DFT Study of Adsorption Behavior of Nitro Species on Carbon-Doped Boron Nitride Nanoribbons for Toxic Gas Sensing
Abstract
1. Introduction
2. Computation Details
3. Results
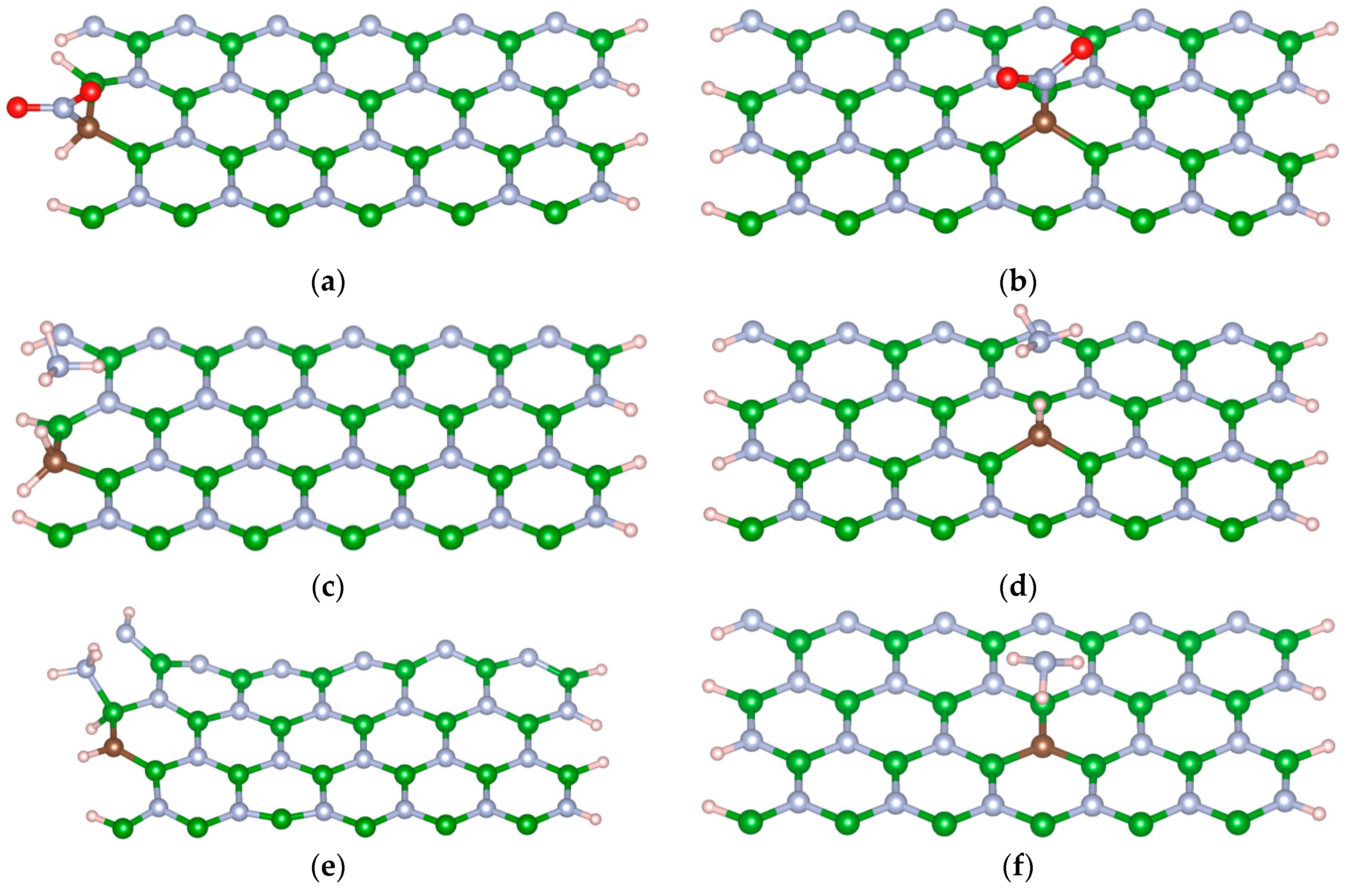
3.1. System Description
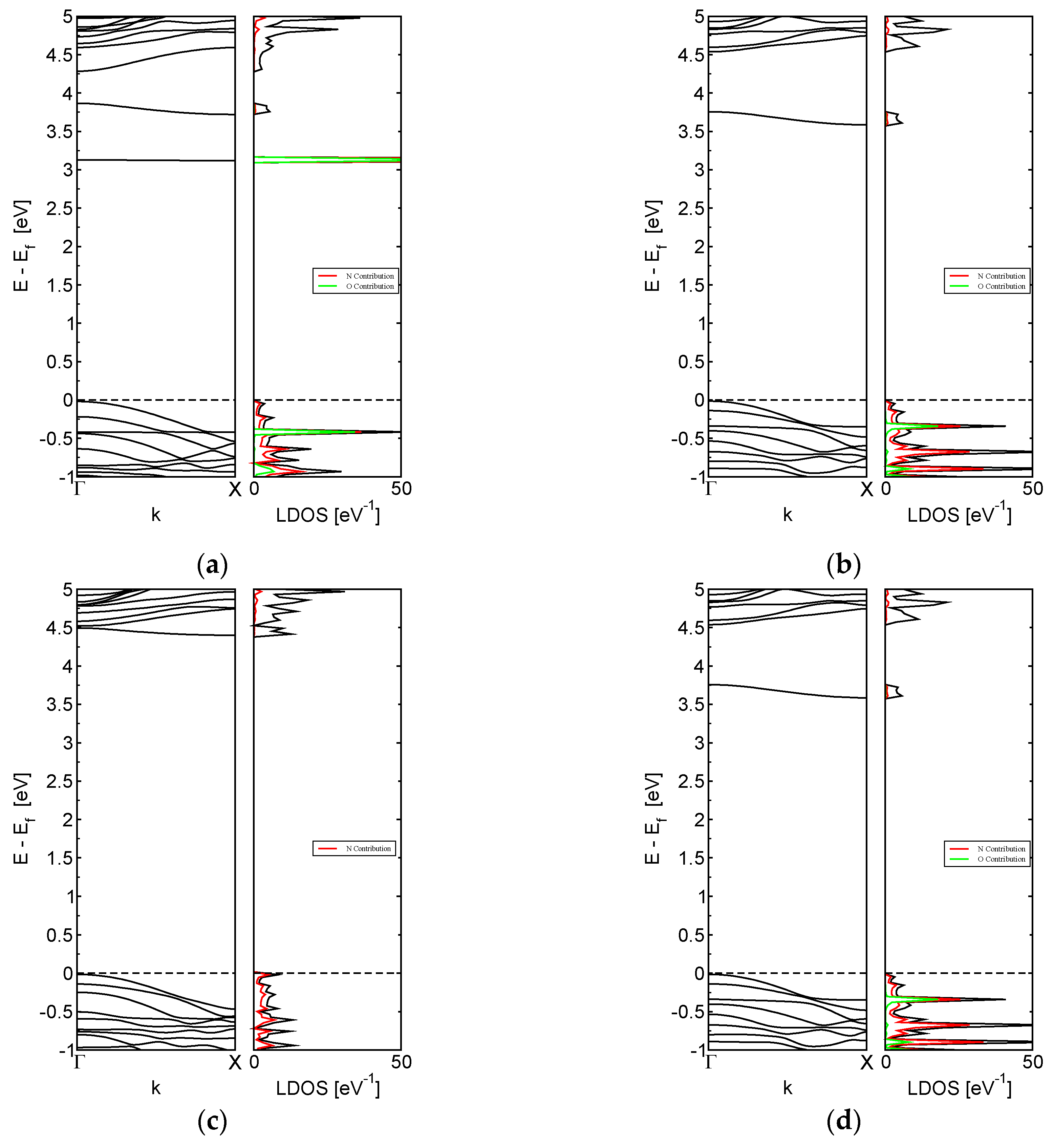
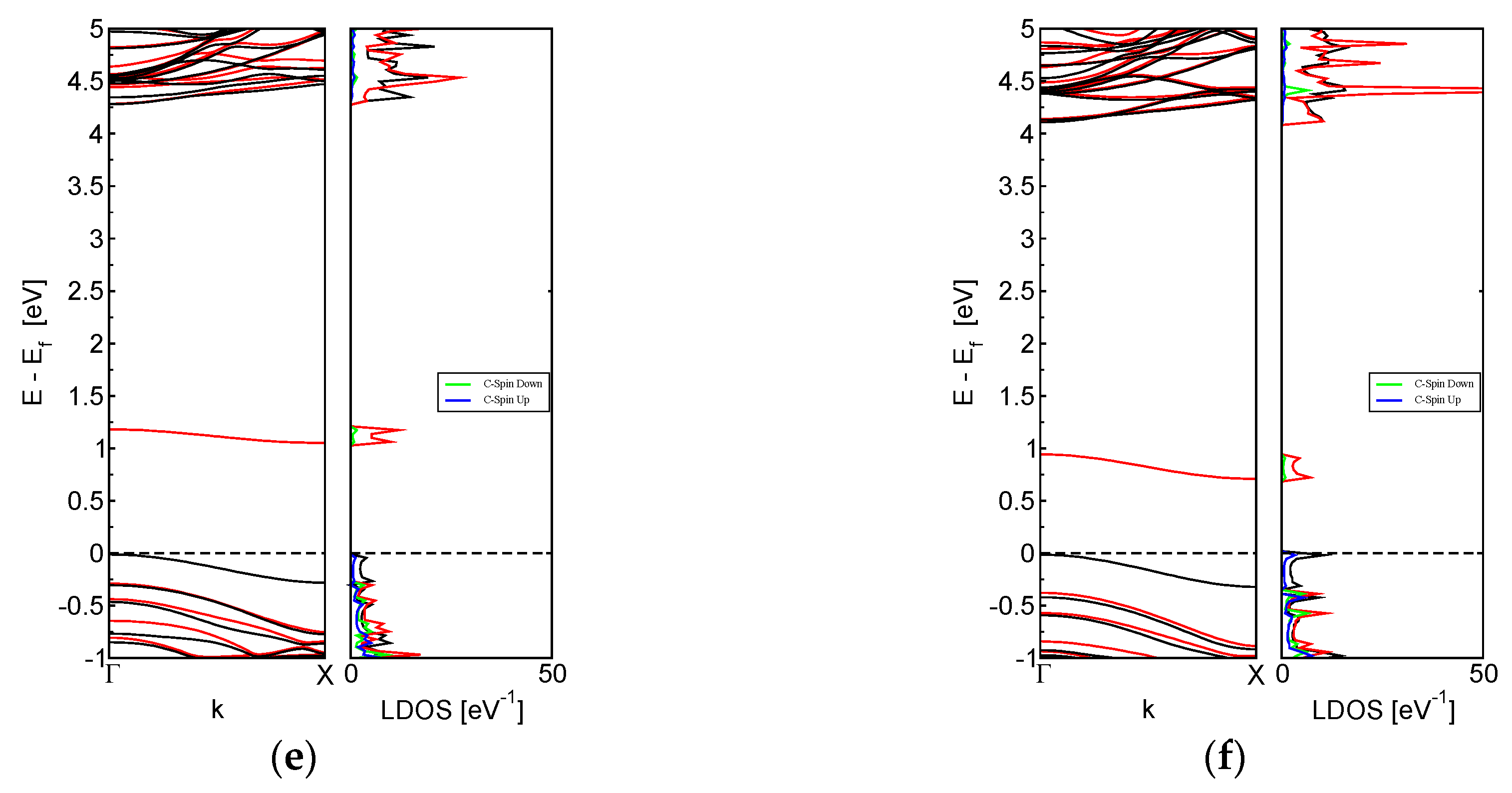
3.2. System Electronic Properties
3.3. Energetic Stability
3.4. Interaction of Toxic Gases on M-BNNRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramanathan, A.A. Defect Functionalization of MoS2 nanostructures as toxic gas sensors: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012001. [Google Scholar] [CrossRef][Green Version]
- Tsujita, W.; Yoshino, A.; Ishida, H.; Moriizumi, T. Gas sensor network for air-pollution monitoring. Sens. Actuators B Chem. 2005, 110, 304–311. [Google Scholar] [CrossRef]
- Kumar, V.; Azhikodan, D.; Roy, D.R. 2D Sb2C3 monolayer: A promising material for the recyclable gas sensor for environmentally toxic nitrogen-containing gases (NCGs). J. Hazard. Mater. 2021, 405, 124168. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef]
- Winiwarter, W.; Klimont, Z. The role of N-gases (N2O, NOx, NH3) in cost-effective strategies to reduce greenhouse gas emissions and air pollution in Europe. Curr. Opin. Environ. Sustain. 2011, 3, 438–445. [Google Scholar] [CrossRef]
- He, Q.; Zeng, Z.; Yin, Z.; Li, H.; Wu, S.; Huang, X.; Zhang, H. Fabrication of Flexible MoS2 Thin-Film Transistor Arrays for Practical Gas-Sensing Applications. Small 2012, 8, 2994–2999. [Google Scholar] [CrossRef]
- Jappor, H.R.; Khudair, S.A.M. Electronic Properties of Adsorption of CO, CO2, NH3, NO, NO2 and SO2 on Nitrogen Doped Graphene for Gas Sensor Applications. Sens. Lett. 2017, 15, 432–439. [Google Scholar] [CrossRef]
