Improved Visible-Light Photocatalytic H2 Evolution of G-C3N4 Nanosheets by Constructing Heterojunctions with Nano-Sized Poly(3-Thiophenecarboxylic Acid) and Coordinating Fe(III)
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of G-C3N4 Nanosheets
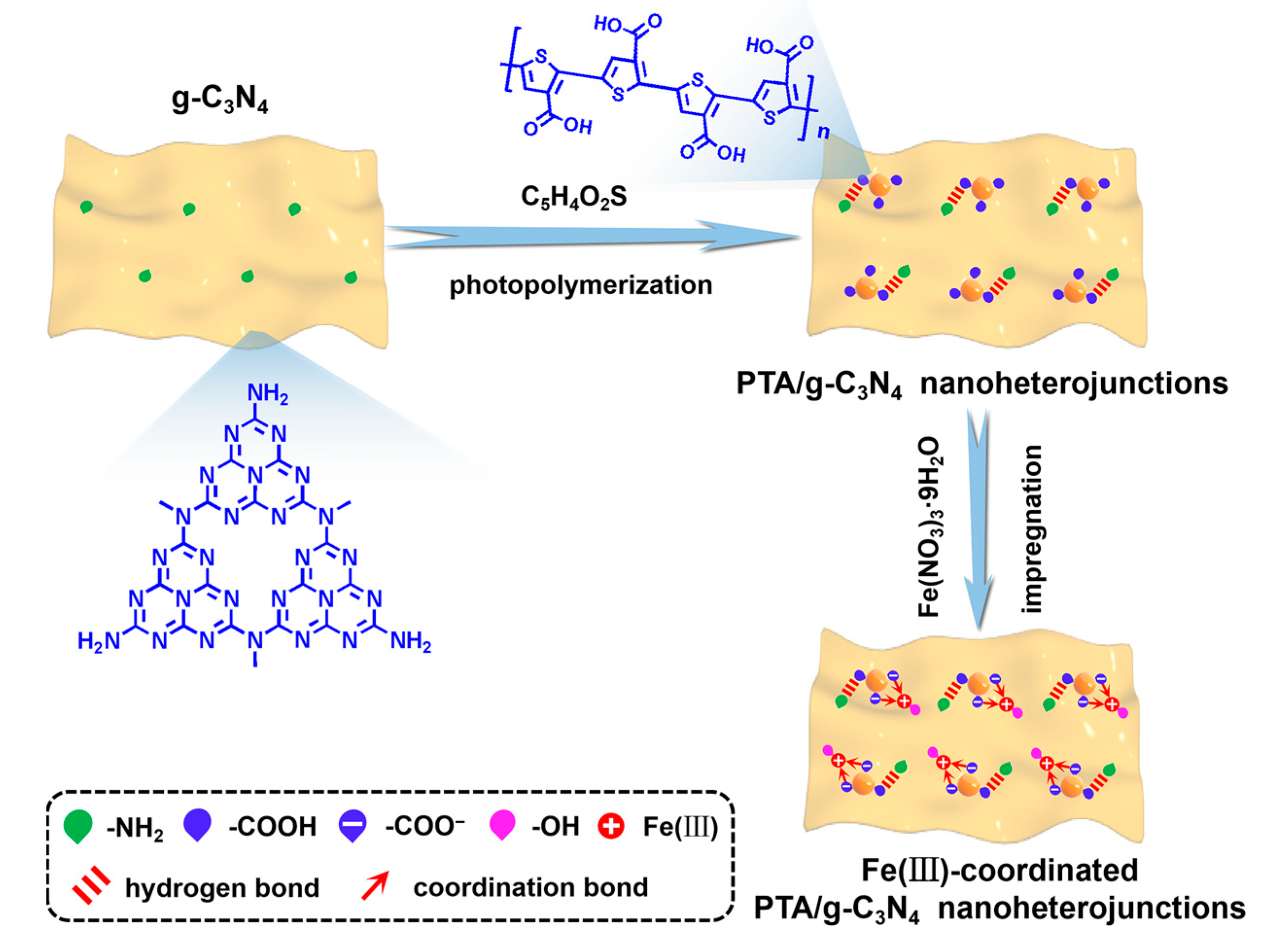
2.2. Synthesis of PTA/g-C3N4 Nanoheterojunctions
2.3. Synthesis of Fe(III)-Coordinated PTA/g-C3N4 Nanoheterojunctions
2.4. Evaluation of Photocatalytic Activities for H2 Evolution
3. Results and Discussion
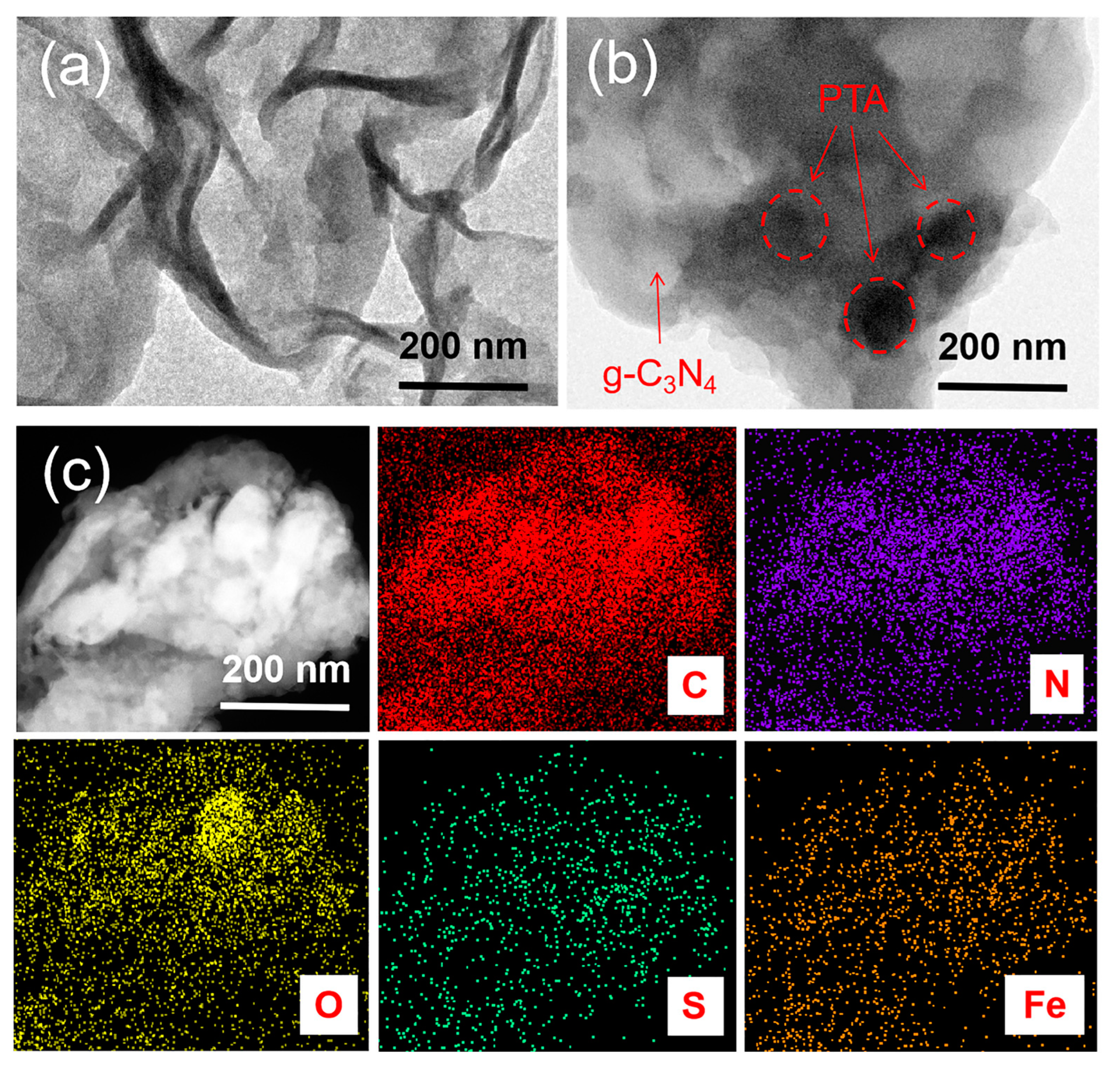
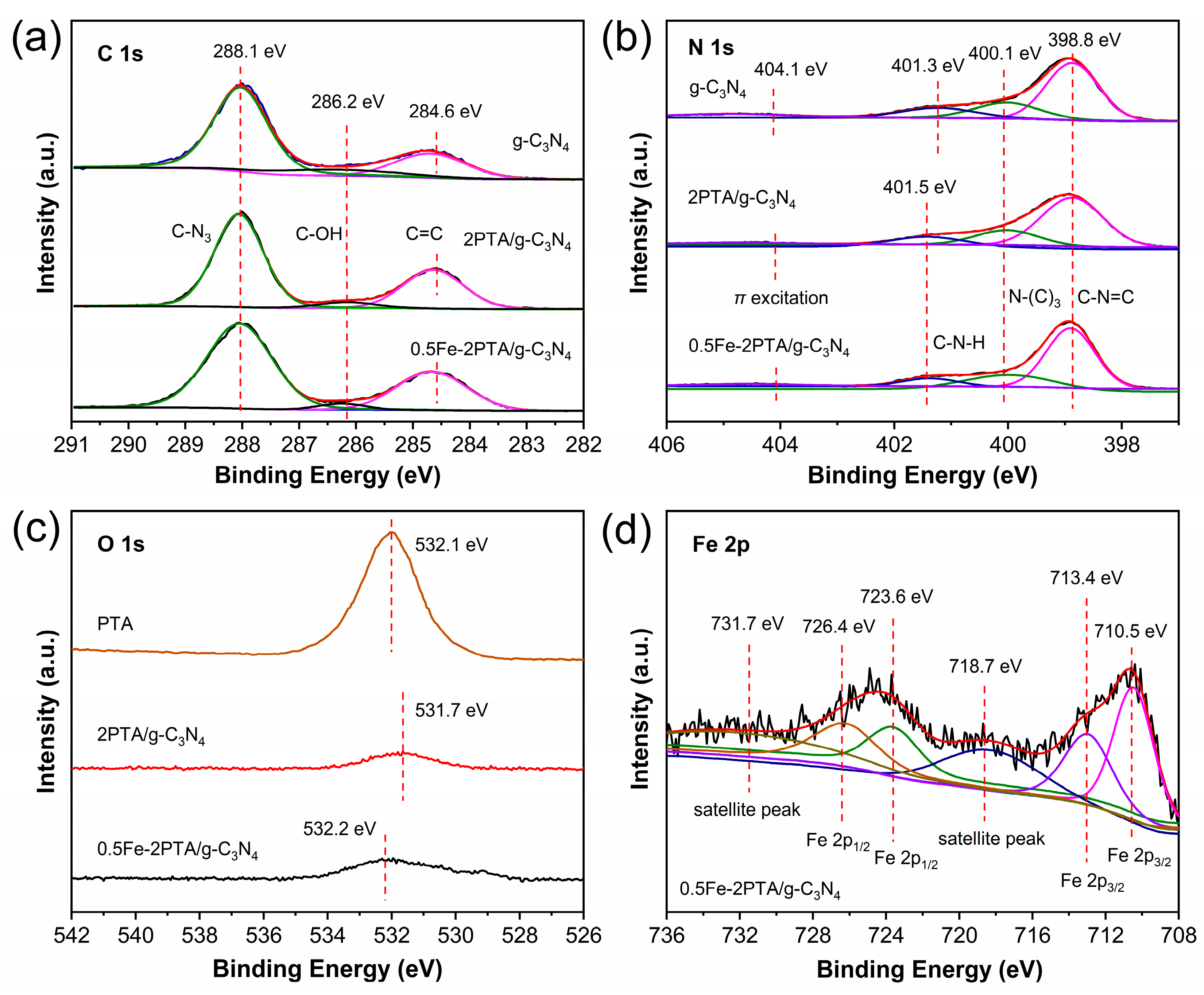
3.1. Structure Characterization
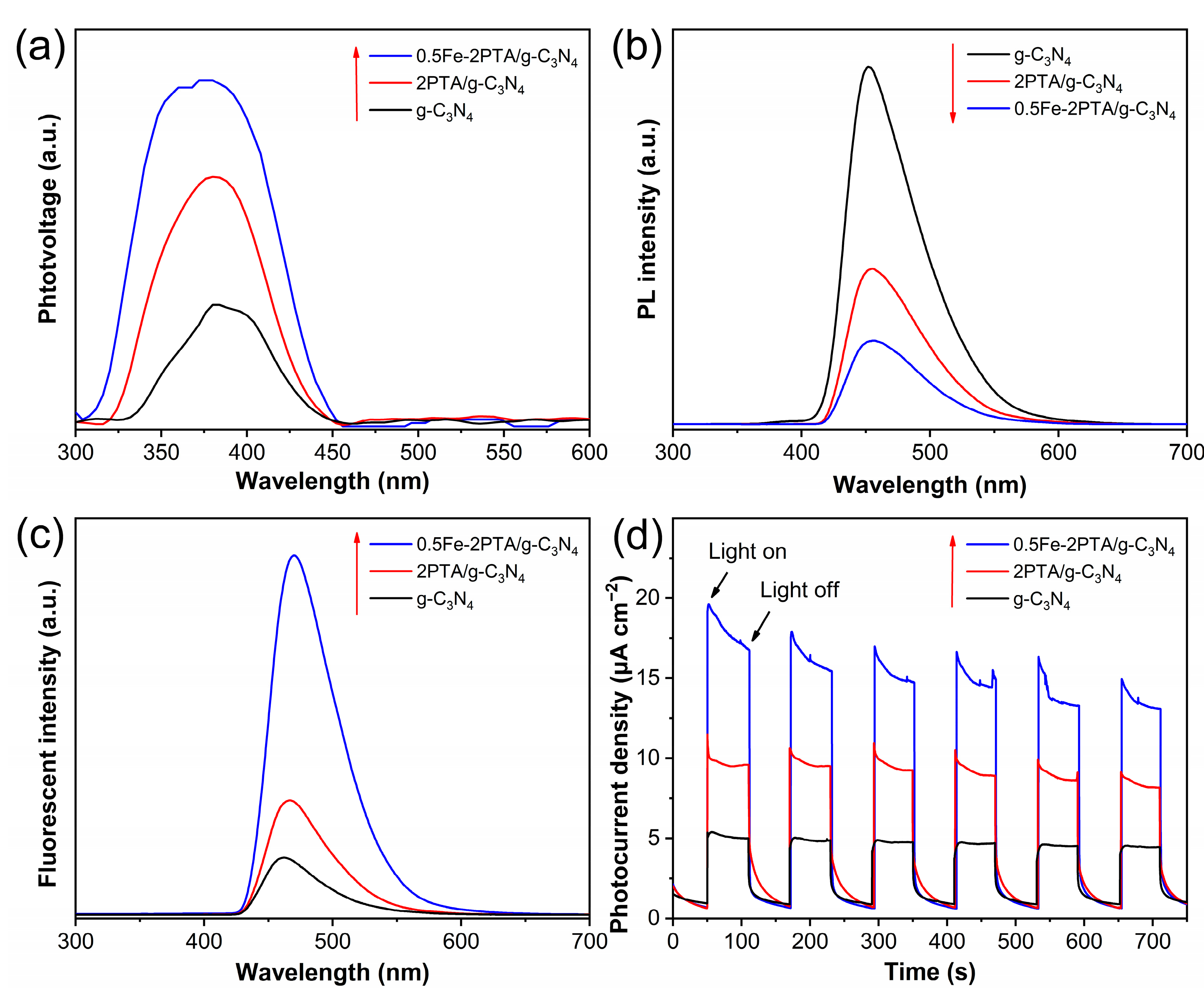
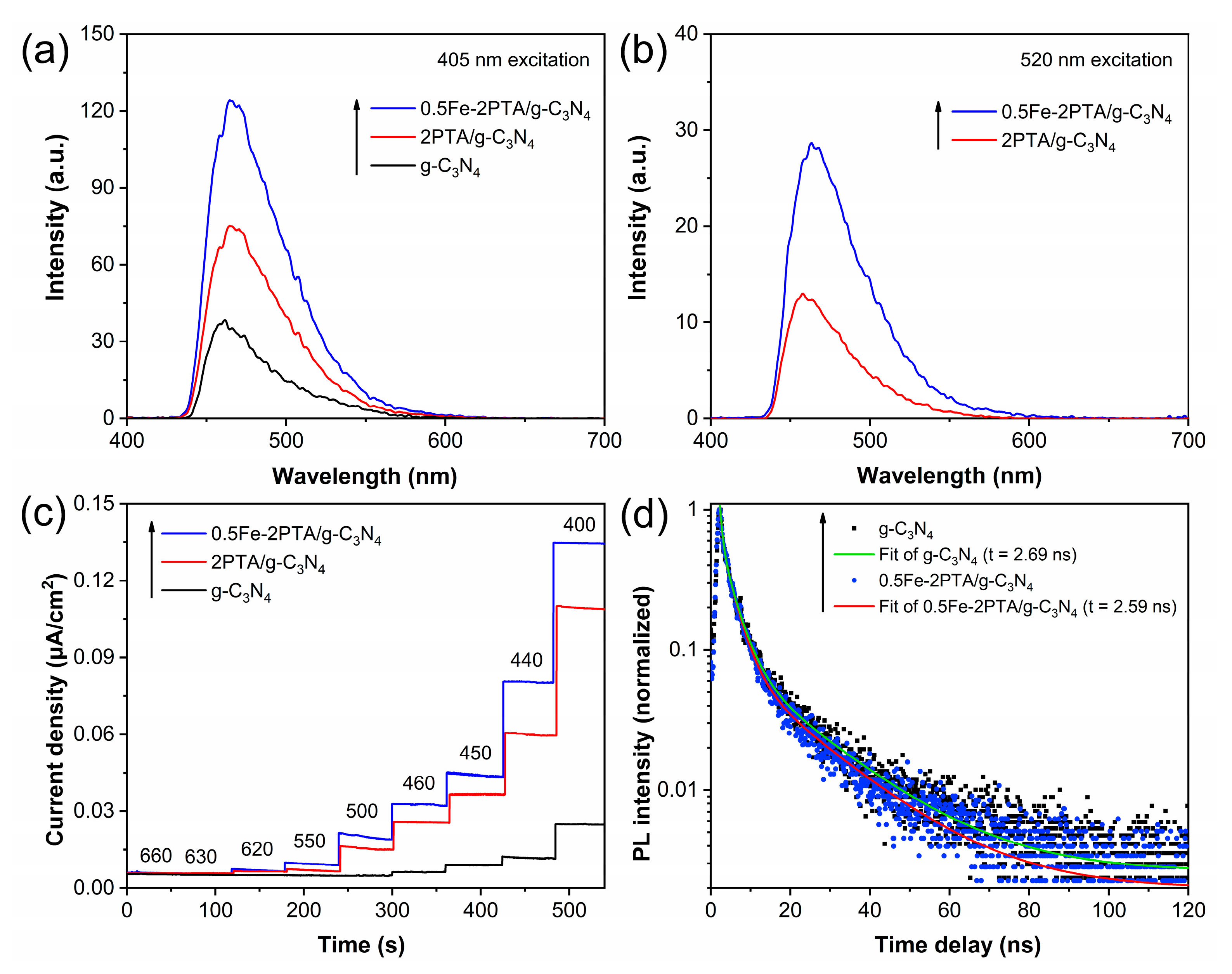
3.2. Photophysical and Photochemical Properties
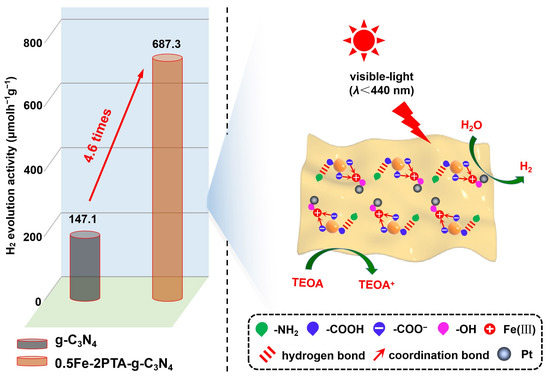
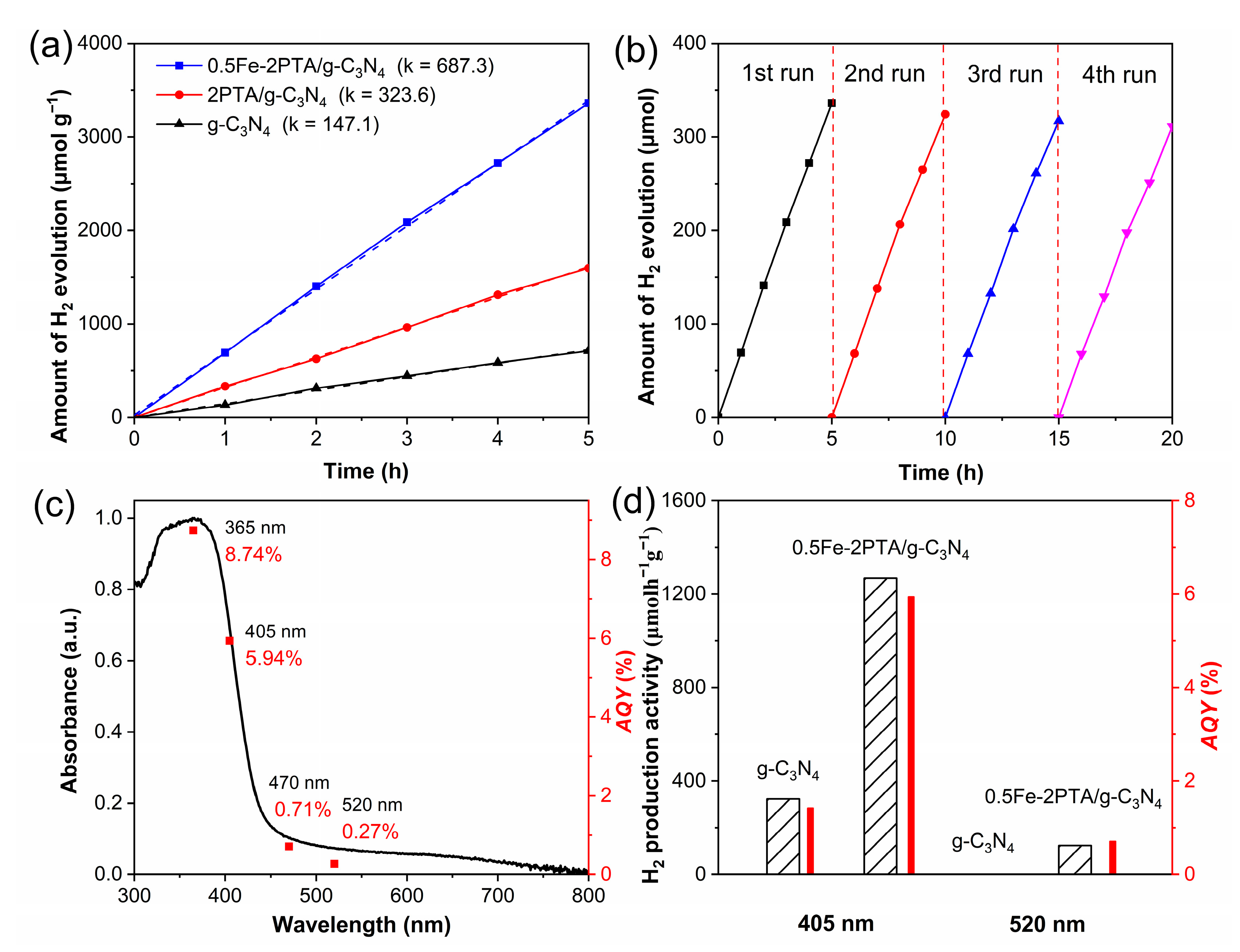
3.3. Photocatalytic Activities for H2 Evolution
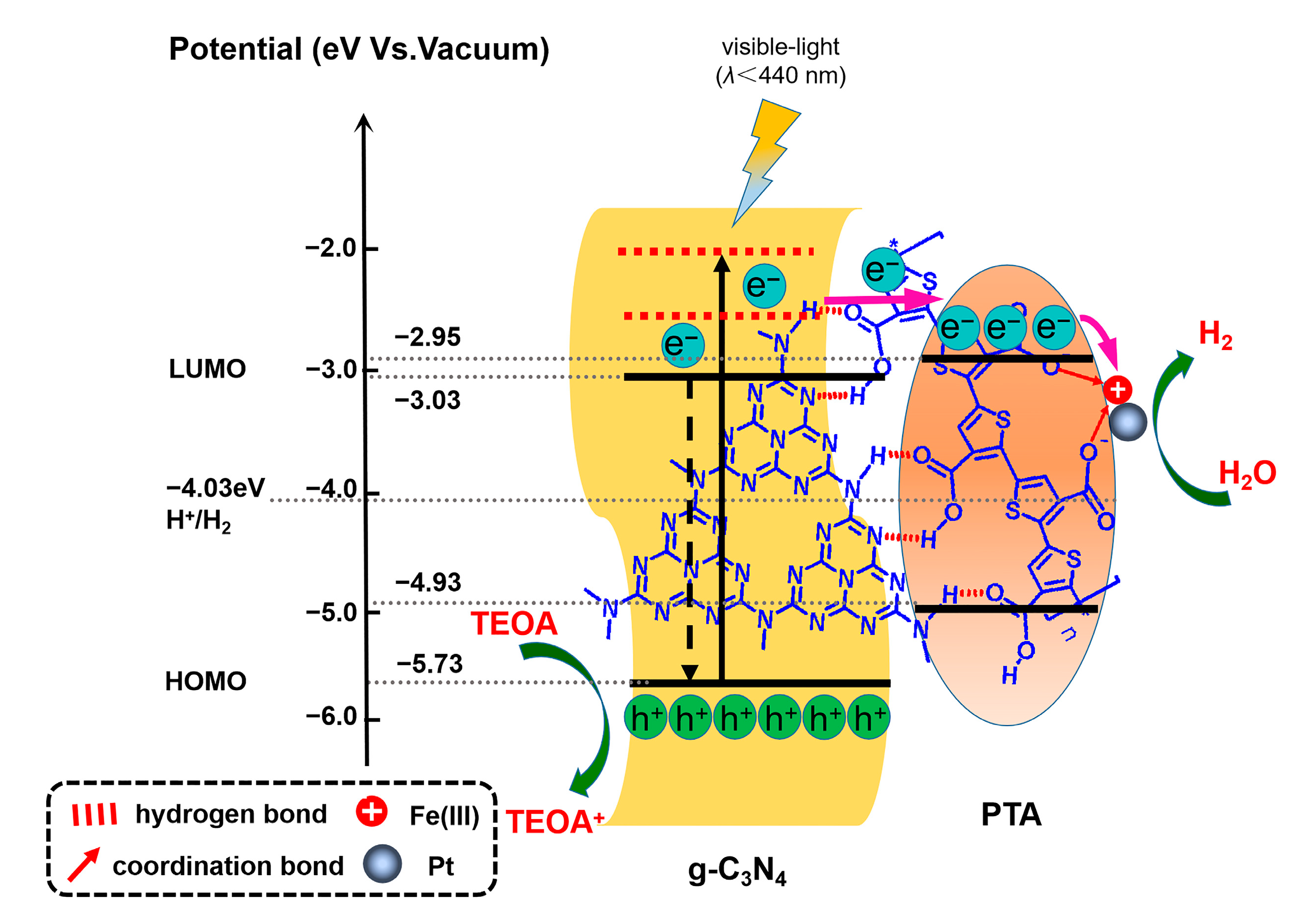
3.4. Discussion on Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.; Liu, Y.; Wu, J.; Zhang, Z.; Li, X.; Wang, X.; Fan, H. Concurrent H2 generation and formate production assisted by CO2 absorption in one electrolyzer. Small Methods 2021, 5, 2100871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ke, J.; Wu, H.; Liu, J.; Xu, D.; Zou, X. Manganese tungstate/graphitic carbon nitride S-scheme heterojunction for boosting hydrogen evolution and mechanism exploration. Mater. Today Energy 2022, 23, 100918. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, J.; Sun, J.; Bian, J.; Wang, J.; Qu, Y.; Yan, R.; Qin, C.; Jing, L. Exceptional visible-light activities of g-C3N4 nanosheets dependent on the unexpected synergistic effects of prolonging charge lifetime and catalyzing H2 evolution with H2O. Appl. Catal. B Environ. 2018, 237, 50–58. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Y.; Gao, X.; Bai, H.; Meng, X. Interfacial charge transfer and enhanced photocatalytic mechanisms for Pt nanoparticles loaded onto sulfur-doped g-C3N4 in H2 evolution. Mater. Today Energy 2021, 22, 100881. [Google Scholar] [CrossRef]
