Multi-Technique Approach for Work Function Exploration of Sc2O3 Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization Techniques
3. Results and Discussion
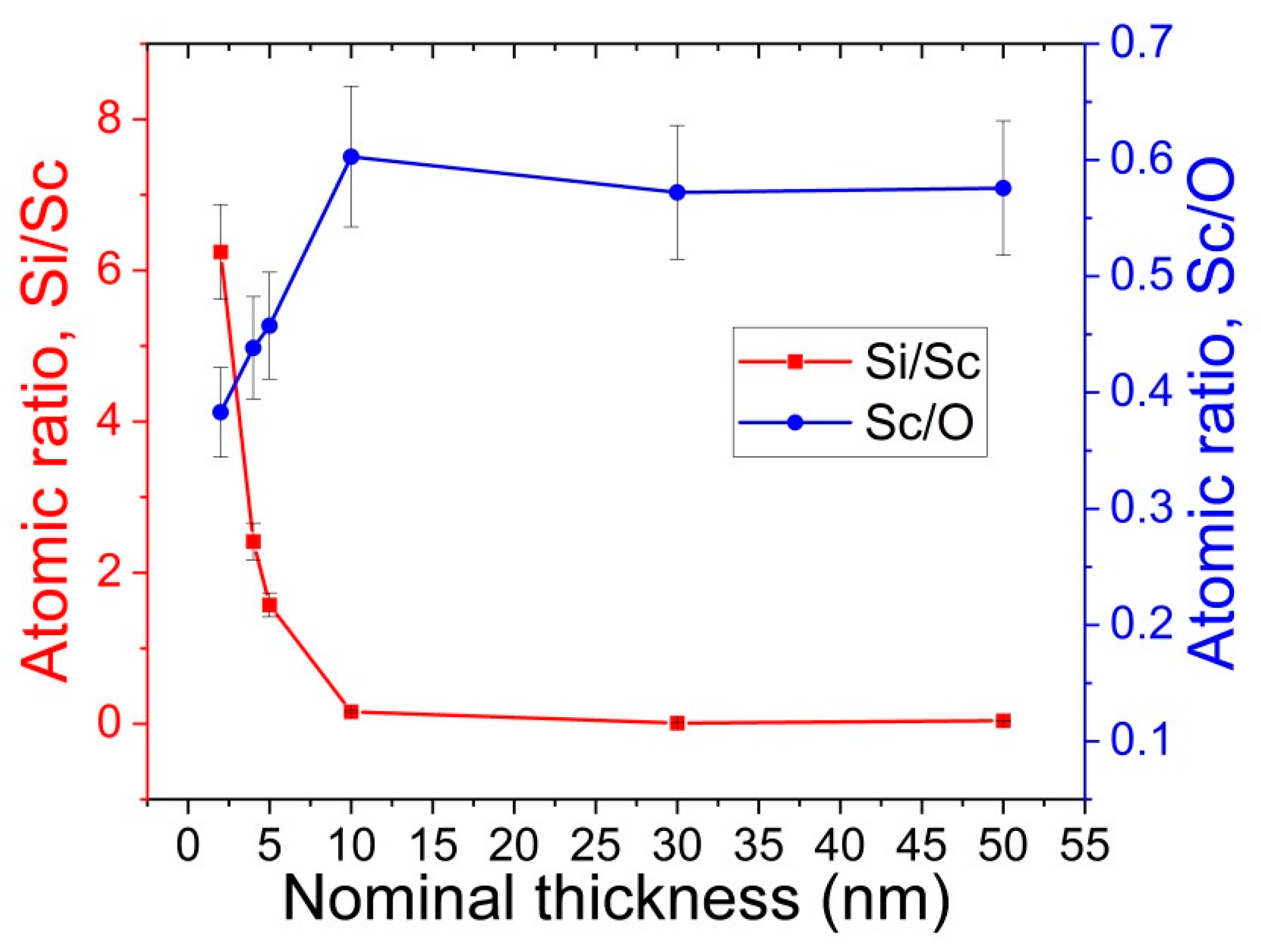
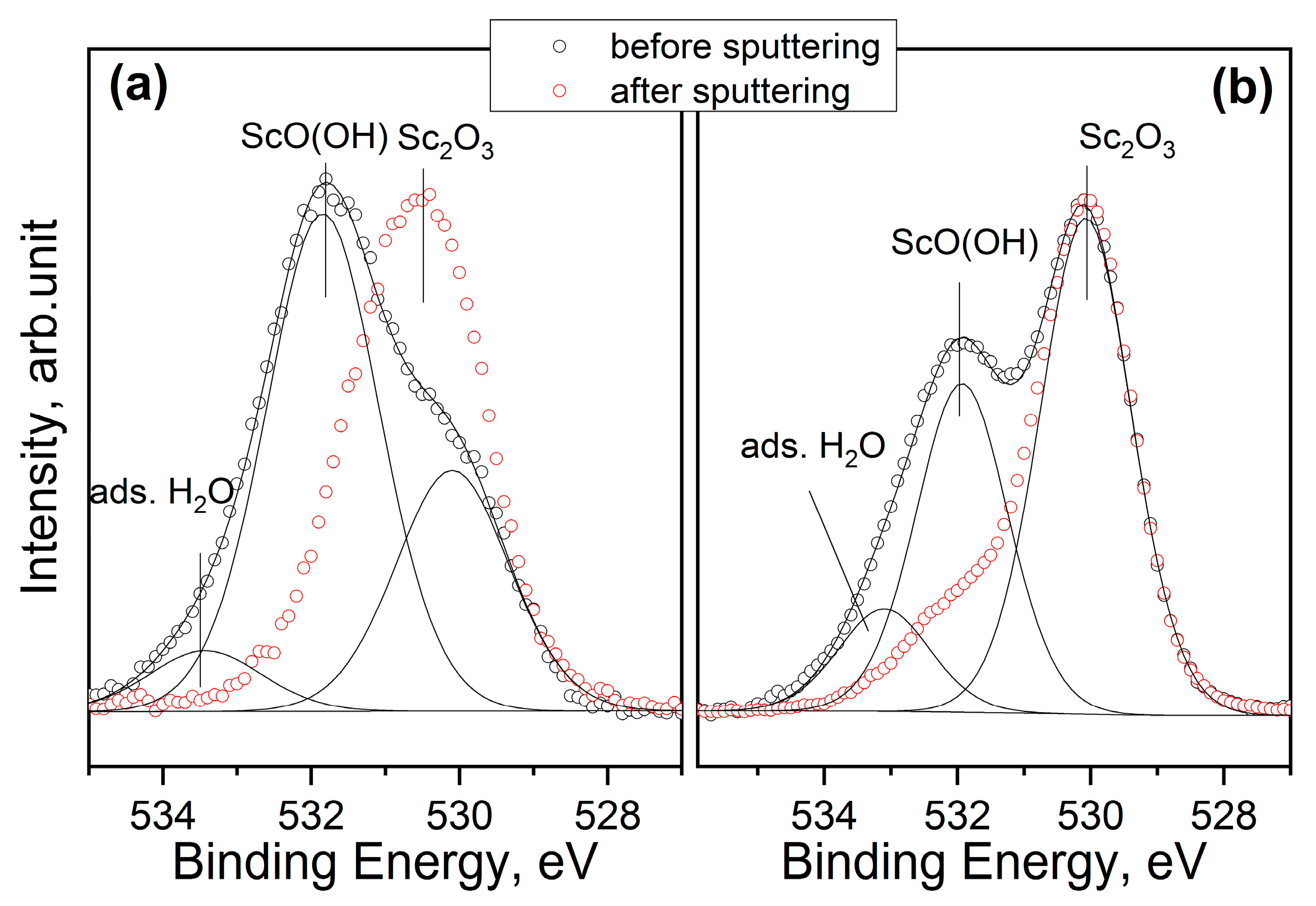
3.1. X-ray Photoelectron Spectroscopy (XPS)
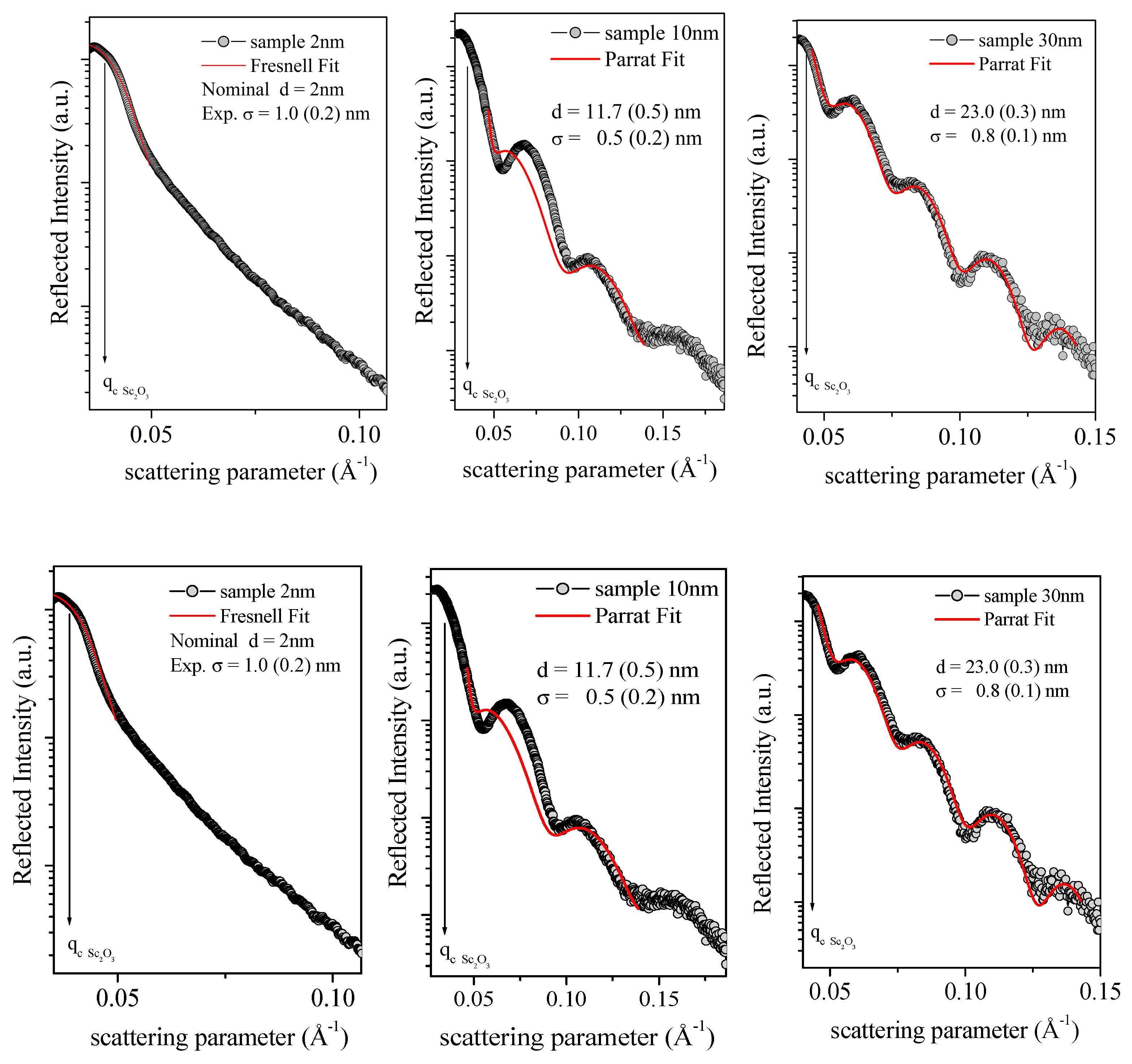
3.2. X-ray Diffraction (XRD) and Energy Dispersive X-ray Reflectivity (EDXR)
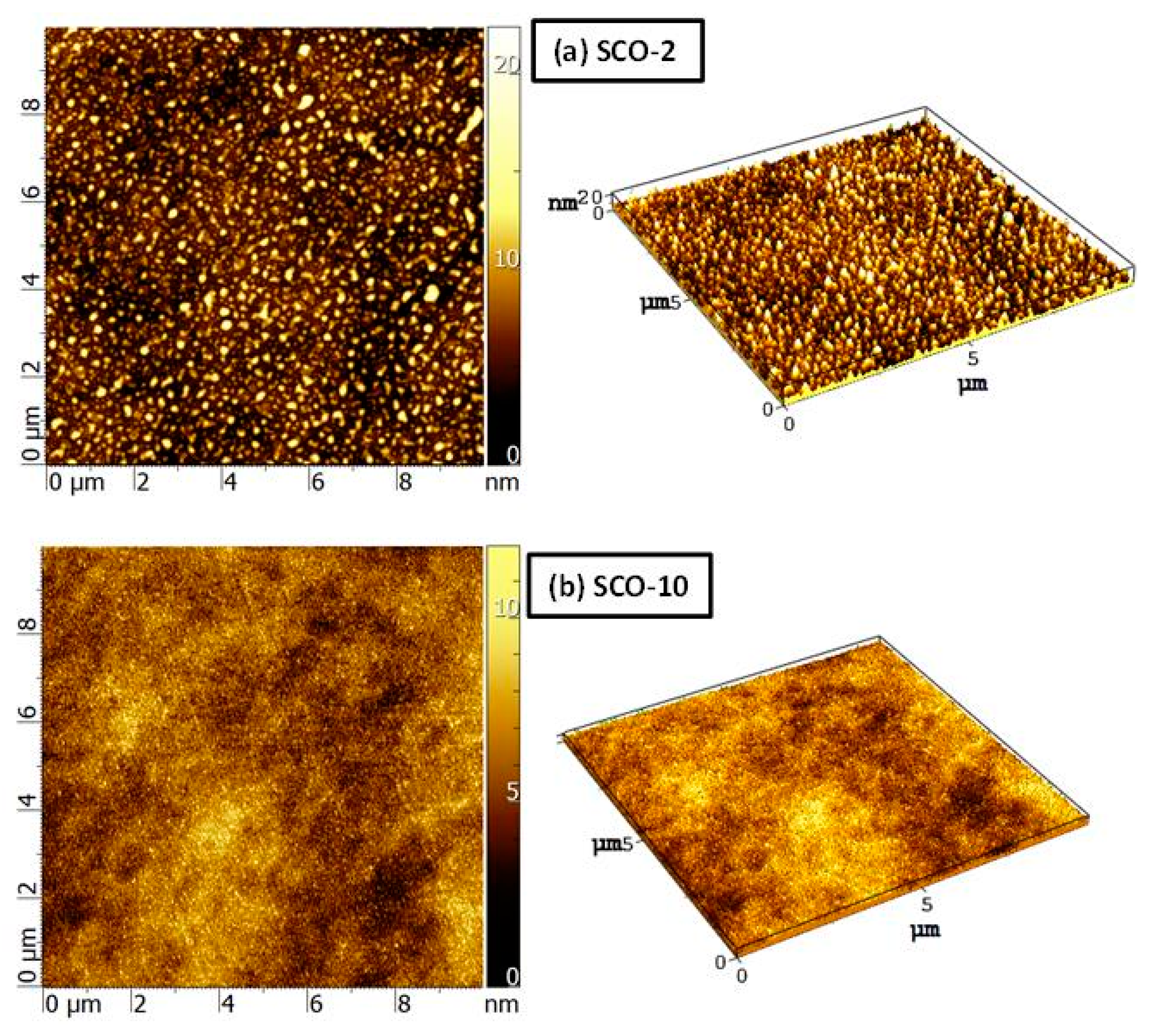
3.3. Atomic Force Microscopy (AFM)
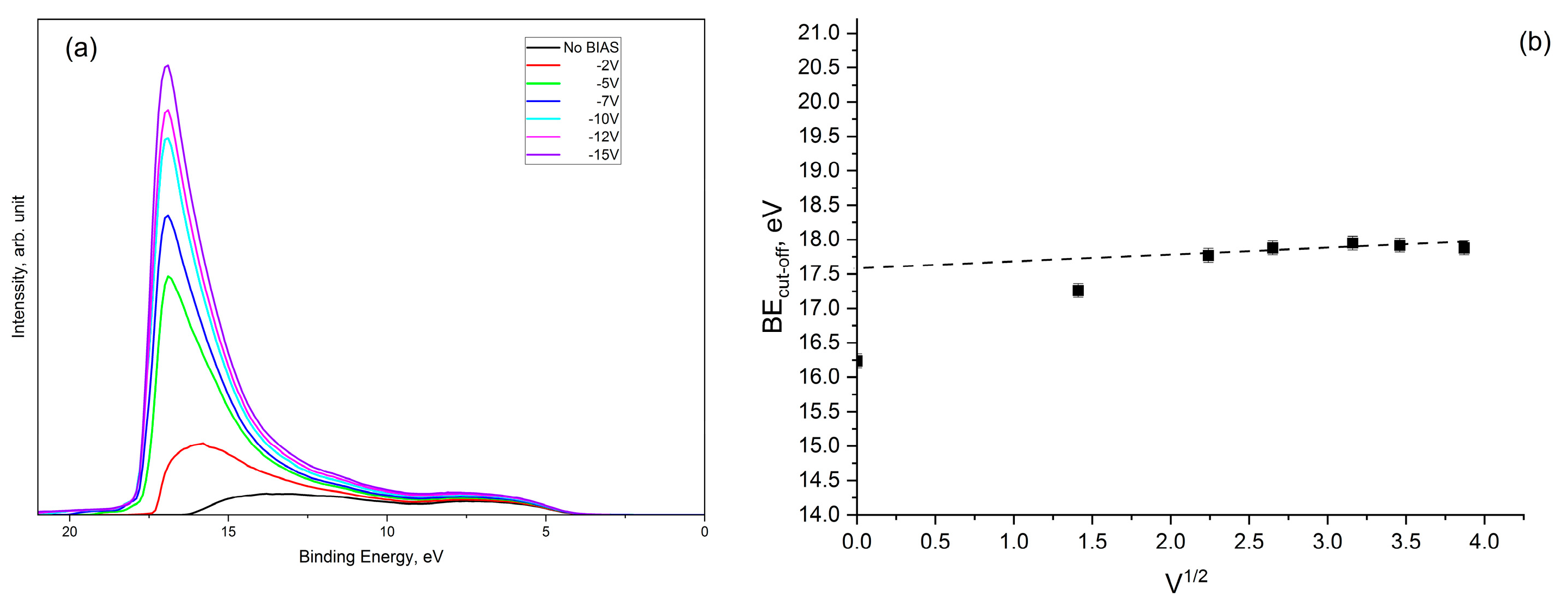
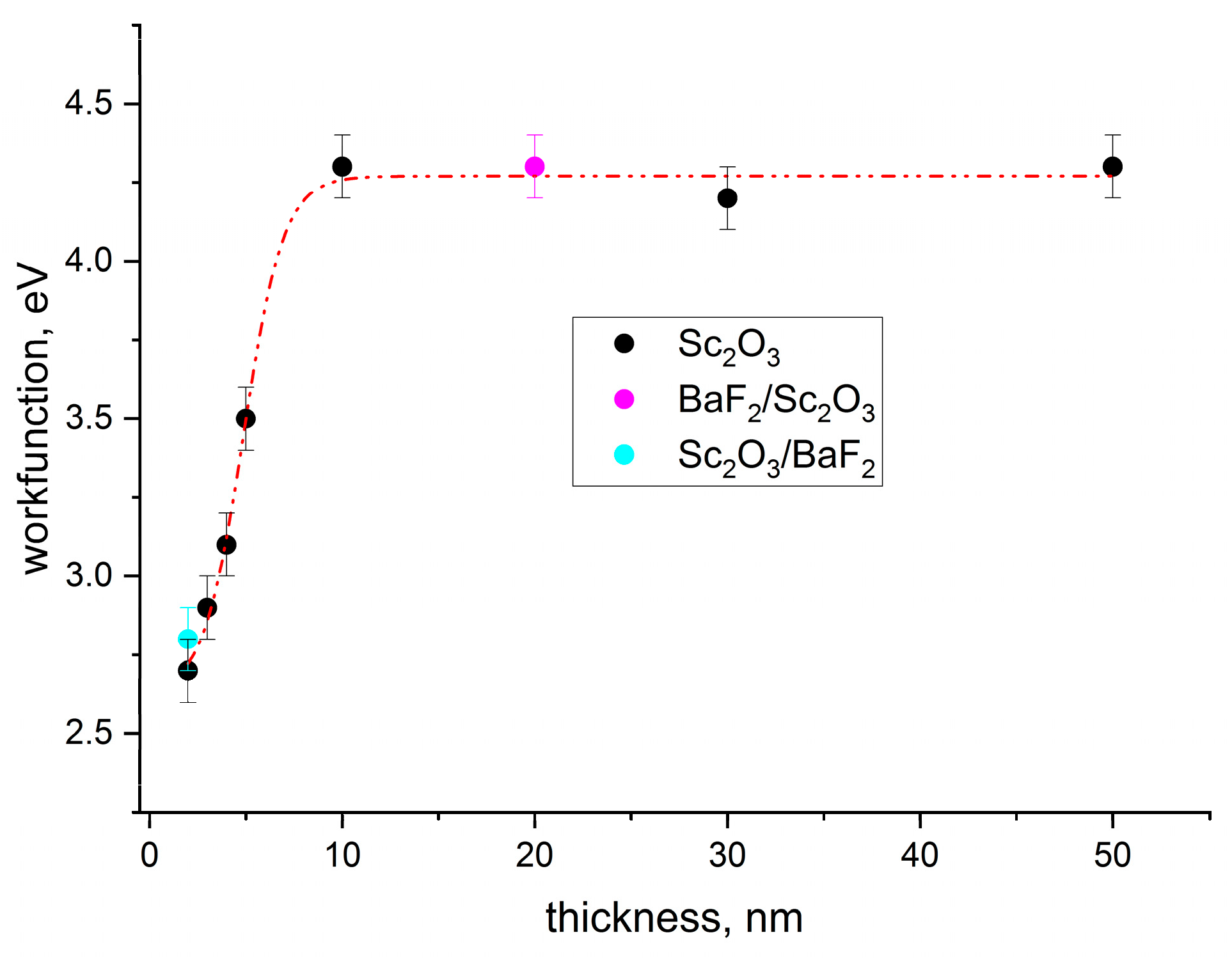
3.4. Ultra-Violet Photoelectron Spectroscopy (UPS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivek, C.M.; Ramkumar, P.; Srividhya, P.K.; Sivasubramanian, M. Recent