The Photocatalytic Activity of CaTiO3 Derived from the Microwave-Melting Heating Process of Blast Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Experimental Design of Photocatalysis
2.3. Instrumental Characterization
2.4. First-Principles Theoretical Analysis
3. Results and Discussion
3.1. Characterization
3.2. Photocatalytic Performance of MM-CaTiO3
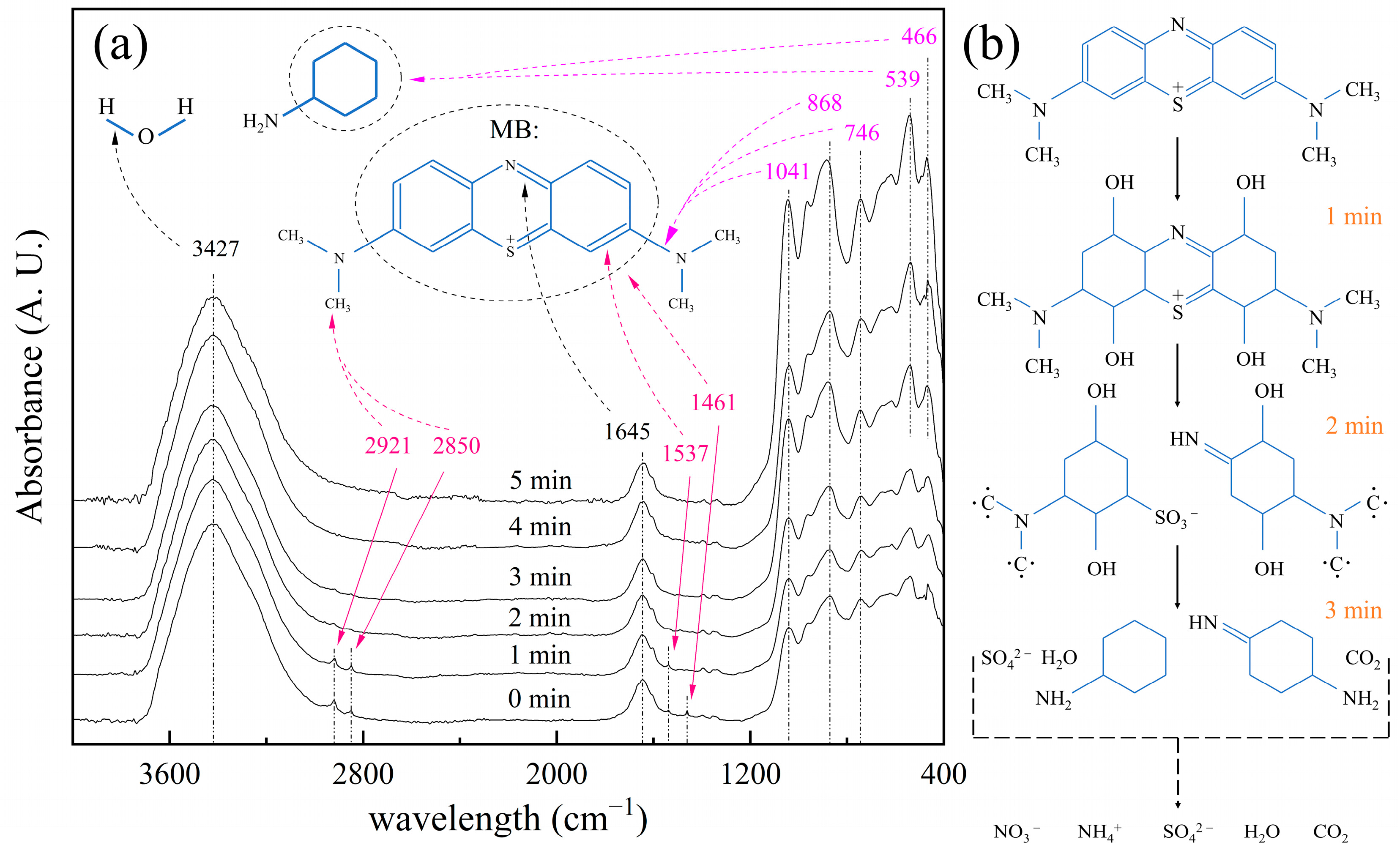
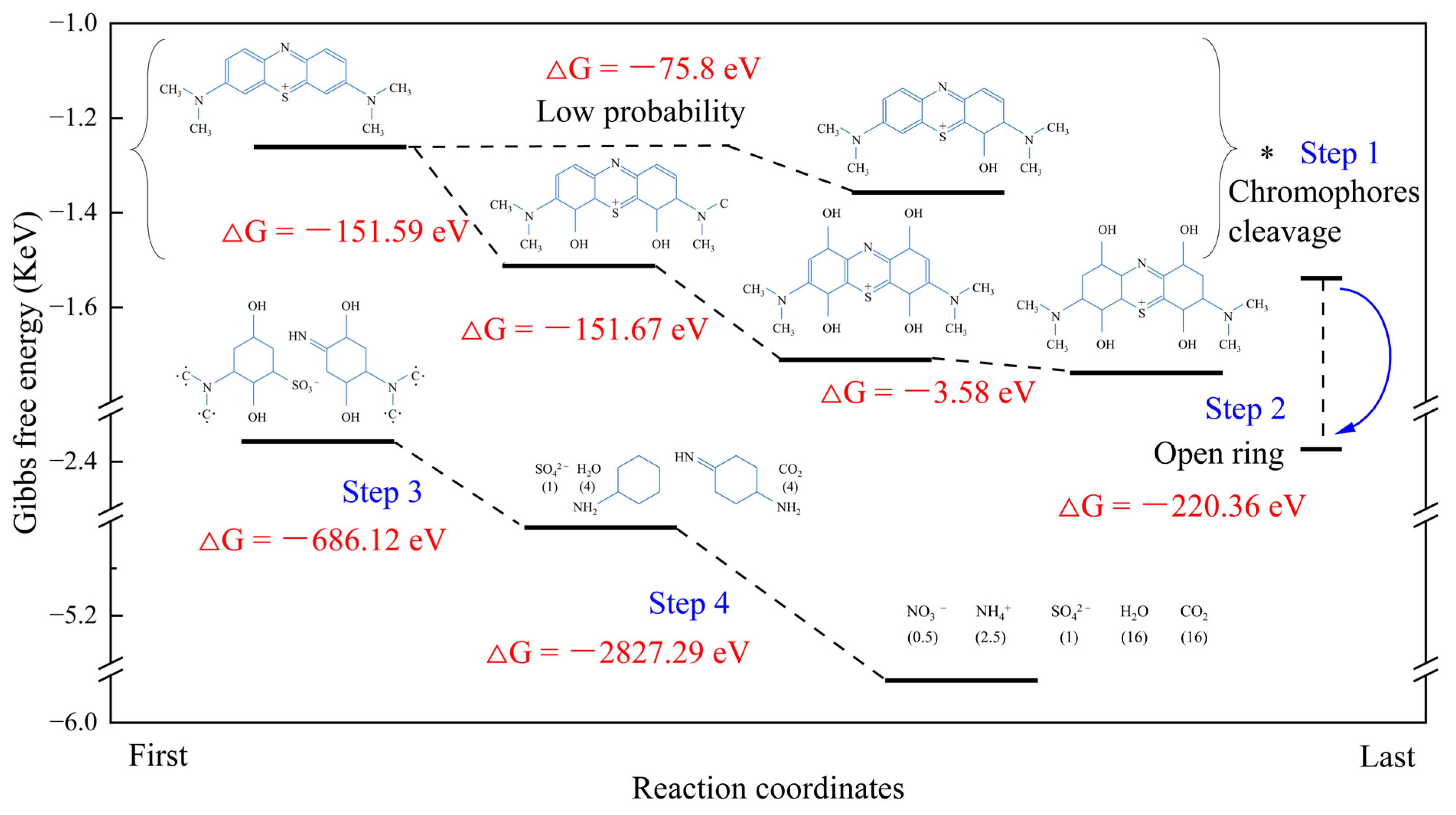
3.3. Degradation Mechanism of MB Using MM-CaTiO3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, G.; Wang, M.; Dang, J.; Zhang, R.; Lv, Z.; He, W.; Lv, X. A novel recycling approach for efficient extraction of titanium from high-titanium-bearing blast furnace slag. Waste Manag. 2021, 120, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Guo, Y.; Qiu, G.; Chen, F.; Wang, S.; Sui, Y.; Jiang, T.; Yang, L. A novel process for preparation of titanium dioxide from Ti-bearing electric furnace slag: NH4HF2-HF leaching and hydrolyzing process. J. Hazard. Mater. 2018, 344, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Feng, Y.; Li, H.; Wu, H. Efficient separation of vanadium, titanium, and iron from vanadium-bearing titanomagnetite by pressurized pyrolysis of ammonium chloride-acid leaching-solvent extraction process. Sep. Purif. Technol. 2021, 255, 117169. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, S.; Zhang, Y.; Fang, Z.Z.; Zhang, M.; Sun, P.; Li, Q.; Zhang, Y.; Li, P.; Jin, W. Potentially more ecofriendly chemical pathway for production of high-purity TiO2 from titanium slag. ACS Sustain. Chem. Eng. 2019, 7, 4821–4830. [Google Scholar] [CrossRef]
- Li, Z.; Lei, Y.; Ma, W.; Zhang, Y.; Wang, S.; Ren, Y.; Lv, G. An approach to prepare high-purity TiSi2 for clean utilization of Ti-bearing blast furnace slag. Green Chem. 2022, 24, 3344–3357. [Google Scholar] [CrossRef]
- Tian, M.; Liu, Y.; Wang, L.; Chen, D.; Zhao, H.; Meng, F.; Zhen, Y.; Qi, T. Role of aluminum salt on thermal hydrolysis of titanyl sulfuric–chloric mixture acid solution. J. Mater. Res. Technol. 2021, 14, 2486–2496. [Google Scholar] [CrossRef]
- Li, L.; Zhu, F.; Deng, P.; Zhang, D.; Jia, Y.; Li, K.; Kong, L.; Liu, D. Behavior of magnesium impurity during carbochlorination of magnesium-bearing titanium slag in chloride media. J. Mater. Res. Technol. 2021, 13, 204–215. [Google Scholar] [CrossRef]
- Deng, P.; Liu, D.; Chen, X.; Jiang, W.; Kong, L.; Li, L. Carbochlorination mechanism of low-grade titanium slag: Ab initio molecular dynamic simulation. J. Mater. Res. Technol. 2022, 17, 459–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Huang, Z.; Lefler, H.; Zhang, T.; Sun, P.; Free, M.L.; Guo, J. A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag. Chem. Eng. J. 2016, 286, 517–527. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Passi, M.; Pal, B. A review on CaTiO3 photocatalyst: Activity enhancement methods and photocatalytic applications. Powder Technol. 2021, 388, 274–304. [Google Scholar] [CrossRef]
- Wei, K.; Faraj, Y.; Yao, G.; Xie, R.; Lai, B. Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress. Chem. Eng. J. 2021, 414, 128783. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W.; Fujishima, A.; Terashima, C. Enhanced Photocatalytic Degradation Activity Using the V2O5/RGO Composite. Nanomaterials 2023, 13, 338. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.; Song, H.; Hao, D.; Xu, B.; Ren, J.; Wang, M.; Dai, C.; Wang, Y.; Liu, W. Size effect of gold nanoparticles in bimetallic ZIF catalysts for enhanced photo-redox reactions. Chem. Eng. J. 2023, 455, 140909. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, R.S.; Rai, S.B. Enhanced photoluminescence in a Eu3+ doped CaTiO3 perovskite phosphor via incorporation of alkali ions for white LEDs. J. Phys. Chem. Solids 2021, 151, 109916. [Google Scholar] [CrossRef]