- Harada, N.; Sato, S. Electronic properties of NH4-adsorbed graphene nanoribbon as a promising candidate for a gas sensor. AIP Adv. 2016, 6, 055023. [Google Scholar] [CrossRef][Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef]
- Dan, Y.; Lu, Y.; Kybert, N.J.; Luo, Z.; Johnson, A.T.C. Intrinsic Response of Graphene Vapor Sensors. Nano Lett. 2009, 9, 1472–1475. [Google Scholar] [CrossRef][Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Galashev, A.E.; Rakhmanova, O.R. Mechanical and thermal stability of graphene and graphene-based materials. Physics-Uspekhi 2014, 57, 970–989. [Google Scholar] [CrossRef]
- Gan, T.; Hu, S. Electrochemical sensors based on graphene materials. Microchim. Acta 2011, 175, 1–19. [Google Scholar] [CrossRef]
- Fu, X.-W.; Liao, Z.-M.; Zhou, Y.-B.; Wu, H.-C.; Bie, Y.-Q.; Xu, J.; Yu, D.-P. Graphene/ZnO nanowire/graphene vertical structure based fast-response ultraviolet photodetector. Appl. Phys. Lett. 2012, 100, 223114. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef][Green Version]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A Mater. 2013, 1, 10078. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef] [PubMed]
- Abergel, D.S.L.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T. Properties of graphene: A theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar] [CrossRef][Green Version]
- Paine, R.T.; Narula, C.K. Synthetic routes to boron nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.P.; Shen, Z.X. Structural and electronic properties of h -BN. Phys. Rev. B 2003, 68, 104102. [Google Scholar] [CrossRef]
- Ooi, N.; Rajan, V.; Gottlieb, J.; Catherine, Y.; Adams, J.B. Structural properties of hexagonal boron nitride. Model. Simul. Mater. Sci. Eng. 2006, 14, 515–535. [Google Scholar] [CrossRef]
- Barone, V.; Peralta, J.E. Magnetic Boron Nitride Nanoribbons with Tunable Electronic Properties. Nano Lett. 2008, 8, 2210–2214. [Google Scholar] [CrossRef][Green Version]