strategies and trends in implanting of renewable energy sources for sustainability—A review. Mat. Today Proc. 2021, 46, 8204–8208. [Google Scholar] [CrossRef]
- Nema, P.; Nema, S.; Roya, P. An overview of global climate changing in current scenario and mitigation action. Ren. Sust. Energy Rev. 2012, 16, 2329–2336. [Google Scholar] [CrossRef]
- Dixit, S. Solar technologies and their implementations: A review. Mater. Today Proc. 2020, 28, 2137–2148. [Google Scholar] [CrossRef]
- Hussaina, A.; Arif, S.M.; Aslam, M. Emerging renewable and sustainable energy technologies: State of the art. Ren. Sust. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Bellucci, A.; Mastellone, M.; Serpente, V.; Girolami, M.; Kaciulis, S.; Mezzi, A.; Trucchi, D.M.; Antolín, E.; Villa, J.; Linares, P.G.; et al. Photovoltaic Anodes for Enhanced Thermionic Energy Conversion. ACS Energy Lett. 2020, 5, 1364–1370. [Google Scholar] [CrossRef]
- Bellucci, A.; Linares, P.G.; Villa, J.; Martí, A.; Datas, A.; Trucchi, D.M. Hybrid thermionic-photovoltaic converter with an In0.53Ga0.47As anode. Sol. Energy Mater. Sol. Cells 2022, 238, 111588. [Google Scholar] [CrossRef]
- Bellucci, A.; Mastellone, M.; Orlando, S.; Girolami, M.; Generosi, A.; Paci, B.; Soltani, P.; Mezzi, A.; Kaciulis, S.; Polini, R.; et al. Lanthanum (oxy)boride thin films