- Yin, H.; Tang, Z. Ultrathin two-dimensional layered metal hydroxides: An emerging platform for advanced catalysis, energy conversion and storage. Chem. Soc. Rev. 2016, 45, 4873–4891. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Hu, K.; Guo, X.; Qu, Y.; Li, Z.; Yang, F.; Liu, H.; Qin, C.; Jing, L. Controlled synthesis of nitro-terminated poly [2-(3-thienyl)-ethanol]/g-C3N4 nanosheet heterojunctions for efficient visible-light photocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2021, 9, 7306–7317. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhang, D.; Cheng, L.; Xiang, Q. Crystalline carbon nitride supported copper single atoms for photocatalytic CO2 reduction with nearly 100% CO selectivity. ACS Nano 2020, 14, 10552–10561. [Google Scholar] [CrossRef]
- Azoulay, A.; Baldovi, A.; Albero, J.; Azaria, N.; Tzadikov, J.; Tashakory, A.; Karjule, N.; Hayun, S.; García, H.; Shalom, M. Carbon-phosphorus-nitrogen materials as highly thermally stable catalyst supports for CO2 hydrogenation to methanol. ACS Appl. Energy Mater. 2023, 6, 439–446. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 Layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Barrio, J.; Mateo, D.; Albero, J.; García, H.; Shalom, M. A heterogeneous carbon nitride-nickel photocatalyst for efficient low-temperature CO2 Methanation. Adv. Energy Mater. 2019, 9, 1902738. [Google Scholar] [CrossRef]
- Ran, J.; Guo, W.; Wang, H.; Zhu, B.; Yu, J.; Qiao, S. Metal-free 2D/2D phosphorene/g-C3N4 van der waals heterojunction for highly enhanced visible-light photocatalytic H2 Production. Adv. Mater. 2018, 30, 1800128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. g-C3N4-based 2D/2D composite heterojunction photocatalyst. Small Struct. 2021, 2, 2100086. [Google Scholar] [CrossRef]
- She, X.; Xu, H.; Yu, Y.; Li, L.; Zhu, X.; Mo, Z.; Song, Y.; Wu, J.; Yuan, S.; Li, H. Accelerating photogenerated charge kinetics via the synergetic utilization of 2D semiconducting structural advantages and noble-metal-free schottky junction effect. Small 2019, 15, 1804613. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.; Ivanov, I.; Ji, H.; Qin, Z.; Wu, Z. 2D/2D heterojunction of Ti3C2/g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, H.; Li, X.; Fan, J.; Xiang, Q. Carbon-graphitic carbon nitride hybrids for heterogeneous photocatalysis. Small 2021, 17, 2005231. [Google Scholar] [CrossRef]