- Miranda-Hernández, J.G.; Vargas-Hernández, J.; Casarrubias-Vargas, H.; González-Morán, C.O.; Refugio-García, E.; Flores Cuautle, J.d.J.A. Synthesis and effect of CaTiO3 formation in CaO·Al2O3 by solid-state reaction from CaCO3·Al2O3 and Ti. Mater. Chem. Phys. 2019, 232, 57–64. [Google Scholar] [CrossRef]
- Shawky, A.; Alhaddad, M.; Al-Namshah, K.S.; Mohamed, R.M.; Awwad, N.S. Synthesis of Pt-decorated CaTiO3 nanocrystals for efficient photoconversion of nitrobenzene to aniline under visible light. J. Mol. Liq. 2020, 304, 112704. [Google Scholar] [CrossRef]
- Cesconeto, F.R.; Borlaf, M.; Nieto, M.I.; de Oliveira, A.P.N.; Moreno, R. Synthesis of CaTiO3 and CaTiO3/TiO2 nanoparticulate compounds through Ca2+/TiO2 colloidal sols: Structural and photocatalytic characterization. Ceram. Int. 2018, 44, 301–309. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Dai, F.; Zhao, R.; Wang, L. Fabrication of CdSe/CaTiO3 nanocomposties in aqueous solution for improved photocatalytic hydrogen production. Appl. Surf. Sci. 2018, 459, 520–526. [Google Scholar] [CrossRef]
- Jiang, E.; Yang, L.; Song, N.; Zhang, X.; Liu, C.; Dong, H. Multi-shelled hollow cube CaTiO3 decorated with Bi12O17Cl2 towards enhancing photocatalytic performance under the visible light. J. Colloid Interface Sci. 2020, 576, 21–33. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.; Huynen, I.; Mahmoud, M.Z.; Akhtar, M.N. Electromagnetic performance, optical and physiochemical features of CaTiO3/NiO and SrFe12O19/NiO nanocomposites based bilayer absorber. J. Colloid Interface Sci. 2022, 610, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ding, B.; Ni, W.; Li, X.; He, X.; Chen, Z.; Ran, S.; Lü, H. Tailoring the crystal structure of CaTiO3 by multielement doping for photo-assisted activation of NO. Chem. Eng. J. 2022, 450, 138255. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Wang, X.; Li, N.; Zhang, Z.; Liu, Y. The active pairs of Co-Co2C adjusted by La-doped CaTiO3 with perovskite phase for higher alcohol synthesis from syngas. Chem. Eng. J. 2022, 439, 135635. [Google Scholar] [CrossRef]
- Chamanehpour, E.; Hossein Sayadi, M.; Hajiani, M. Metal-organic framework coordinated with g-C3N4 and metal ions for boosting photocatalytic H2 production under sunlight. J. Photochem. Photobiol. A Chem. 2023, 434, 114221. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Li, P.; Wang, Y.; Zhao, X.; Safaei, J.; Tian, H.; Zhou, D.; Li, B.; Kang, F.; et al. Biomimetic Dendrite-Free Multivalent Metal Batteries. Adv. Mater. 2022, 34, 2206970. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Z.; Saito, M.; Shibata, N.; Ikuhara, Y. Atomistic mechanisms of nonstoichiometry-induced twin boundary structural transformation in titanium dioxide. Nat. Commun. 2015, 6, 7120. [Google Scholar] [CrossRef]
- Abbad, S.; Guergouri, K.; Gazaout, S.; Djebabra, S.; Zertal, A.; Barille, R.; Zaabat, M. Effect of silver doping on the photocatalytic activity of TiO2 nanopowders synthesized by the sol-gel route. J. Environ. Chem. Eng. 2020, 8, 103718. [Google Scholar] [CrossRef]
- Nekooie, R.; Shamspur, T.; Mostafavi, A. Novel CuO/TiO2/PANI nanocomposite: Preparation and photocatalytic investigation for chlorpyrifos degradation in water under visible light irradiation. J. Photochem. Photobiol. A Chem. 2021, 407, 113038. [Google Scholar] [CrossRef]
- Vanthana Sree, G.; Nagaraaj, P.; Kalanidhi, K.; Aswathy, C.A.; Rajasekaran, P. Calcium oxide a sustainable photocatalyst derived from eggshell for efficient photo-degradation of organic pollutants. J. Clean. Prod. 2020, 270, 122294. [Google Scholar] [CrossRef]
- Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, B.; Sun, Y.; Li, F.; Song, W. Understanding the roles of copper dopant and oxygen vacancy in promoting nitrogen oxides removal over iron-based catalyst surface: A collaborative experimental and first-principles study. J. Colloid Interface Sci. 2022, 612, 584–597. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K.; Huang, W.; Wang, L. In Situ Formation of Oxygen Vacancies Achieving Near-Complete Charge Separation in Planar BiVO4 Photoanodes. Adv. Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef]
- Chen, L.; Xu, B.; Yang, Y.; Bellaiche, L. Macroscopic and Microscopic Structures of Cesium Lead Iodide Perovskite from Atomistic Simulations. Adv. Funct. Mater. 2020, 30, 1909496. [Google Scholar] [CrossRef]
- Kahmann, S.; Nazarenko, O.; Shao, S.; Hordiichuk, O.; Kepenekian, M.; Even, J.; Kovalenko, M.V.; Blake, G.R.; Loi, M.A. Negative Thermal Quenching in FASnI3 Perovskite Single Crystals and Thin Films. ACS Energy Lett. 2020, 5, 2512–2519. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Chen, J.; Han, W.; Zhang, W.; Li, H.; Zhang, X.; Zhang, B. K+ alkalization promoted Ca2+ intercalation in V2CTx MXene for enhanced Li storage. J. Energy Chem. 2020, 49, 358–364. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, Y.; Chi, J.; Wang, Y.; Zhao, Y. Bioinspired Bilayer Structural Color Hydrogel Actuator with Multienvironment Responsiveness and Survivability. Small Methods 2019, 3, 1900519. [Google Scholar] [CrossRef]
- Wang, R.; Ni, S.; Liu, G.; Xu, X. Hollow CaTiO3 cubes modified by La/Cr co-doping for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 225, 139–147. [Google Scholar] [CrossRef]
- Hu, S.; Shaner, M.R.; Beardslee, J.A.; Lichterman, M.; Brunschwig, B.S.; Lewis, N.S. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 2014, 344, 1005–1009. [Google Scholar] [CrossRef]
- Yang, G.; Ren, Z.; Liu, K.; Qin, M.; Deng, W.; Zhang, H.; Wang, H.; Liang, J.; Ye, F.; Liang, Q.; et al. Stable and low-photovoltage-loss perovskite solar cells by multifunctional passivation. Nat. Photonics 2021, 15, 681–689. [Google Scholar] [CrossRef]
- Ahmad, N.; Anae, J.; Khan, M.Z.; Sabir, S.; Yang, X.J.; Thakur, V.K.; Campo, P.; Coulon, F. Visible light-conducting polymer nanocomposites as efficient photocatalysts for the treatment of organic pollutants in wastewater. J. Environ. Manag. 2021, 295, 113362. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, J.; Li, M.; Yuan, J.; Wu, P.; Chang, X.; Liu, C.; Wang, X. Bismuth silicate photocatalysts with enhanced light harvesting efficiency by photonic crystal. J. Alloy. Compd. 2019, 810, 151839. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, X.; Zhang, C.; Li, R.; Wang, Y.; Fan, C. One-step synthesis of novel Bi/Bi2SiO5 flower-like composites with highly-efficient CO2 photoreduction performance. Compos. Commun. 2020, 20, 100366. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, R.; Hu, H.; Fan, X.; Zhang, D.; Wang, D. 0D/2D Heterojunctions of Ti3C2 MXene QDs/SiC as an Efficient and Robust Photocatalyst for Boosting the Visible Photocatalytic NO Pollutant Removal Ability. ACS Appl. Mater. Interfaces 2020, 12, 40176–40185. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Han, C.; Xue, X. Photocatalytic activity of Fe-doped CaTiO3 under UV–visible light. J. Environ. Sci. 2014, 26, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, Y.; Sun, X.; Fu, C.; Prakash Bhoi, Y.; Xiong, W.; Huang, W. TiO2 Facet-dependent reconstruction and photocatalysis of CuOx/TiO2 photocatalysts in CO2 photoreduction. Appl. Surf. Sci. 2021, 564, 150407. [Google Scholar] [CrossRef]
- Spectroscopic Tools. Available online: https://www.science-and-fun.de/tools/ (accessed on 14 April 2023).