for thermionic emission applications. Appl. Surf. Sci. 2019, 479, 296–302. [Google Scholar] [CrossRef]
- Bellucci, A.; García-Linares, P.; Martí, A.; Trucchi, D.M.; Datas, A. A Three-Terminal Hybrid Thermionic-Photovoltaic Energy Converter Advanced. Adv. Energy Mat. 2022, 12, 2200357. [Google Scholar] [CrossRef]
- Crowell, C.R. The Richardson constant for thermionic emission in Schottky barrier diodes. Solid State Electron. 1965, 8, 395–399. [Google Scholar] [CrossRef]
- Bellucci, A.; Girolami, M.; Mastellone, M.; Orlando, S.; Polini, R.; Santagata, A.; Serpente, V.; Valentini, V.; Trucchi, D.M. Novel concepts and nanostructured materials for thermionic-based solar and thermal energy converters. Nanotechnology 2020, 32, 024002. [Google Scholar] [CrossRef]
- Bellucci, A.; Sabbatella, G.; Girolami, M.; Mastellone, M.; Serpente, V.; Mezzi, A.; Kaciulis, S.; Paci, B.; Generosi, A.; Polini, R.; et al. Dielectric Micro- and Sub-Micrometric Spacers for High-Temperature Energy Converters. Energy Technol. 2021, 9, 2000788. [Google Scholar] [CrossRef]
- Zeneli, M.; Bellucci, A.; Sabbatella, G.; Fotopoulou, M.; Apostolopoulos, V.; Stamatopoulos, P.; Trucchi, D.M.; Nikolopoulos, A.; Rakopoulos, D. Thermal Assessment of Dielectric Microspacer Technology Using an Advanced Three-Dimensional Simulation Model. Sustainability 2023, 15, 1786. [Google Scholar] [CrossRef]