- Ma, T.; Dai, S.; Jaroniec, M.; Qiao, S. Graphitic carbon nitride nanosheet-carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. 2014, 126, 7409–7413. [Google Scholar] [CrossRef]
- Chen, J.; Dong, C.; Zhao, D.; Huang, Y.; Wang, X.; Samad, L.; Dang, L.; Shearer, M.; Shen, S.; Guo, L. Molecular design of polymer heterojunctions for efficient solar-hydrogen conversion. Adv. Mater. 2017, 29, 1606198. [Google Scholar] [CrossRef]
- Chu, X.; Liu, H.; Yu, H.; Bai, L.; Yang, F.; Zhao, L.; Zhao, Z.; Jiao, Y.; Li, W.; Zhang, G.; et al. Improved visible-light activities of ultrathin CoPc/g-C3N4 heterojunctions by N-doped graphene modulation for selective benzyl alcohol oxidation. Mater. Today Energy 2022, 25, 100963. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, S.; Ye, H.; Kong, K.; Gong, X.; Hua, J.; Tian, H. Molecular engineering of donor-acceptor conjugated polymer/g-C3N4 heterostructures for significantly enhanced hydrogen evolution under visible-light irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, B.; Zhang, S.; Peng, T. Robust wide visible-light-responsive photoactivity for H2 production over a polymer/polymer heterojunction photocatalyst: The significance of sacrificial reagent. ACS Sustain. Chem. Eng. 2015, 3, 1501–1509. [Google Scholar] [CrossRef]
- Bai, X.; Sun, C.; Wu, S.; Zhu, Y. Enhancement of photocatalytic performance via a P3HT-g-C3N4 heterojunction. J. Mater. Chem. A 2015, 3, 2741–2747. [Google Scholar] [CrossRef]
- Miao, H.; Yang, J.; Sheng, Y.; Li, W.; Zhu, Y. Controlled synthesis of higher interfacial electron transfer graphite-like carbon nitride/perylenetetracarboxylic diimide heterogeneous for enhanced photocatalytic activity. Sol. RRL 2021, 5, 2000453. [Google Scholar] [CrossRef]