- Yu, Z.; Chuang, S.S.C. Probing Methylene Blue Photocatalytic Degradation by Adsorbed Ethanol with In Situ IR. J. Phys. Chem. C 2007, 111, 13813–13820. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-E.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef]
- Xiang, H.; Ren, G.; Zhong, Y.; Xu, D.; Zhang, Z.; Wang, X.; Yang, X. Fe3O4@C Nanoparticles Synthesized by In Situ Solid-Phase Method for Removal of Methylene Blue. Nanomaterials 2021, 11, 330. [Google Scholar] [CrossRef]

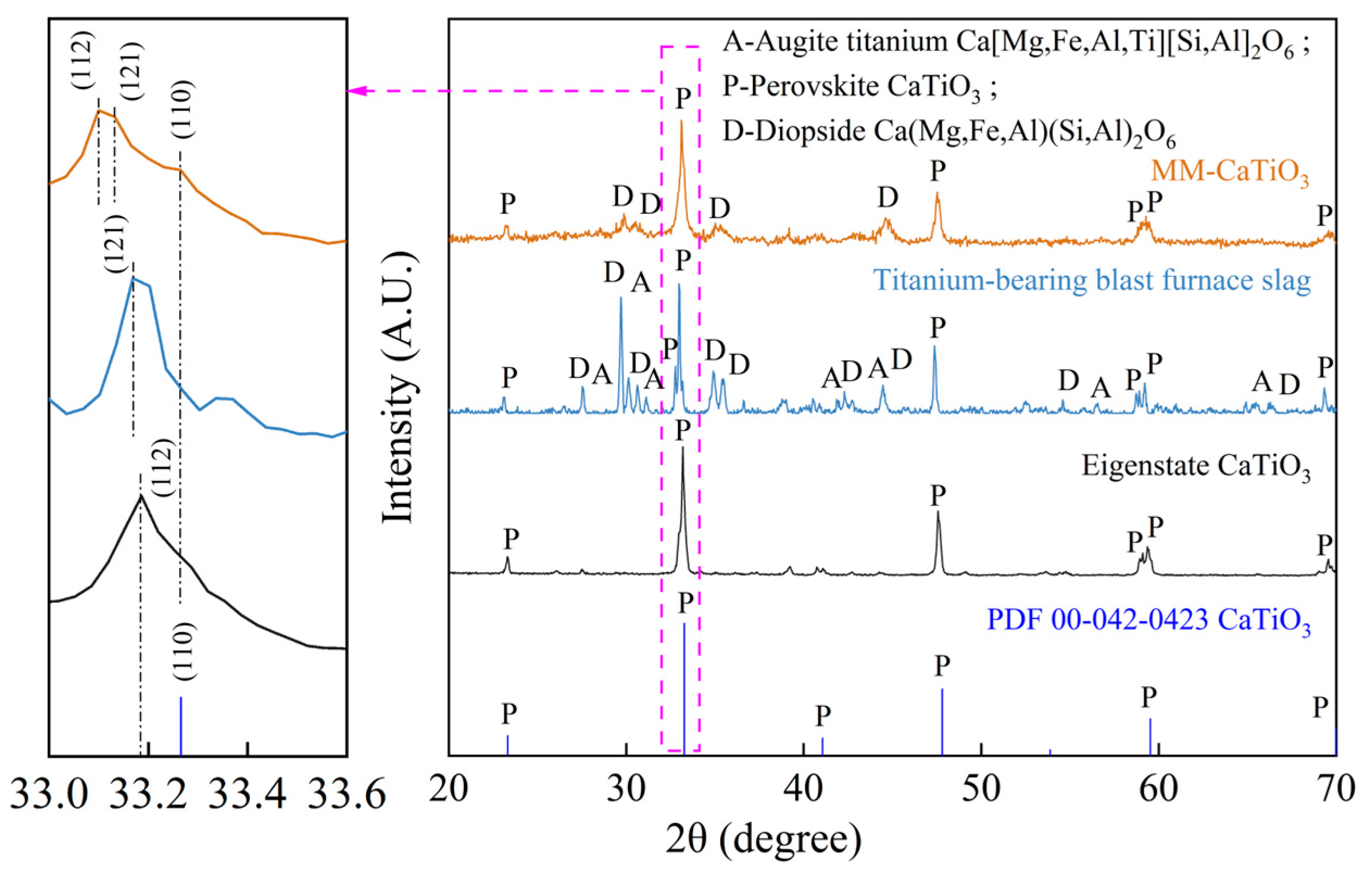
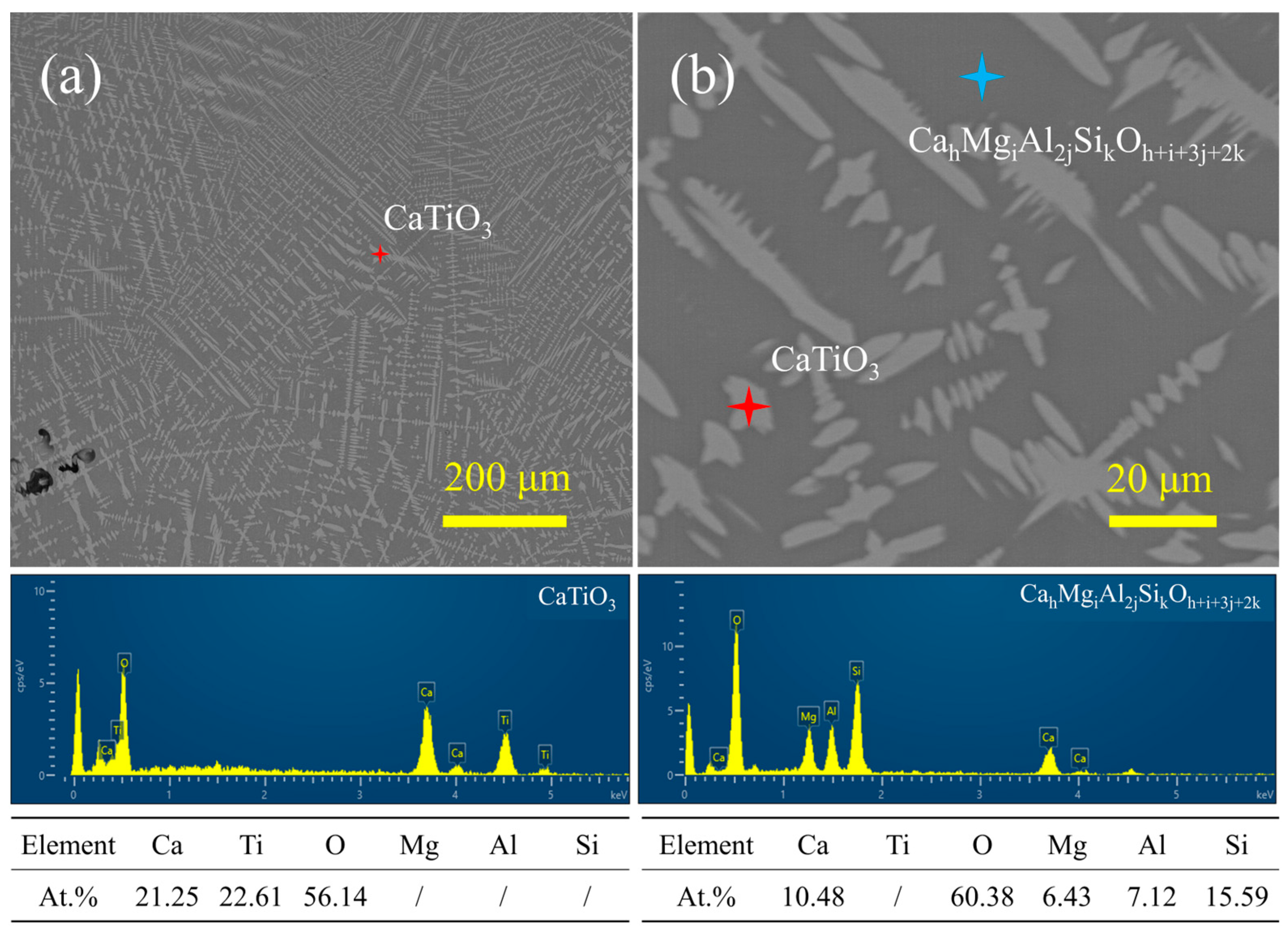
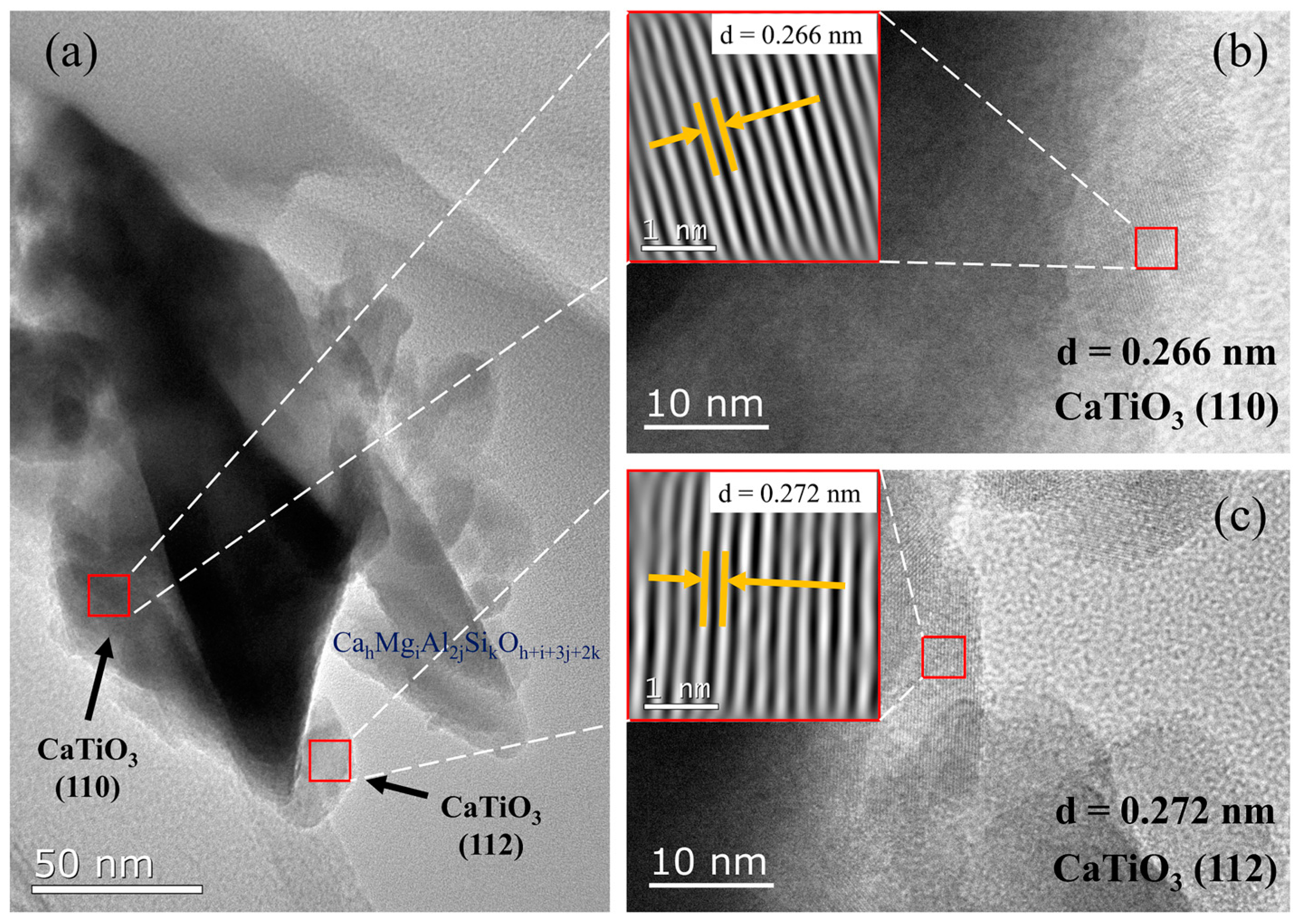

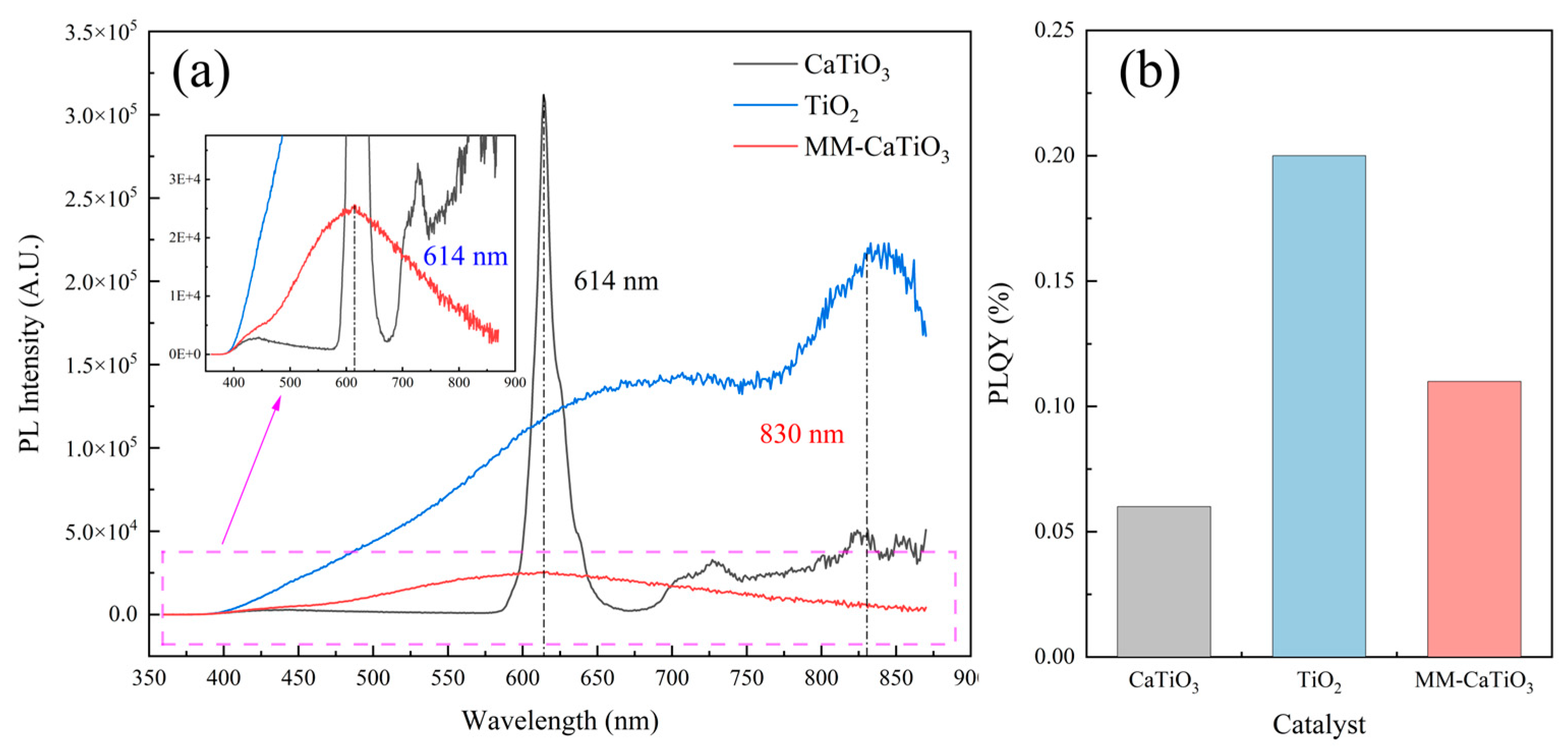
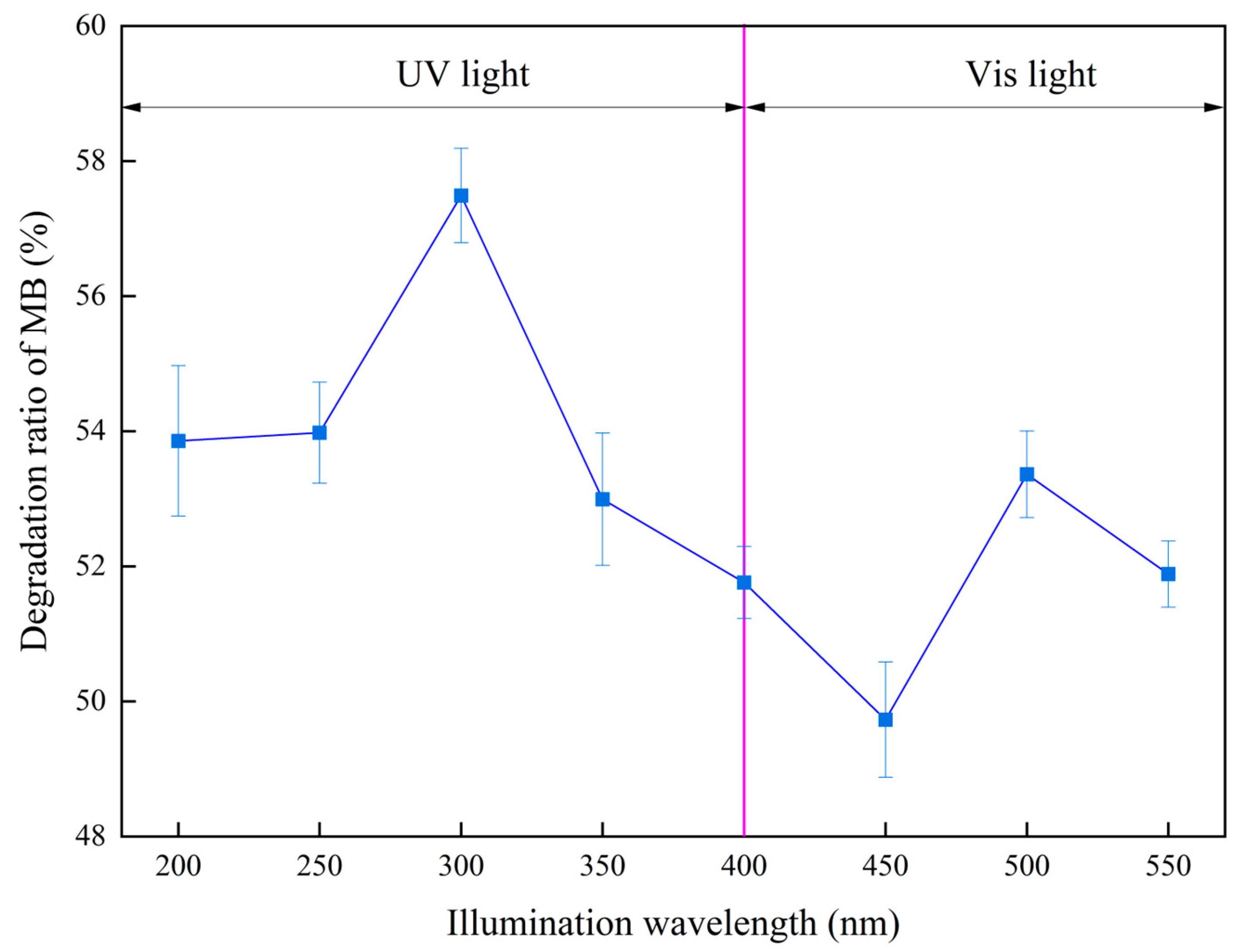
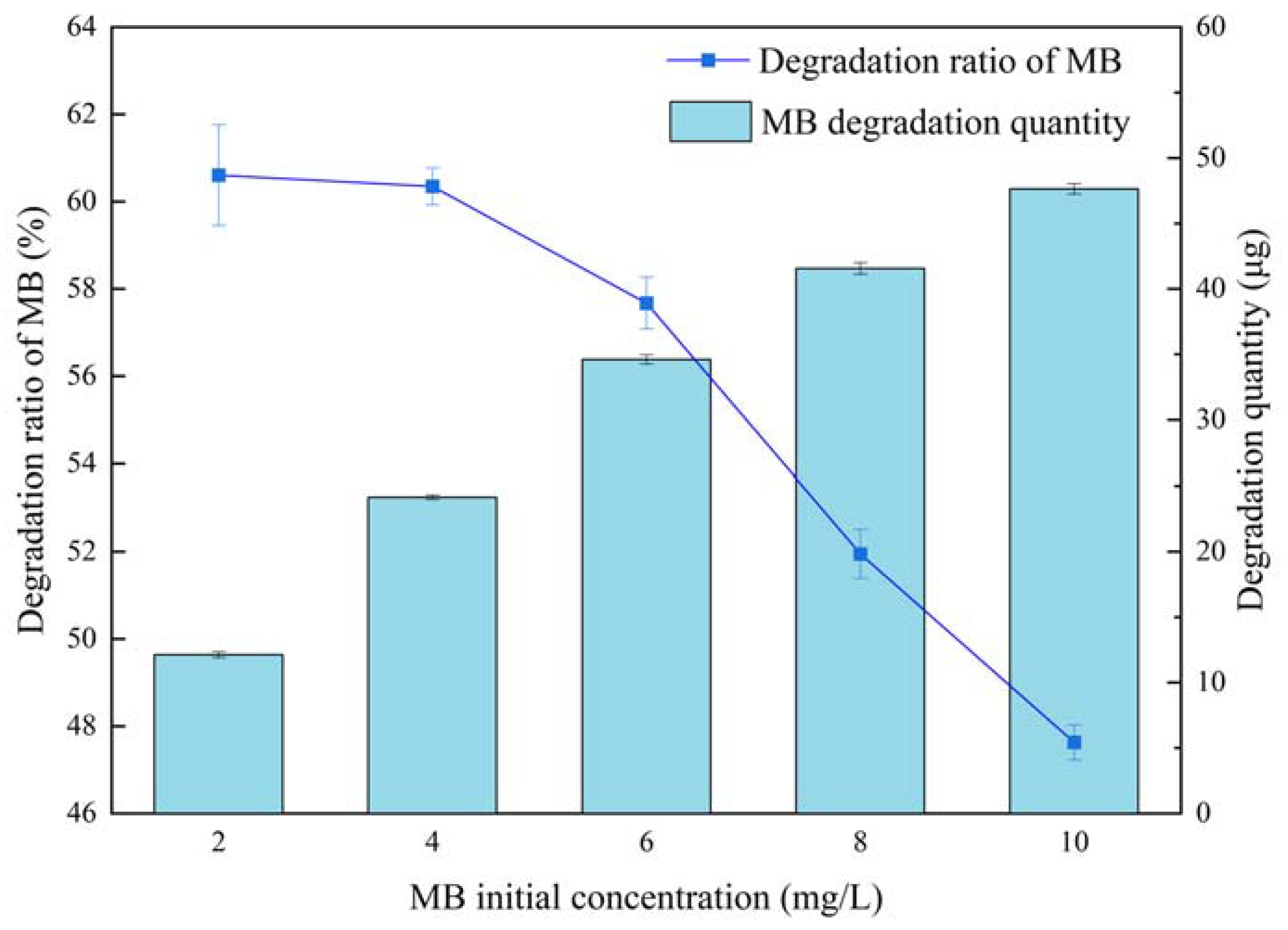
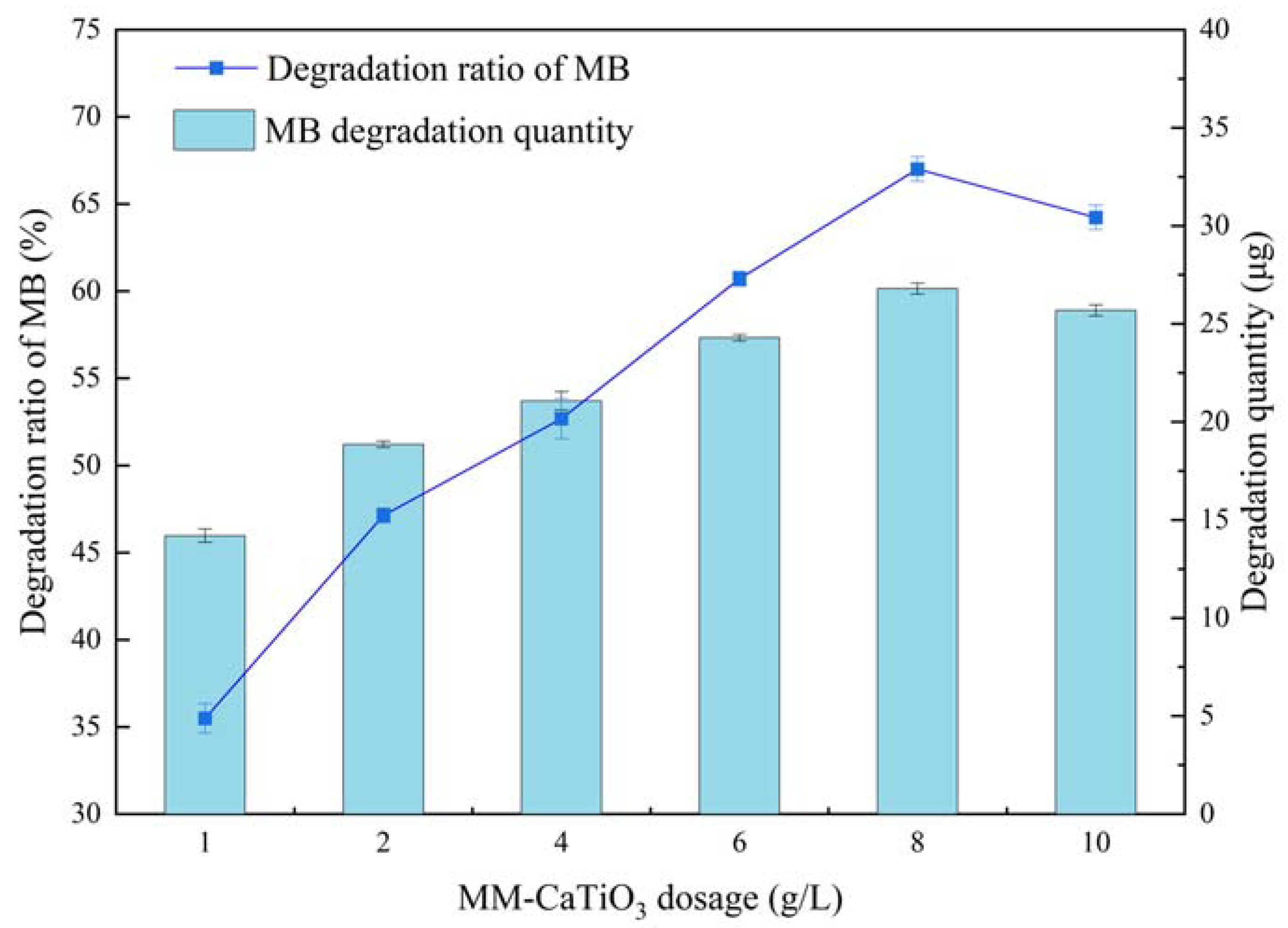
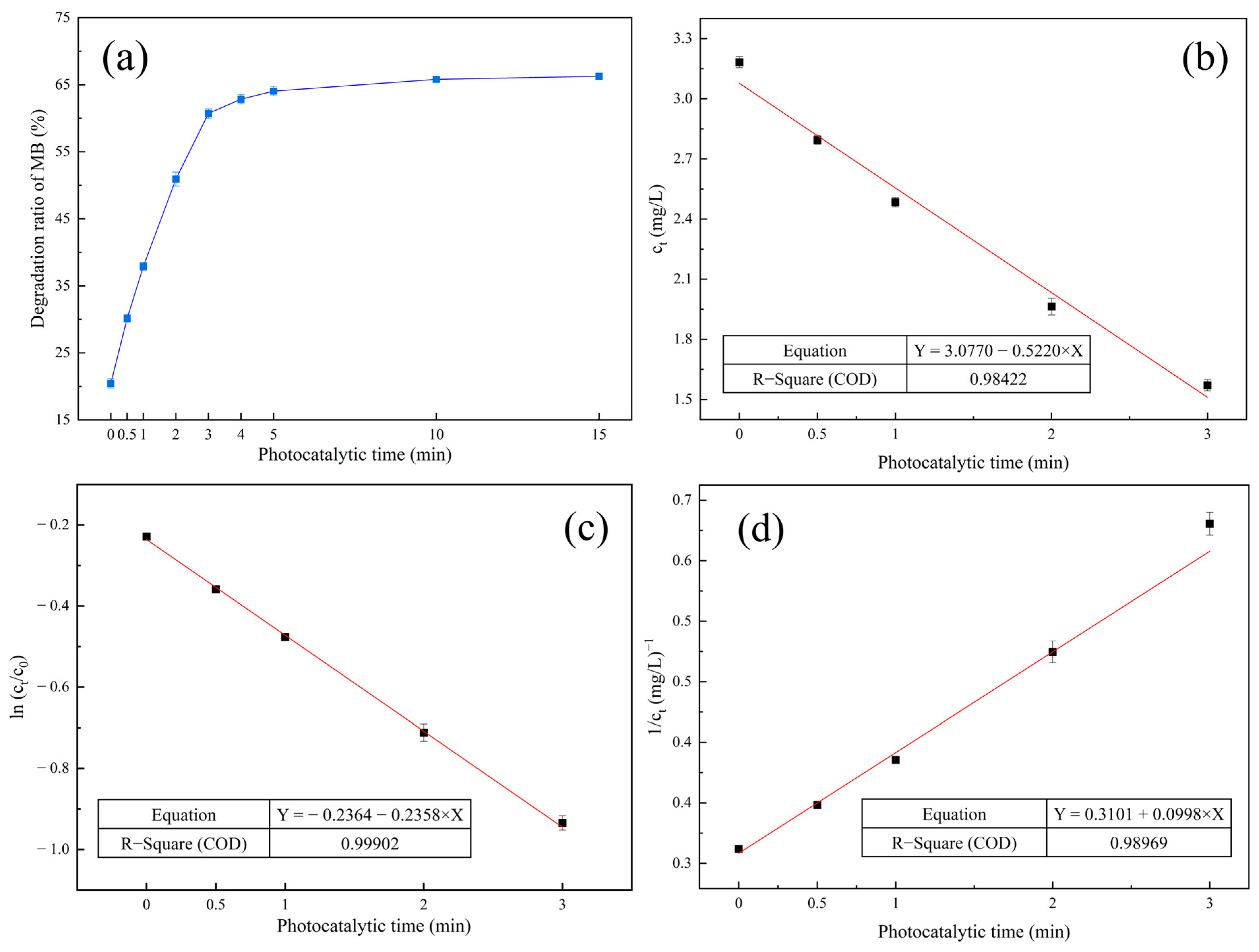

