Scalable Wettability Modification of Aluminum Surface through Single-Shot Nanosecond Laser Processing
Abstract
1. Introduction
2. Materials and Methods
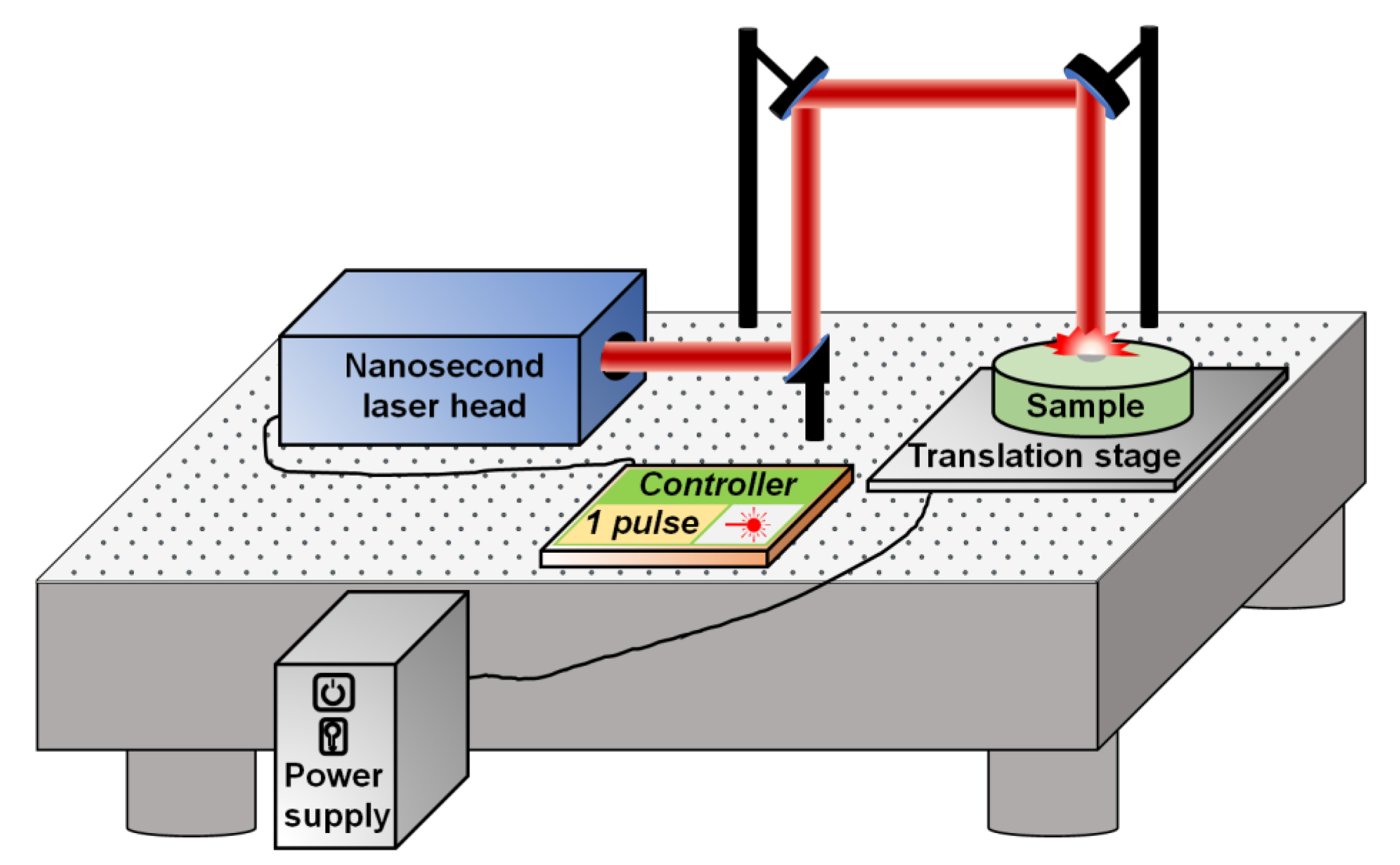
2.1. Fabrication Process
2.2. Surface Characterization
3. Results
3.1. Change of Surface Morphology
3.2. Change of Surface Compositions
3.3. Surface Wettability Conversion
3.4. Mechanism of Wettability Conversion with Single-Shot Nanosecond Laser Irradiation
3.5. Potential Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; Asm International: Almere, The Netherlands, 1999. [Google Scholar]
- Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cole, I.S.; Marney, D. The science of pipe corrosion: A review of the literature on the corrosion of ferrous metals in soils. Corros. Sci. 2012, 56, 5–16. [Google Scholar] [CrossRef]
- Leidheiser, H., Jr. Corrosion of painted metals—A review. Corrosion 1982, 38, 374–383. [Google Scholar] [CrossRef]
- Walter, G. A critical review of the protection of metals by paints. Corros. Sci. 1986, 26, 27–38. [Google Scholar] [CrossRef]
- Fadeeva, E.; Schlie, S.; Koch, J.; Chichkov, B.; Vorobyev, A.; Guo, C. Femtosecond laser-induced surface structures on platinum and their effects on surface wettability and fibroblast cell proliferation. Contact Angle Wettability Adhes. 2009, 6, 163–171. [Google Scholar]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, M.; Kaakkunen, J.; Paivasaari, K.; Vahimaa, P.; Jaaskelainen, T. Controlling the hydrophobic properties of material surface using femtosecond ablation. J. Laser Micro Nanoeng. 2010, 5, 97–98. [Google Scholar] [CrossRef]
- Vorobyev, A.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Vorobyev, A.; Guo, C. Multifunctional surfaces produced by femtosecond laser pulses. J. Appl. Phys. 2015, 117, 033103. [Google Scholar] [CrossRef]