- Sachs, M.; Sprick, R.; Pearce, D.; Hillman, S.; Monti, A.; Guilbert, A.; Brownbill, N.; Dimitrov, S.; Shi, X.; Blanc, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Commun. 2018, 9, 4968. [Google Scholar] [CrossRef]
- Guo, X.; Hu, K.; Chu, M.; Li, Y.; Bian, J.; Qu, Y.; Chu, X.; Yang, F.; Zhao, Q.; Qin, C.; et al. Mg-O-bridged polypyrrole/g-C3N4 nanocomposites as efficient visible-light catalysts for hydrogen evolution. ChemSusChem 2020, 13, 3707–3717. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Hong, W.; Zong, R.; Yao, W.; Zhu, Y. Visible light photoactivity enhancement via CuTCPP hybridized g-C3N4 nanocomposite. Appl. Catal. B Environ. 2015, 166–167, 366–373. [Google Scholar] [CrossRef]
- Lin, L.; Hou, C.; Zhang, X.; Wang, Y.; Chen, Y.; He, T. Highly efficient visible-light driven photocatalytic reduction of CO2 over g-C3N4 nanosheets/tetra (4-carboxyphenyl)porphyrin iron (Ⅲ) chloride heterogeneous catalysts. Appl. Catal. B Environ. 2018, 221, 312–319. [Google Scholar] [CrossRef]
- Yan, H.; Huang, Y. Polymer composites of carbon nitride and poly (3-hexylthiophene) to achieve enhanced hydrogen production from water under visible light. Chem. Commun. 2011, 47, 4168–4170. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Chen, X.; Zhang, H.; Wang, J. A full-spectrum metal-free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv. Mater. 2018, 30, 1806626. [Google Scholar] [CrossRef]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.; Jiang, H. From metal-organic frameworks to single-atom Fe implanted N-doped porous carbons: Efficient oxygen reduction in both alkaline and acidic media. Angew. Chem. Int. Ed. 2018, 57, 8525–8529. [Google Scholar] [CrossRef]
- Sardar, S.; Sarkar, S.; Myint, M.; Al-Harthi, S.; Dutta, J.; Pal, S. Role of central metal ions in hematoporphyrin-functionalized titania in solar energy conversion dynamics. Phys. Chem. Chem. Phys. 2013, 15, 18562. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-responsive gel-sol/sol-gel transition in poly (acrylic acid) aqueous solution containing Fe (Ⅲ) ions switched by light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, B.; Ahmadi, M. Coordination geometry in metallo-supramolecular polymer networks. Coord. Chem. Rev. 2022, 471, 214733. [Google Scholar] [CrossRef]