- Liu, N.; Liu, J.B.; Gao, G.Y.; Yao, K.L. Carbon doping induced giant low bias negative differential resistance in boron nitride nanoribbon. Phys. Lett. A 2014, 378, 2217–2221. [Google Scholar] [CrossRef]
- Park, C.H.; Louie, S.G. Energy Gaps and Stark Effect in Boron Nitride Nanoribbons. Nano Lett. 2008, 8, 2200–2203. [Google Scholar] [CrossRef][Green Version]
- Villanueva-Mejia, F.; Navarro-Santos, P.; Rodríguez-Kessler, P.L.; Herrera-Bucio, R.; Rivera, J.L. Reactivity of atomically functionalized C-doped boron nitride nanoribbons and their interaction with organosulfur compounds. Nanomaterials 2019, 9, 452. [Google Scholar] [CrossRef][Green Version]
- Dabhi, S.D.; Roondhe, B.; Jha, P.K. Nucleobases-decorated boron nitride nanoribbons for electrochemical biosensing: A dispersion-corrected DFT study. Phys. Chem. Chem. Phys. 2018, 20, 8943–8950. [Google Scholar] [CrossRef]
- Roondhe, B.; Dabhi, S.D.; Jha, P.K. Sensing properties of pristine boron nitride nanostructures towards alkaloids: A first principles dispersion corrected study. Appl. Surf. Sci. 2018, 441, 588–598. [Google Scholar] [CrossRef]
- Srivastava, P.; Jaiswal, N.K.; Sharma, V. First-principles investigation of armchair boron nitride nanoribbons for sensing PH3 gas molecules. Superlattices Microstruct 2014, 73, 350–358. [Google Scholar] [CrossRef]
- Li, Y.; Nie, J.; Gao, D.; Zhao, S.; Zhang, Y.; Bian, B.; Guo, Z.; Huang, Y.; Fang, Y.; Tang, C. Hexagonal boron nitride nanoribbon as a novel metal-free catalyst for high-efficiency NO reduction to NH3. Fuel 2023, 339, 126943. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Tang, S.; Cao, Z. Adsorption of nitrogen oxides on graphene and graphene oxides: Insights from density functional calculations. J. Chem. Phys. 2011, 134, 044710. [Google Scholar] [CrossRef]