- Fang, R.; Vorobyev, A.; Guo, C. Direct visualization of the complete evolution of femtosecond laser-induced surface structural dynamics of metals. Light Sci. Appl. 2017, 6, e16256. [Google Scholar] [CrossRef]
- Jalil, S.A.; Lai, B.; ElKabbash, M.; Zhang, J.; Garcell, E.M.; Singh, S.; Guo, C. Spectral absorption control of femtosecond laser-treated metals and application in solar-thermal devices. Light Sci. Appl. 2020, 9, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, G.; Guo, C.; Ngo, C.-V.; Li, W. Femtosecond Laser Fabrication and Chemical Coating of Anti-Corrosion Ethylene-Glycol Repellent Aluminum Surfaces. Mater. Lett. 2022, 323, 132562. [Google Scholar] [CrossRef]
- Chen, Z.; Segev, M. Highlighting photonics: Looking into the next decade. ELight 2021, 1, 2. [Google Scholar] [CrossRef]
- Verma, G.; Yadav, G.; Saraj, C.S.; Li, L.; Miljkovic, N.; Delville, J.P.; Li, W. A versatile interferometric technique for probing the thermophysical properties of complex fluids. Light Sci. Appl. 2022, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.-V.; Chun, D.-M. Fast wettability transition from hydrophilic to superhydrophobic laser-textured stainless steel surfaces under low-temperature annealing. Appl. Surf. Sci. 2017, 409, 232–240. [Google Scholar] [CrossRef]
- Xie, F.; Yang, J.; Ngo, C.-V. The effect of femtosecond laser fluence and pitches between V-shaped microgrooves on the dynamics of capillary flow. Results Phys. 2020, 19, 103606. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Zhu, Z.; Xiang, N.; Wang, Z.; Sun, G. Switchable wettability control of titanium via facile nanosecond laser-based surface texturing. Surf. Interfaces 2021, 24, 101122. [Google Scholar] [CrossRef]