- Wang, J.; Qin, C.; Wang, H.; Chu, M.; Zada, A.; Zhang, X.; Li, J.; Raziq, F.; Qu, Y.; Jing, L. Exceptional photocatalytic activities for CO2 conversion on Al-O bridged g-C3N4/α-Fe2O3 Z-scheme nanocomposites and mechanism insight with isotopes. Appl. Catal. B Environ. 2018, 221, 459–466. [Google Scholar] [CrossRef]
- Piletsky, S.; Piletska, E.; Karim, K.; Davis, F.; Higson, S.; Turner, A. Photochemical polymerization of thiophene derivatives in aqueous solution. Chem. Commun. 2004, 19, 2222–2223. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yi, M.; Duan, H.; Xu, J.; Wang, Q. Tough TiO2-rGO-PDMAA nanocomposite hydrogel via one-pot UV polymerization and reduction for photodegradation of methylene blue. Carbon 2016, 108, 394–403. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.; Carins, G.; Irvine, J. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, S.; Jiang, L.; Liu, Y.; Li, H.; Hu, W.; Wang, E.; Yan, S.; Wei, Z.; Xu, W.; et al. Nanowire crystals of a rigid rod conjugated polymer. J. Am. Chem. Soc. 2009, 131, 17315–17320. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI-g-C3N4 composite photocatalysts. J. Mater. Chem. 2012, 22, 11843–11850. [Google Scholar] [CrossRef]
- Wajima, T.; Murakami, K.; Kato, T.; Sugawara, K. Heavy metal removal from aqueous solution using carbonaceous K2S-impregnated adsorbent. J. Environ. Sci. 2009, 21, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Lin, D.; Wu, R. Synthesis of high-quality carboxyl end-functionalized poly (3-hexylthiophene)/CdSe nanocomposites. J. Nanomater. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Charpentier, P.; Xu, W. Synthesis and photocatalytic antibacterial properties of poly [2,11′-thiophene-ethylene -thiophene-alt-2, 5-(3-carboxyl)-thiophene]. ACS Appl. Polym. Mater. 2020, 2, 1886–1896. [Google Scholar] [CrossRef]