- Lin, X.; Ni, J.; Fang, C. Adsorption capacity of H2O, NH3, CO, and NO2 on the pristine graphene. J. Appl. Phys. 2013, 113, 034306. [Google Scholar] [CrossRef]
- Hosseinian, A.; Asadi, Z.; Edjlali, L.; Bekhradnia, A.; Vessally, E. NO2 sensing properties of a borazine doped nanographene: A DFT study. Comput Theor Chem. 2017, 1106, 36–42. [Google Scholar] [CrossRef]
- Esrafili, M.D. Boron and nitrogen co-doped graphene nanosheets for NO and NO2 gas sensing. Phys. Lett. A 2019, 383, 1607–1614. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H. Different elements doped graphene sensor for CO2 greenhouse gases detection: The DFT study. Chem. Phys. Lett. 2019, 721, 33–37. [Google Scholar] [CrossRef]
- Nasehnia, F.; Seifi, M. Adsorption of molecular oxygen on VIIIB transition metal-doped graphene: A DFT study. Mod. Phys. Lett. B 2014, 28, 1450237. [Google Scholar] [CrossRef]
- Borisova, D.; Antonov, V.; Proykova, A. Hydrogen sulfide adsorption on a defective graphene. Int. J. Quantum. Chem. 2013, 113, 786–791. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Shen, Z.; Chen, W.; Ma, D.; Dai, X. Adsorption sensitivity of metal atom decorated bilayer graphene toward toxic gas molecules (CO, NO, SO2 and HCN). Sens. Actuators B Chem. 2017, 238, 182–195. [Google Scholar] [CrossRef]
- Bo, Z.; Guo, X.; Wei, X.; Yang, H.; Yan, J.; Cen, K. Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene. Phys. E Low Dimens. Syst. Nanostruct. 2019, 109, 156–163. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order- N materials simulation. J. Phys. Condens. Matter. 2002, 14, 2745–2779. [Google Scholar] [CrossRef][Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef][Green Version]
- Du, A.J.; Smith, S.C.; Lu, G.Q. First-principle studies of electronic structure and C-doping effect in boron nitride nanoribbon. Chem. Phys. Lett. 2007, 447, 181–186. [Google Scholar] [CrossRef]