| Materials | Titanium-Bearing Blast Furnace Slag | MM-CaTiO3 | Pristine CaTiO3 | |
|---|---|---|---|---|
| Space group|number | Pnma|62 | Pbnm|62 | Pm-3m|221 | Pbnm|62 |
| a(Å) | 5.4424 | 5.3796 | 3.7950 | 5.3780 |
| b(Å) | 7.6417 | 5.4423 | 3.7950 | 5.4440 |
| c(Å) | 5.3807 | 7.6401 | 3.7950 | 7.6370 |
| α/β/γ(°) | 90.0000 | 90.0000 | 90.0000 | 90.0000 |
| Z | 4.00 | 4.00 | 1.00 | 4.00 |
| (h k l) | (121) | (112) | (110) | (112) |
| d(Å) | 2.7025 | 2.7030 | 2.6835 | 2.7030 |
| Lattice Plane | (112) | (110) |
|---|---|---|
| Molecular model | 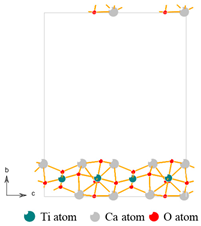 | 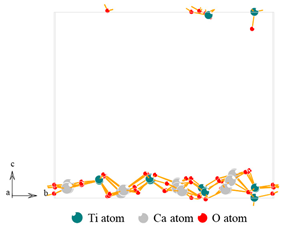 |
| Epure | −609.95 eV | −595.48 eV |
| Eov-1 | −601.76 eV | −587.59 eV |
| Oxygen vacancy formation energy | 8.152 eV | 7.852 eV |
| Model | Equation | R2 |
|---|---|---|