- Gomes, A.; Zakia, M.; Filho, J.; Armelin, E.; Alemmán, C.; Campos, J. Preparation and characterization of semiconducting polymeric blends. Photochemical synthesis of poly (3-alkylthiophenes) using host microporous matrices of poly (vinylidene fluoride). Polym. Chem. 2012, 3, 1334–1343. [Google Scholar] [CrossRef]
- Zong, X.; Miao, X.; Hua, S.; An, L.; Gao, X.; Jiang, W.; Qu, D.; Zhou, Z.; Liu, X.; Sun, Z. Structure defects assisted photocatalytic H2 production for polythiophene nanofibers. Appl. Catal. B Environ. 2017, 211, 98–105. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.; Kang, F.; Yang, Q. Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.; Wu, L.; Tung, C.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe (Ⅲ)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Bian, J.; Feng, J.; Zhang, Z.; Li, Z.; Zhang, Y.; Liu, Y.; Ali, S.; Qu, Y.; Bai, L.; Xie, J.; et al. Dimension-matched zinc phthalocyanine/BiVO4 ultrathin nano-composites for CO2 reduction as efficient wide-visible-light-driven photocatalysts via a cascade charge transfer. Angew. Chem. 2019, 131, 10989. [Google Scholar] [CrossRef]
- Christoforidis, K.; Syrgiannis, Z.; Parola, V.; Montini, T.; Petit, C.; Stathatos, E.; Godin, R.; Durrant, J.; Prato, M.; Fornasiero, P. Metal-free dual-phase full organic carbon nanotubes/g-C3N4 heteroarchitectures for photocatalytic hydrogen production. Nano Energy 2018, 50, 468–478. [Google Scholar] [CrossRef]
- Lin, L.; Ou, H.; Zhang, Y.; Wang, X. Tri-s-triazine-based crystalline graphitic carbon nitrides for highly efficient hydrogen evolution photocatalysis. ACS Catal. 2016, 6, 3921–3931. [Google Scholar] [CrossRef]
- Li, K.; Xie, X.; Zhang, W. Porous graphitic carbon nitride derived from melamine-ammonium oxalate stacking sheets with excellent photocatalytic hydrogen evolution activity. ChemCatChem 2016, 8, 2128–2135. [Google Scholar] [CrossRef]