- Rajan, R.A.; Ngo, C.-V.; Yang, J.; Liu, Y.; Rao, K.; Guo, C. Femtosecond and picosecond laser fabrication for long-term superhydrophilic metal surfaces. Opt. Laser Technol. 2021, 143, 107241. [Google Scholar] [CrossRef]
- Jagdheesh, R.; García-Ballesteros, J.; Ocaña, J. One-step fabrication of near superhydrophobic aluminum surface by nanosecond laser ablation. Appl. Surf. Sci. 2016, 374, 2–11. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Bian, H.; Ou, Y.; Si, J.; Du, G.; Hou, X. Stable superhydrophobic surface with hierarchical mesh-porous structure fabricated by a femtosecond laser. Appl. Phys. A 2013, 111, 243–249. [Google Scholar] [CrossRef]
- Moradi, S.; Kamal, S.; Englezos, P.; Hatzikiriakos, S.G. Femtosecond laser irradiation of metallic surfaces: Effects of laser parameters on superhydrophobicity. Nanotechnology 2013, 24, 415302. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhong, M.; Zhang, H.; Fan, P. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cai, M.; Luo, X.; Chen, C.; Pan, R.; Zhang, H.; Zhong, M. Wettability transition modes of aluminum surfaces with various micro/nanostructures produced by a femtosecond laser. J. Laser Appl. 2019, 31, 022503. [Google Scholar] [CrossRef]
- Rajab, F.H.; Liu, Z.; Li, L. Long term superhydrophobic and hybrid superhydrophobic/superhydrophilic surfaces produced by laser surface micro/nano surface structuring. Appl. Surf. Sci. 2019, 466, 808–821. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Yang, Z. Stability Mechanism of Laser-induced Fluorinated Super-hydrophobic Coating in Alkaline Solution. J. Bionic Eng. 2022, 19, 113–125. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, Y.; Ngo, C.-V.; Guo, C. Rapid fabrication of anti-corrosion and self-healing superhydrophobic aluminum surfaces through environmentally friendly femtosecond laser processing. Opt. Express 2020, 28, 35636–35650. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.-L.; Wang, L.-K. Application of femtosecond laser micromachining in silicon carbide deep etching for fabricating sensitive diaphragm of high temperature pressure sensor. Sens. Actuators A Phys. 2020, 309, 112017. [Google Scholar] [CrossRef]