- Bagheri, Z.; Peyghan, A.A. DFT study of NO2 adsorption on the AlN nanocones. Comput. Theor. Chem. 2013, 1008, 20–26. [Google Scholar] [CrossRef]
- Salih, E.; Ayesh, A.I. Enhancing the Sensing Performance of Zigzag Graphene Nanoribbon to Detect NO, NO2, and NH3 Gases. Sensors 2020, 20, 3932. [Google Scholar] [CrossRef]


| M × N | Pristine | E_M-BBNR | C_M-BNNR |
|---|---|---|---|
| 12 × 2 | 6.32 (−5.5) | 6.30 (−5.39) | 6.29 (−5.38) |
| M-BNNR | NO2 | NH4 | NH3 |
|---|---|---|---|
| E_M-BNNR | −50.04 | −87.09 | −80.47 |
| C_M-BNNR | −44.23 | −85.02 | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Mejia, F.; Guevara-Martínez, S.J.; Arroyo-Albiter, M.; Alvarado-Flores, J.J.; Zamudio-Ojeda, A. DFT Study of Adsorption Behavior of Nitro Species on Carbon-Doped Boron Nitride Nanoribbons for Toxic Gas Sensing. Nanomaterials 2023, 13, 1410. https://doi.org/10.3390/nano13081410
Villanueva-Mejia F, Guevara-Martínez SJ, Arroyo-Albiter M, Alvarado-Flores JJ, Zamudio-Ojeda A. DFT Study of Adsorption Behavior of Nitro Species on Carbon-Doped Boron Nitride Nanoribbons for Toxic Gas Sensing. Nanomaterials. 2023; 13(8):1410. https://doi.org/10.3390/nano13081410
Chicago/Turabian StyleVillanueva-Mejia, Francisco, Santiago José Guevara-Martínez, Manuel Arroyo-Albiter, José Juan Alvarado-Flores, and Adalberto Zamudio-Ojeda. 2023. "DFT Study of Adsorption Behavior of Nitro Species on Carbon-Doped Boron Nitride Nanoribbons for Toxic Gas Sensing" Nanomaterials 13, no. 8: 1410. https://doi.org/10.3390/nano13081410
APA StyleVillanueva-Mejia, F., Guevara-Martínez, S. J., Arroyo-Albiter, M., Alvarado-Flores, J. J., & Zamudio-Ojeda, A. (2023). DFT Study of Adsorption Behavior of Nitro Species on Carbon-Doped Boron Nitride Nanoribbons for Toxic Gas Sensing. Nanomaterials, 13(8), 1410. https://doi.org/10.3390/nano13081410