- Li, B.; Dong, Y.; Li, L. Preparation and catalytic performance of Fe (Ⅲ)-citric acid-modified cotton fiber complex as a novel cellulose fiber-supported heterogeneous photo-fenton catalyst. Cellulose 2015, 22, 1295–1309. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Li, Q.; Li, F.; Fan, Z.; Wang, H.; Wang, Y.; Zheng, B.; Wu, G. Fe3+ doping promoted N2 photofixation ability of honeycombed graphitic carbon nitride: The experimental and density functional theory simulation analysis. Appl. Catal. B Environ. 2017, 201, 58–69. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, L.; Li, Z.; Qu, Y.; Wu, W.; Jing, L. Porous two-dimension MnO2-C3N4/titanium phosphate nanocomposites as efficient photocatalsyts for CO oxidation and mechanisms. Appl. Catal. B Environ. 2021, 282, 119563. [Google Scholar] [CrossRef]
- Li, Y.; Pang, X.; Zhao, Q.; Zhang, B.; Guo, X.; Zhang, Y.; Xie, Y.; Qin, C.; Jing, L. Controlled synthesis of nitro-terminated oligothiophene/crystallinity-improved g-C3N4 heterojunctions for enhanced visible-light catalytic H2 production. ACS Appl. Mater. Interfaces 2023, 15, 5365–5377. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Q.; Nan, J.; Shi, W.; Cui, F.; Zhu, Y. Perylenetetracarboxylic acid nanosheets with internal electric fields and anisotropic charge migration for photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2067. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Song, E.; Lin, J.; Zhou, J.; Suenaga, K.; Zhou, W.; Liu, Z.; Liu, J.; Lou, J.; et al. Enhanced performance of in-plane transition metal dichalcogenides monolayers by configuring local atomic structures. Nat. Commun. 2020, 11, 2253. [Google Scholar] [CrossRef]
- Yang, C.; Ma, B.; Zhang, L.; Lin, S.; Ghasimi, S.; Landfester, K.; Zhang, K.; Wang, X. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew. Chem. Int. Ed. 2016, 55, 9202–9206. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Wang, X. g-C3N4/Ti3C2Tx (MXenes) composite with oxidized surface groups for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Liu, Z.; Li, N.; Chen, X.; Hu, J.; Li, X. Multi-functional Ni3C cocatalyst/g-C3N4 nanoheterojunctions for robust photocatalytic H2 evolution under visible light. J. Mater. Chem. A 2018, 6, 13110–13122. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, Z.; Feng, J.; Thangamuthu, M.; Yang, F.; Sun, L.; Li, Z.; Qu, Y.; Tang, D.; Lin, Z.; et al. Energy platform for directed charge transfer in the cascade Z-scheme heterojunction: CO2 photoreduction without a cocatalyst. Angew. Chem. Int. Ed. 2021, 60, 20906–20914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, T.; Shi, H.; Yu, D.; Hong, W.; Chen, X. Conjugated polymer dots/graphitic carbon nitride nanosheet heterojunctions for metal-free hydrogen evolution photocatalysis. J. Mater. Chem. A 2019, 7, 303–311. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Wu, Z.; Liang, J.; Chen, X.; Leng, L.; Wang, H.; Wang, H. Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 221, 715–725. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, B.; Pang, X.; Li, Z.; Zhang, Y.; Hao, M.; Zhu, Y.; Qin, C.; Jing, L. Improved Visible-Light Photocatalytic H2 Evolution of G-C3N4 Nanosheets by Constructing Heterojunctions with Nano-Sized Poly(3-Thiophenecarboxylic Acid) and Coordinating Fe(III). Nanomaterials 2023, 13, 1338. https://doi.org/10.3390/nano13081338
Li Y, Zhang B, Pang X, Li Z, Zhang Y, Hao M, Zhu Y, Qin C, Jing L. Improved Visible-Light Photocatalytic H2 Evolution of G-C3N4 Nanosheets by Constructing Heterojunctions with Nano-Sized Poly(3-Thiophenecarboxylic Acid) and Coordinating Fe(III). Nanomaterials. 2023; 13(8):1338. https://doi.org/10.3390/nano13081338
Chicago/Turabian StyleLi, Yong, Bingmiao Zhang, Xulong Pang, Zhijun Li, Yi Zhang, Ming Hao, Yan Zhu, Chuanli Qin, and Liqiang Jing. 2023. "Improved Visible-Light Photocatalytic H2 Evolution of G-C3N4 Nanosheets by Constructing Heterojunctions with Nano-Sized Poly(3-Thiophenecarboxylic Acid) and Coordinating Fe(III)" Nanomaterials 13, no. 8: 1338. https://doi.org/10.3390/nano13081338
APA StyleLi, Y., Zhang, B., Pang, X., Li, Z., Zhang, Y., Hao, M., Zhu, Y., Qin, C., & Jing, L. (2023). Improved Visible-Light Photocatalytic H2 Evolution of G-C3N4 Nanosheets by Constructing Heterojunctions with Nano-Sized Poly(3-Thiophenecarboxylic Acid) and Coordinating Fe(III). Nanomaterials, 13(8), 1338. https://doi.org/10.3390/nano13081338