- Epperlein, N.; Menzel, F.; Schwibbert, K.; Koter, R.; Bonse, J.; Sameith, J.; Krüger, J.; Toepel, J. Influence of femtosecond laser produced nanostructures on biofilm growth on steel. Appl. Surf. Sci. 2017, 418, 420–424. [Google Scholar] [CrossRef]
- Cardoso, J.; Aguilar-Morales, A.; Alamri, S.; Huerta-Murillo, D.; Cordovilla, F.; Lasagni, A.; Ocaña, J. Superhydrophobicity on hierarchical periodic surface structures fabricated via direct laser writing and direct laser interference patterning on an aluminium alloy. Opt. Lasers Eng. 2018, 111, 193–200. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Fan, P.; Gong, D.; Zhang, H. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27 (Suppl. S2), S29107. [Google Scholar] [CrossRef]
- Dunn, A.; Wasley, T.J.; Li, J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Esenturk, E.; Connaughton, C.; Shephard, J.D. Laser textured surface gradients. Appl. Surf. Sci. 2016, 371, 583–589. [Google Scholar]
- Dong, C.; Gu, Y.; Zhong, M.; Li, L.; Sezer, K.; Ma, M.; Liu, W. Fabrication of superhydrophobic Cu surfaces with tunable regular micro and random nano-scale structures by hybrid laser texture and chemical etching. J. Mater. Process. Technol. 2011, 211, 1234–1240. [Google Scholar] [CrossRef]
- Lei, Z.; Tian, Z.; Chen, X.; Chen, Y.; Bi, J.; Wu, S.; Sun, H. Large spot diameter nanosecond laser treatment of aluminum alloy sheets for high-speed superhydrophobic hierarchical micro-and nanostructured surface preparation. Surf. Coat. Technol. 2019, 361, 249–254. [Google Scholar] [CrossRef]
