Flexer, V. Water Kefir Grains—Microbial Biomass Source for Carbonaceous Materials Used as Sulfur-Host Cathode in Li-S Batteries. Materials 2022, 15, 8856. [Google Scholar] [CrossRef] [PubMed]
- Benítez, A.; Morales, J.; Caballero, Á. Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries. Nanomaterials 2020, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Xia, G.H.; Wang, D. Biomass-derived self-supporting sulfur host with NiS/C composite for high-loading Li-S battery cathode. Sci. China Technol. Sci. 2023, 66, 181–192. [Google Scholar] [CrossRef]
- Lama, F.L.; Marangon, V.; Caballero, Á. Diffusional Features of a Lithium-Sulfur Battery Exploiting Highly Microporous Activated Carbon. ChemSusChem 2023, 16, e202202095. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Xia, G.H.; Wang, D. Self-supporting Biomass Li–S Cathodes Decorated with Metal Phosphides–Higher Sulfur Loading, Better Stability, and Longer Cycle Life. ACS Appl. Energy Mater. 2022, 5, 15401–15411. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhang, M.; Ding, Y.; Xue, Y.; Wang, H.; Li, P.; Wu, D. Green Production of Biomass-Derived Carbon Materials for High-Performance Lithium–Sulfur Batteries. Nanomaterials 2023, 13, 1768. https://doi.org/10.3390/nano13111768
Ma C, Zhang M, Ding Y, Xue Y, Wang H, Li P, Wu D. Green Production of Biomass-Derived Carbon Materials for High-Performance Lithium–Sulfur Batteries. Nanomaterials. 2023; 13(11):1768. https://doi.org/10.3390/nano13111768
Chicago/Turabian StyleMa, Chao, Mengmeng Zhang, Yi Ding, Yan Xue, Hongju Wang, Pengfei Li, and Dapeng Wu. 2023. "Green Production of Biomass-Derived Carbon Materials for High-Performance Lithium–Sulfur Batteries" Nanomaterials 13, no. 11: 1768. https://doi.org/10.3390/nano13111768
APA StyleMa, C., Zhang, M., Ding, Y., Xue, Y., Wang, H., Li, P., & Wu, D. (2023). Green Production of Biomass-Derived Carbon Materials for High-Performance Lithium–Sulfur Batteries. Nanomaterials, 13(11), 1768. https://doi.org/10.3390/nano13111768