| Zero-order reaction kinetics | Ct = −0.5220 t + 3.0770 | 0.98422 |
| First-order reaction kinetics | ln(Ct/C0) = −0.2358 t − 0.2364 | 0.99902 |
| Second-order reaction kinetics | 1/Ct = 0.0998 t + 0.3101 | 0.98969 |
| Raw Material | Synthesis Method | Band Gap | Ref. |
|---|---|---|---|
| Calcium carbonate and titanium dioxide. | Calcium carbonate and titanium dioxide are firstly mixed and then calcined at 1400 °C for 2 h. | 3.50 eV | [44] |
| Titanium isoprenoid, calcium acetate and nitric acid. | The Ca2+/TiO2 sol is first synthesized using calcium acetate, nitric acid and titanium isoprenoid, and then aging and calcined after freeze-drying. | 3.44 eV | [18] |
| Ti(C4H9O)4, AgNO3, and CH3COOH. | Ag-doped TiO2 NPs were synthesized by sol–gel technology. | 2.67 eV | [27] |
| C12H28O4Ti, Cu(NO3)2, CH3COOH, C3H7OH, and NaBH4. | Cu(NO3)2 is added to the ultrasonically dispersed TiO2 NPs aqueous suspension and magnetically stirred. Subsequently, add NaBH4 solution and continue stirring for 24 h. Finally, centrifuge, wash, and dry. | 2.40 eV | [28] |
| TiO2 (001), TiO2 (100), TiO2 (101), and Cu(NO3)2 (0.1 mol/L). | CuOx/TiO2 catalysts were synthesized by the initial impregnation method using TiO2 (001), TiO2 (100) and TiO2 (101) as the vector and Cu(NO3)2 (0.1 mol/L) solution as the load. | 2.25 eV | [45] |
| Titanium-bearing blast furnace slag. | Melting pretreatment at 1300 °C for 1 h and then roasted in a microwave field at 1200 °C with a heating time of 20 min. | 2.25 eV | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Ye, Q.; Zhou, J.; Liao, Y.; Qian, G. The Photocatalytic Activity of CaTiO3 Derived from the Microwave-Melting Heating Process of Blast Furnace Slag. Nanomaterials 2023, 13, 1412. https://doi.org/10.3390/nano13081412
Xie J, Ye Q, Zhou J, Liao Y, Qian G. The Photocatalytic Activity of CaTiO3 Derived from the Microwave-Melting Heating Process of Blast Furnace Slag. Nanomaterials. 2023; 13(8):1412. https://doi.org/10.3390/nano13081412
Chicago/Turabian StyleXie, Jun, Qing Ye, Jianghao Zhou, Yue Liao, and Gongming Qian. 2023. "The Photocatalytic Activity of CaTiO3 Derived from the Microwave-Melting Heating Process of Blast Furnace Slag" Nanomaterials 13, no. 8: 1412. https://doi.org/10.3390/nano13081412
APA StyleXie, J., Ye, Q., Zhou, J., Liao, Y., & Qian, G. (2023). The Photocatalytic Activity of CaTiO3 Derived from the Microwave-Melting Heating Process of Blast Furnace Slag. Nanomaterials, 13(8), 1412. https://doi.org/10.3390/nano13081412