- Mezzi, A.; Soltani, P.; Kaciulis, S.; Bellucci, A.; Girolami, M.; Mastellone, M.; Trucchi, D.M. Investigation of work function and chemical composition of thin films of borides and nitrides. Surf. Inter. Anal. 2018, 50, 1138–1144. [Google Scholar] [CrossRef]
- Mezzi, A.; Bolli, E.; Kaciulis, S.; Mastellone, M.; Girolami, M.; Serpente, V.; Bellucci, A.; Carducci, R.; Polini, R.; Trucchi, D.M. Work function and negative electron affinity of ultrathin barium fluoride films. Surf. Inter. Anal. 2020, 52, 968–974. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Melson, G.A.; Youngblood, D.H.; Schock, H.H. Physical Metallurgy. In Scandium–Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology; Horovitz, C.T., Ed.; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1975; p. 76. [Google Scholar]
- Gibson, J.W.; Haas, G.A.; Thomas, R.E. Investigation of scandate cathodes: Emission, fabrication, and activation processes. IEEE Trans. Electron Devices 1989, 36, 209–214. [Google Scholar] [CrossRef]
- Vickerman, J.C.; Gilmore, I.S. (Eds.) Surface Analysis–The Principal Techniques, 2nd ed.; John Wiley & Sons, Ltd.: Teddington, UK, 2009; p. 67. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Swanson, H.E.; Fuyat, R.K.; Ugrinic, G.M. Standard X-ray Diffraction Powder Patterns; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1954; Volume III, p. 27.