- Callendar, L. Oxide Layer on a Polished Surface. Nature 1936, 138, 291. [Google Scholar] [CrossRef]
- Guo, C. Thermal effects in femtosecond laser ablation of metals. In Proceedings of the Ultrafast Phenomena in Semiconductors and Nanostructure Materials X, San Jose, CA, USA, 15 February 2006; SPIE: Bellingham, WA, USA, 2006; Volume 6118, pp. 66–79. [Google Scholar]
- Vorobyev, A.; Kuzmichev, V.; Kokody, N.; Kohns, P.; Dai, J.; Guo, C. Residual thermal effects in Al following single ns-and fs-laser pulse ablation. Appl. Phys. A 2006, 82, 357–362. [Google Scholar] [CrossRef]
- Vorobyev, A.; Guo, C. Residual thermal effects in laser ablation of metals. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2007; Volume 59, p. 089. [Google Scholar]
- Zhang, Y.; Zhang, D.; Wu, J.; He, Z.; Deng, X. A thermal model for nanosecond pulsed laser ablation of aluminum. AIP Adv. 2017, 7, 075010. [Google Scholar] [CrossRef]
- Vorobyev, A.; Guo, C. Nanochemical effects in femtosecond laser ablation of metals. Appl. Phys. Lett. 2013, 102, 074107. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of different organic matter fractions on iron oxide. Geochim. Cosmochim. Acta 1995, 59, 219–229. [Google Scholar] [CrossRef]
- Takeda, S.; Fukawa, M.; Hayashi, Y.; Matsumoto, K. Surface OH group governing adsorption properties of metal oxide films. Thin Solid Film. 1999, 339, 220–224. [Google Scholar] [CrossRef]
- Khare, P.; Kumar, N.; Kumari, K.; Srivastava, S. Atmospheric formic and acetic acids: An overview. Rev. Geophys. 1999, 37, 227–248. [Google Scholar] [CrossRef]
- Royer, S.-J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradaran, S.; Torkmahalleh, M.A.; Saeedi, R.; Aibaghi, R.; Ghasemi, F.F. Suspended fine particulate matter (PM2. 5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implications. Environ. Res. 2021, 192, 110339. [Google Scholar] [CrossRef]
- Kumar, T.V.; Prabhakar, S.; Raju, G.B. Adsorption of oleic acid at sillimanite/water interface. J. Colloid Interface Sci. 2002, 247, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.; Guo, C. Direct observation of enhanced residual thermal energy coupling to solids in femtosecond laser ablation. Appl. Phys. Lett. 2005, 86, 011916. [Google Scholar] [CrossRef]

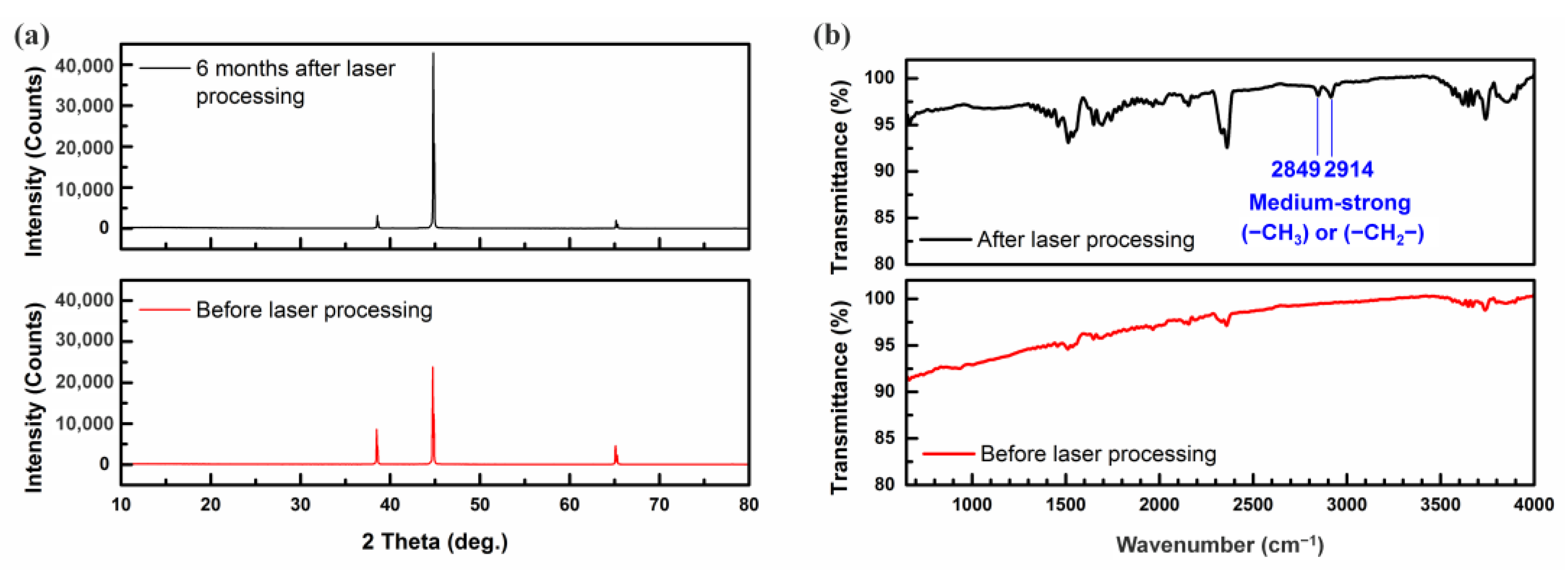
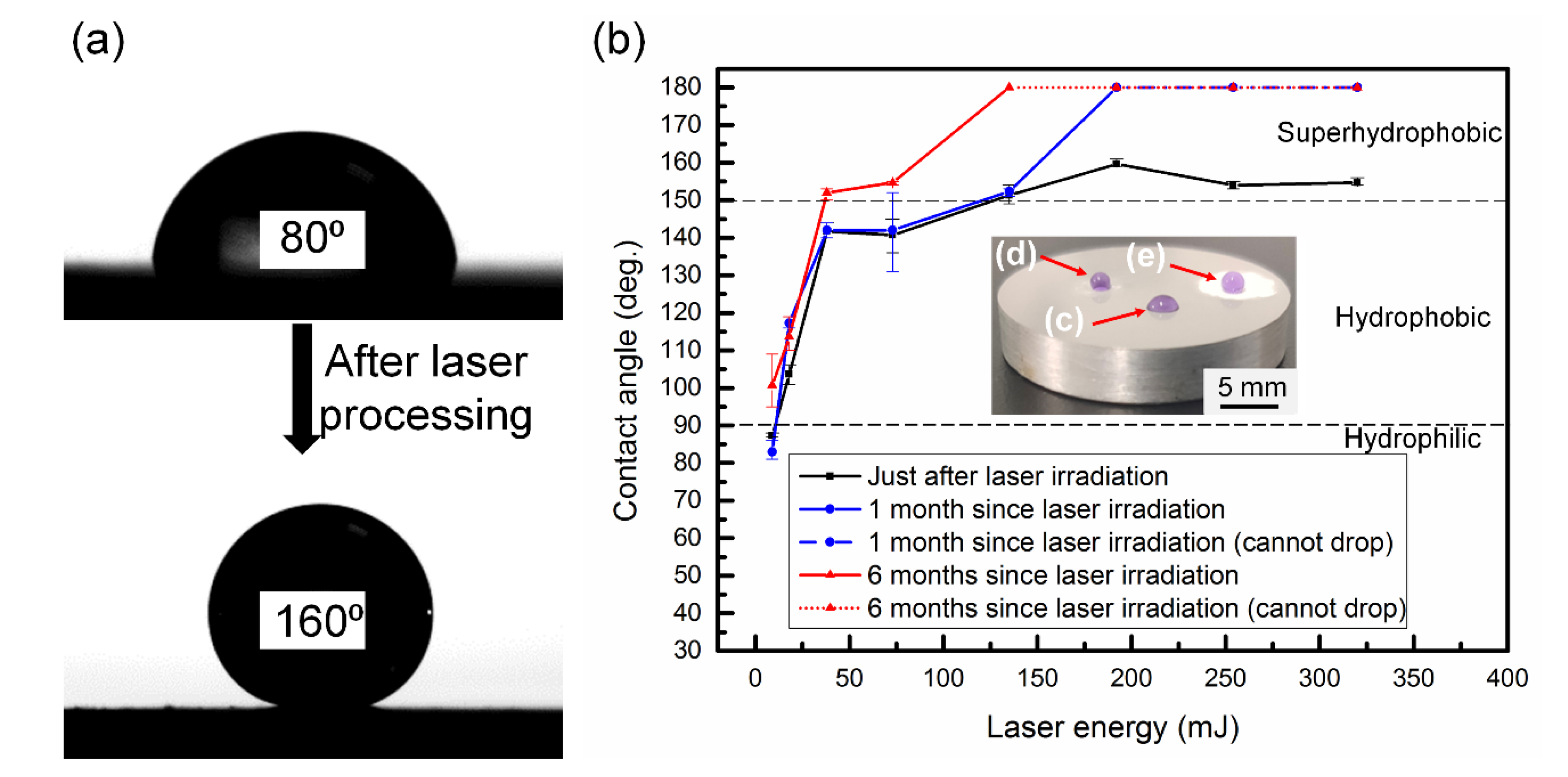


| Element | Before the Laser Irradiation | Just after the Laser Irradiation | 1 Month after the Laser Irradiation | 6 Months after the Laser Irradiation |
|---|---|---|---|---|
| Al | 63.80 | 55.60 | 52.73 | 51.00 |
| O | 32.40 | 36.19 | 34.15 | 32.80 |
| C | 3.80 | 8.21 | 13.12 | 16.20 |
| O/Al | 0.51 | 0.65 | 0.65 | 0.64 |
| C/Al | 0.06 | 0.15 | 0.25 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, C.-V.; Liu, Y.; Li, W.; Yang, J.; Guo, C. Scalable Wettability Modification of Aluminum Surface through Single-Shot Nanosecond Laser Processing. Nanomaterials 2023, 13, 1392. https://doi.org/10.3390/nano13081392
Ngo C-V, Liu Y, Li W, Yang J, Guo C. Scalable Wettability Modification of Aluminum Surface through Single-Shot Nanosecond Laser Processing. Nanomaterials. 2023; 13(8):1392. https://doi.org/10.3390/nano13081392
Chicago/Turabian StyleNgo, Chi-Vinh, Yu Liu, Wei Li, Jianjun Yang, and Chunlei Guo. 2023. "Scalable Wettability Modification of Aluminum Surface through Single-Shot Nanosecond Laser Processing" Nanomaterials 13, no. 8: 1392. https://doi.org/10.3390/nano13081392
APA StyleNgo, C.-V., Liu, Y., Li, W., Yang, J., & Guo, C. (2023). Scalable Wettability Modification of Aluminum Surface through Single-Shot Nanosecond Laser Processing. Nanomaterials, 13(8), 1392. https://doi.org/10.3390/nano13081392