- Lozovoy, K.A.; Korotaev, A.G.; Kokhanenko, A.P.; Dirko, V.V.; Voitsekhovskii, A.V. Kinetics of epitaxial formation of nanostructures by Frank–van der Merwe, Volmer–Weber and Stranski–Krastanow growth modes. Surf. Coat. Technol. 2020, 384, 125289. [Google Scholar] [CrossRef]
- Whitten, J.E. Ultraviolet photoelectron spectroscopy: Practical aspects and best practices. Appl. Surf. Sci. Adv. 2023, 13, 100384. [Google Scholar] [CrossRef]
- Kim, J.S.; Lägel, B.; Moons, E.; Johansson, N.; Baikie, I.D.; Salaneck, W.R.; Friend, R.H.; Cacialli, F. Kelvin probe and ultraviolet photoemission measurements of indium tin oxide work function: A comparison. Synth. Met. 2000, 111–112, 311–314. [Google Scholar] [CrossRef]
- Martinez, E.; Guedj, C.; Mariolle, D.; Licitra, C.; Renault, O.; Bertin, F.; Chabli, A.; Imbert, G.; Delsol, R. Electronic and chemical properties of the TaN/a-SiOC:H stack studied by photoelectron spectroscopy for advanced interconnects. J. Appl. Phys. 2008, 104, 073708. [Google Scholar] [CrossRef]
- Langmuir, I. Vapor pressures, evaporation, condensation and adsorption. J. Am. Chem. Soc. 1932, 54, 2798. [Google Scholar] [CrossRef]
- Gurney, R.W. Theory of electrical double layers in adsorbed films. Phys. Rev. 1935, 47, 479. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzi, A.; Bolli, E.; Kaciulis, S.; Bellucci, A.; Paci, B.; Generosi, A.; Mastellone, M.; Serpente, V.; Trucchi, D.M. Multi-Technique Approach for Work Function Exploration of Sc2O3 Thin Films. Nanomaterials 2023, 13, 1430. https://doi.org/10.3390/nano13081430
Mezzi A, Bolli E, Kaciulis S, Bellucci A, Paci B, Generosi A, Mastellone M, Serpente V, Trucchi DM. Multi-Technique Approach for Work Function Exploration of Sc2O3 Thin Films. Nanomaterials. 2023; 13(8):1430. https://doi.org/10.3390/nano13081430
Chicago/Turabian StyleMezzi, Alessio, Eleonora Bolli, Saulius Kaciulis, Alessandro Bellucci, Barbara Paci, Amanda Generosi, Matteo Mastellone, Valerio Serpente, and Daniele Maria Trucchi. 2023. "Multi-Technique Approach for Work Function Exploration of Sc2O3 Thin Films" Nanomaterials 13, no. 8: 1430. https://doi.org/10.3390/nano13081430
APA StyleMezzi, A., Bolli, E., Kaciulis, S., Bellucci, A., Paci, B., Generosi, A., Mastellone, M., Serpente, V., & Trucchi, D. M. (2023). Multi-Technique Approach for Work Function Exploration of Sc2O3 Thin Films. Nanomaterials, 13(8), 1430. https://doi.org/10.3390/nano13081430









